Effect of Modified Clay on the Growth Dynamics and Physio—Biochemical Response of Newly Hatched Larvae of the Marine Medaka (Oryzias melastigma)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Culture and Modified Clay Preparation
2.2. Experimental Design
2.3. Measurement of Basic Parameters
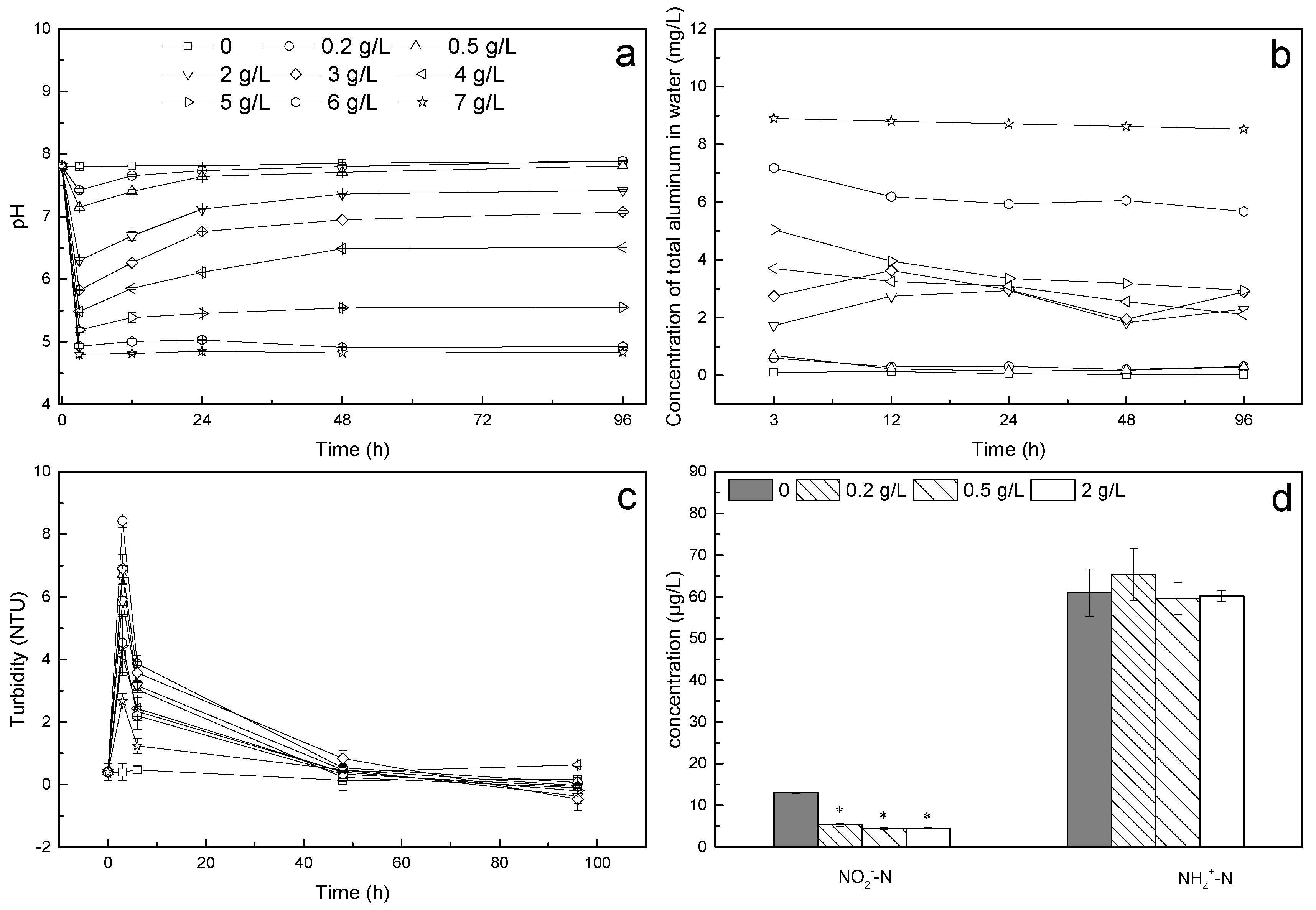
2.3.1. Environmental Parameter Measurement
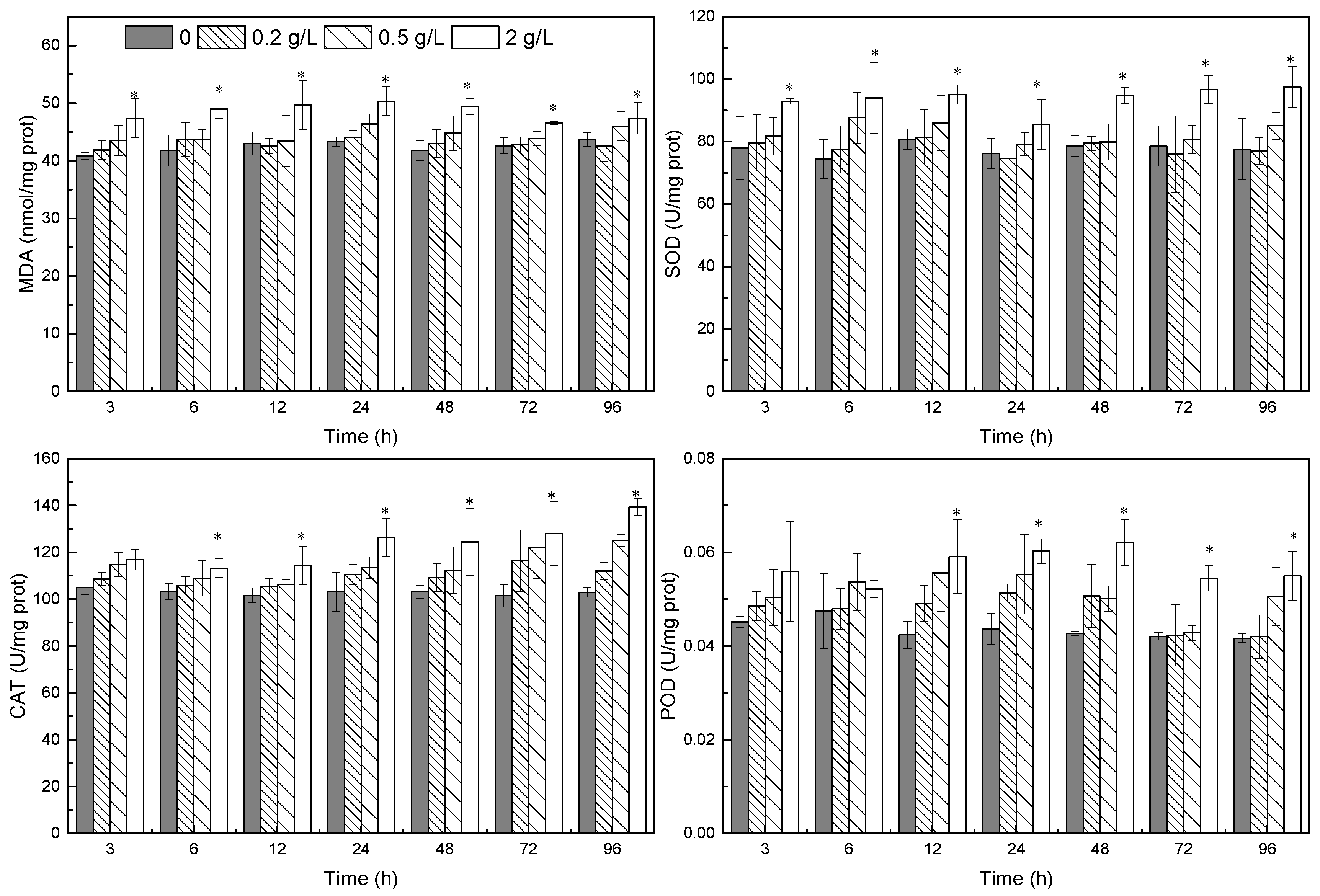
2.3.2. Oxidative Stress Indicators
2.3.3. Aluminum Content of the Fish
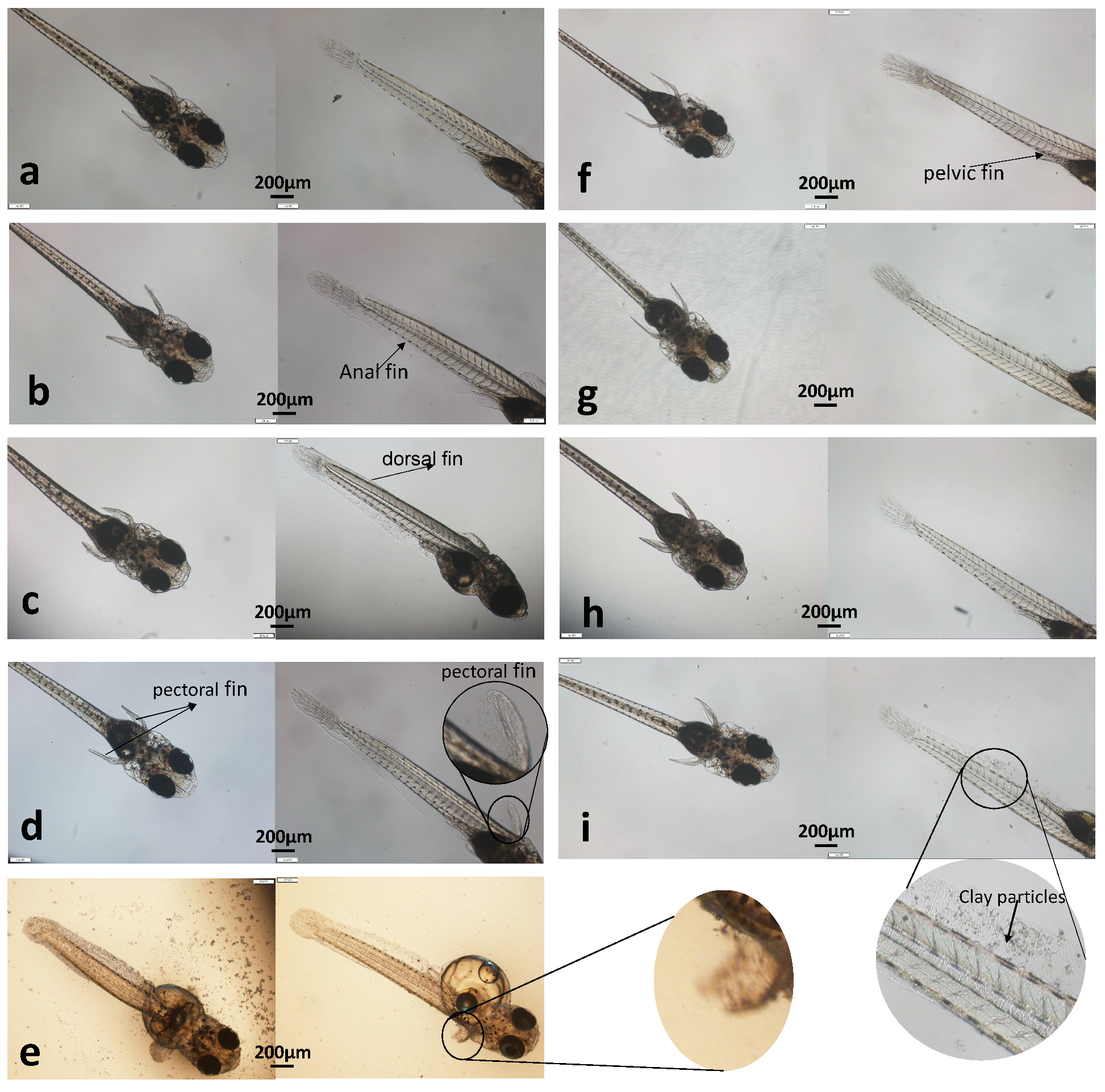
2.3.4. Microscope Observation
2.4. Statistical Analysis
3. Results
3.1. Growth Indicators of Marine Medaka
3.1.1. Survival
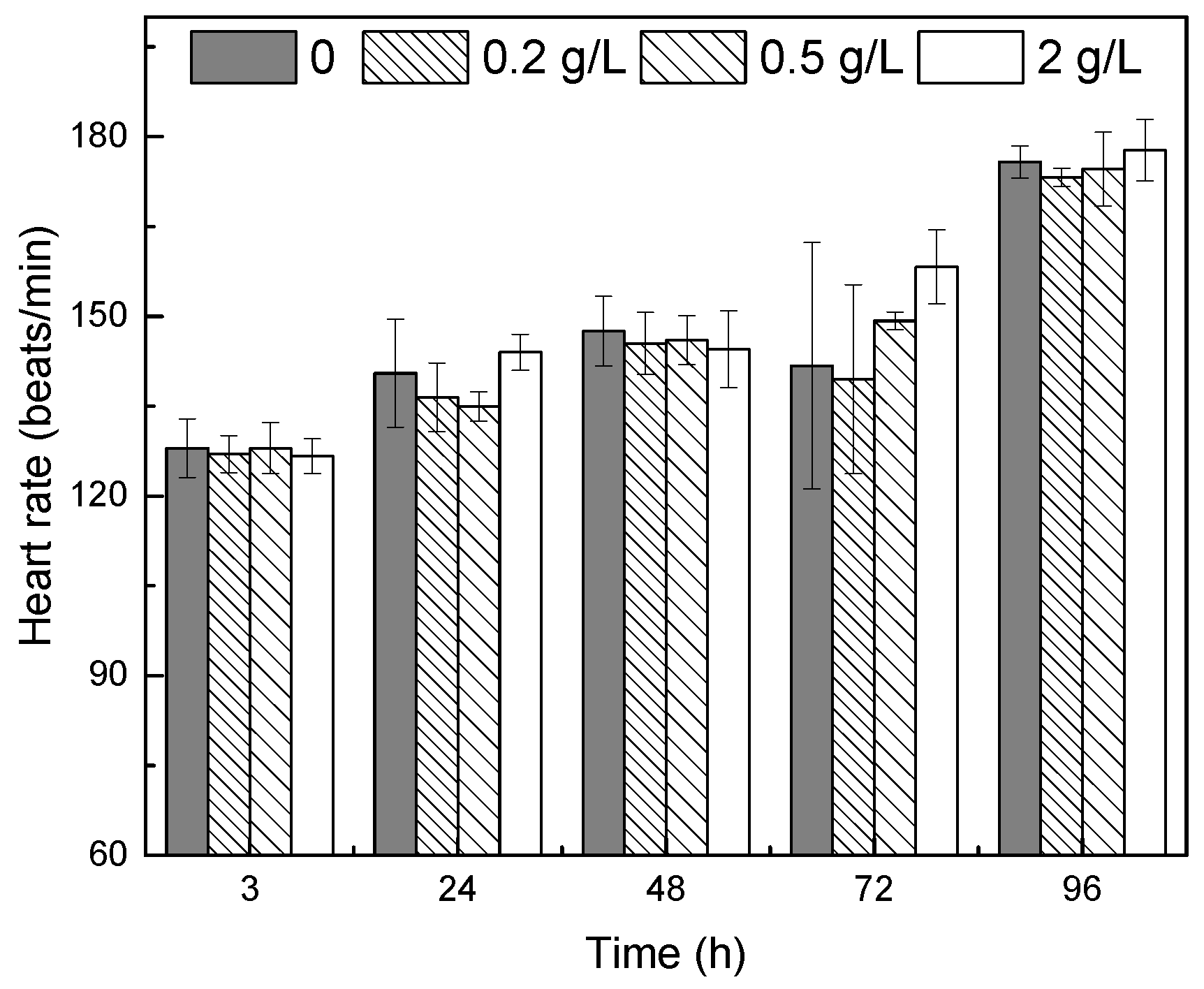
3.1.2. Heart Rate and Total Length of the Larvae
3.1.3. Morphological Observation
3.2. Oxidative Stress Indicators
3.3. Environmental Parameters
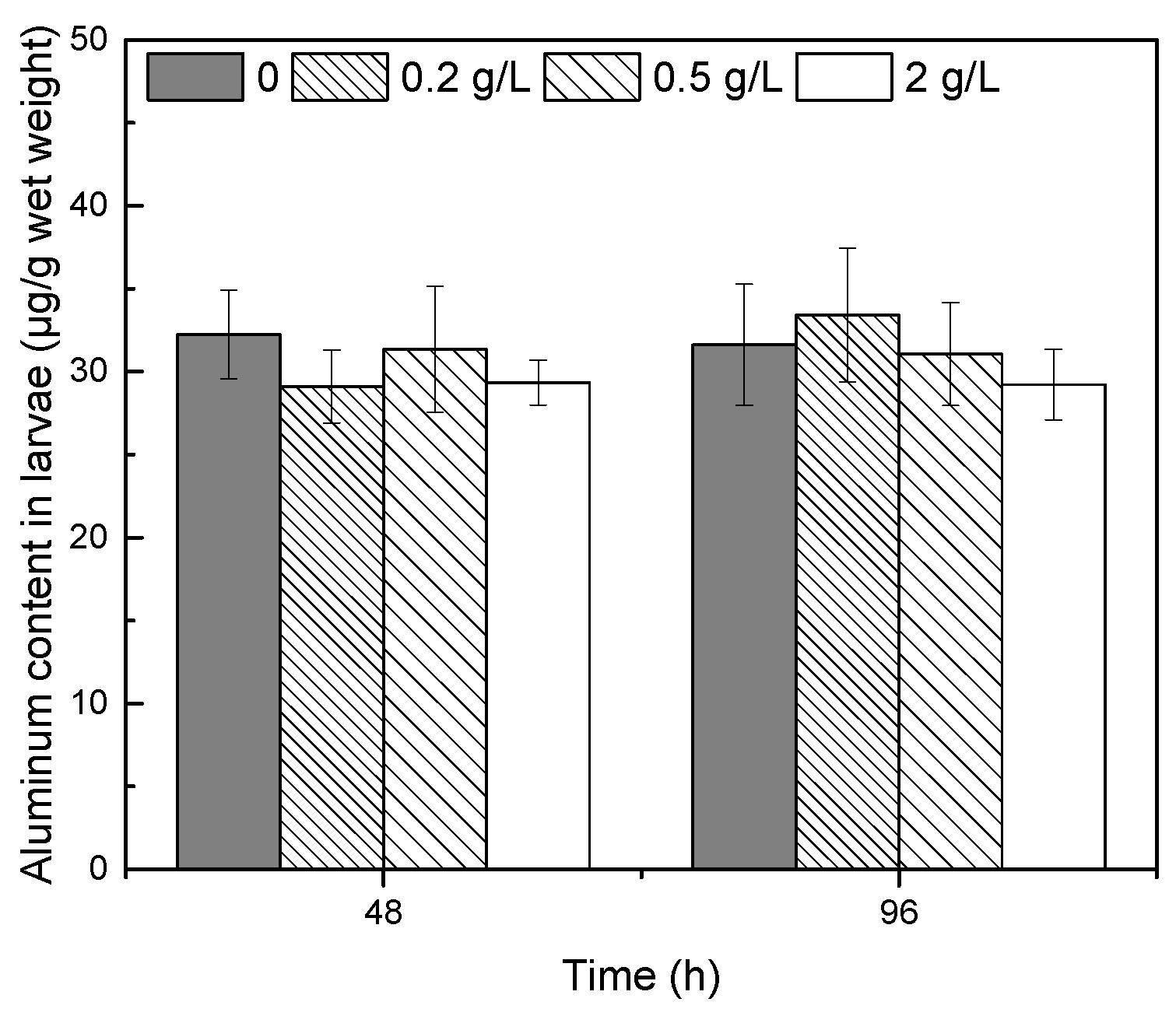
3.4. Changes in the Aluminum Content in Newly Hatched Larvae
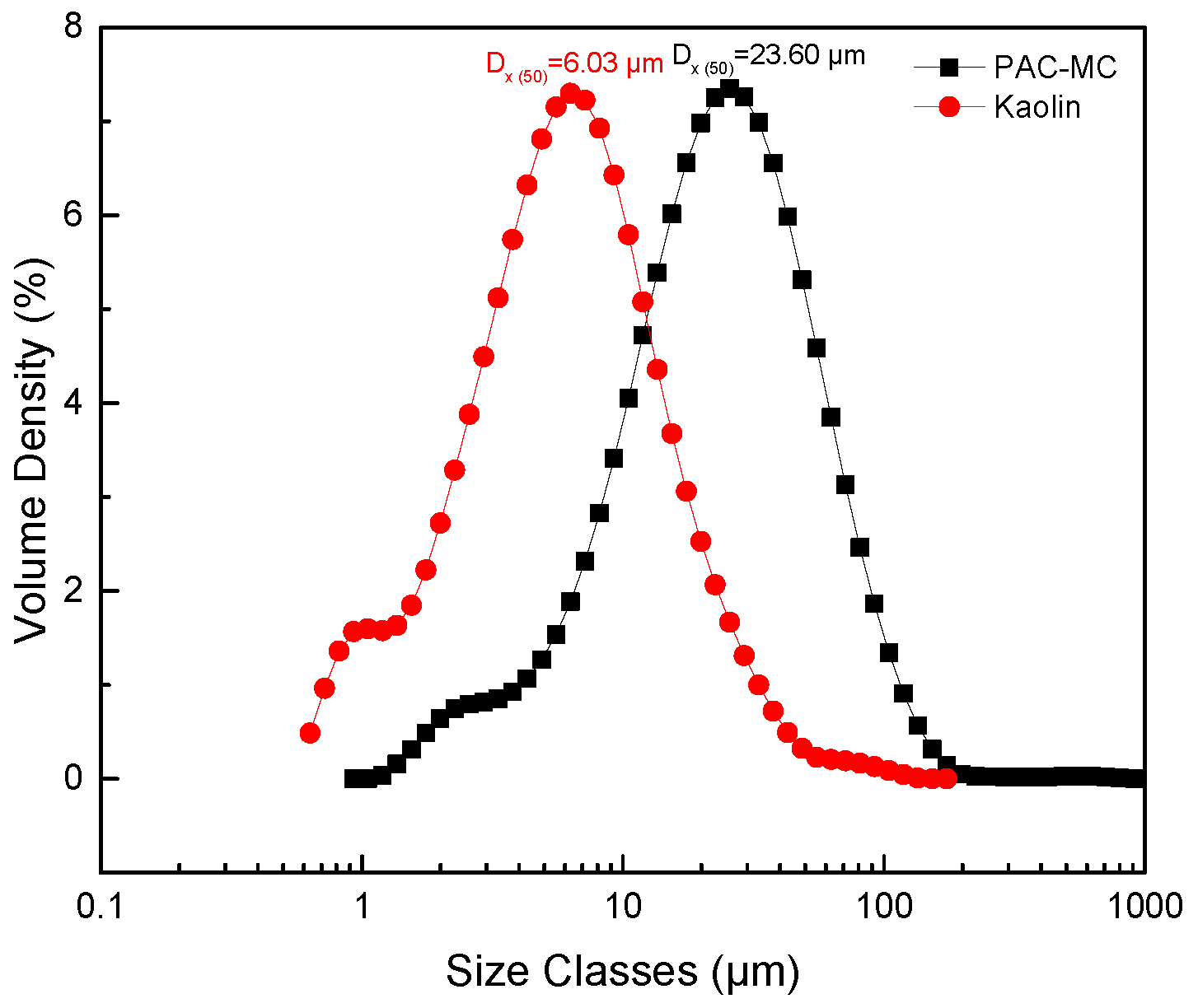
3.5. Changes in Clay Properties before and after PAC Modification
4. Discussion
4.1. The Effect of PAC-MC on the Survival and Morphology of Marine Medaka
4.2. The Effect of PAC-MC on the Oxidative Stress of Marine Medaka
4.3. Effects of PAC-MC on Aluminum Accumulation in Fish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef] [Green Version]
- Shirota, A. Red tide problem and countermeasures (2). Int. J. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Kim, H.G. Mitigation and controls of HABs. Ecol. Harmful Algae 2006, 189, 327–338. [Google Scholar] [CrossRef]
- Chen, W.; Jia, Y.; Li, E.; Shuang, Z.; Zhou, Q.; Liu, L.; Song, L. Soil-Based Treatments of Mechanically Collected Cyanobacterial Blooms from Lake Taihu: Efficiencies and Potential Risks. Environ. Technol. 2012, 46, 13370–13376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, Q. Algal fouling of microfiltration and ultrafiltration membranes and control strategies: A review. Sep. Purif. Technol. 2018, 203, 193–208. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V. Flocculation of algae using chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Sun, X.X.; Lee, Y.J.; Choi, J.K.; Kim, E.K. Synergistic effect of sophorolipid and loess combination in harmful algal blooms mitigation. Mar. Pollut. Bull. 2004, 48, 863–872. [Google Scholar] [CrossRef]
- Ma, J.; Wei, L. Effectiveness and mechanism of potassium ferrate(VI) preoxidation for algae removal by coagulation. Water Res. 2002, 36, 871–878. [Google Scholar] [CrossRef]
- Zhang, S.D.; Song, X.X.; Cao, X.H.; Ming, Y.Z. Inhibitory Effect of Gracilaria lemaneiformis(Bory) Weber Bosse on the Cocultured Scrippsiella trochoidea(Stein) Loeblich III Under Controlled Laboratory Conditions. Environ. Sci. 2008, 29, 2291–2295. [Google Scholar]
- Marcoval, M.A.; Pan, J.; Tang, Y.; Gobler, C.J. The ability of the branchiopod, Artemia salina, to graze upon harmful algal blooms caused by Alexandrium fundyense, Aureococcus anophagefferens, and Cochlodinium polykrikoides. Estuar. Coast. Shelf Sci. 2013, 131, 235–244. [Google Scholar] [CrossRef]
- Kodama, M.; Doucette, G.J.; Green, D.H. Relationships between bacteria and harmful algae. Ecol. Harmful Algae 2006, 189, 243–255. [Google Scholar]
- Yang, Y.F.; Liu, Q.; Chai, Z.Y.; Tang, Y.Z. Inhibition of marine coastal bloom-forming phytoplankton by commercially cultivated Gracilaria lemaneiformis (Rhodophyta). J. Appl. Phycol. 2015, 27, 2341–2352. [Google Scholar] [CrossRef]
- Ichiro, I.; Mineo, Y.; Yutaka, H. Eutrophication and occurrences of harmful algal blooms in the Seto Inland Sea, Japan. Plankton Benthos Res. 2006, 1, 71–84. [Google Scholar]
- Lee, Y.C.; Jin, E.S.; Jung, S.W.; Kim, Y.M.; Chang, K.S.; Yang, J.W.; Kim, S.W.; Kim, Y.O.; Shin, H.J. Utilizing the algicidal activity of aminoclay as a practical treatment for toxic red tides. Sci. Rep. 2013, 3, 1292. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zou, J. A more effective clay for removing red tide organisms. J. Nat. Disasters 1994, 3, 105–109. [Google Scholar]
- Yu, Z.; Zou, J.; Ma, X. A new method to improve the capability of clays for removing red tide organisms. Oceanol. Et Limnol. Sin. 1994, 25, 226–232. [Google Scholar]
- Yu, Z.M.; Zou, J.Z.; Ma, X.N. Application of clays to removal of red tide organisms I. Coagulation of red tide organisms with clays. Chin. J. Oceanol. Limnol. 1994, 12, 316–324. [Google Scholar]
- Yu, Z.M.; Zou, J.Z.; Ma, X.N. Application of clays to removal of red tide organisms II. Coagulation of different species of red tide organisms with montmorillonite and effect of clay pretreatment. Chin. J. Oceanol. Limnol. 1994, 12, 316–324. [Google Scholar]
- Yu, Z.; Song, X.; Cao, X.; Liu, Y. Mitigation of harmful algal blooms using modified clays: Theory, mechanisms, and applications. Harmful Algae 2017, 69, 48–64. [Google Scholar] [CrossRef]
- Orizar, I.S.; Rivera, P.P.L.; San Diego-McGlone, M.L.; Azanza, R.V. Harmful Algal Bloom (HAB) mitigation using ball clay: Effect on non-target organisms. J. Environ. Sci. Manag. 2013, 16, 36–43. [Google Scholar]
- Zhang, Y.; Song, X.; Shen, H.; Cao, X.; Yuan, Y.; Wu, Z.; Yu, Z. The Effects of Modified Clay on Abalone (Haliotis discus hannai) Based on Laboratory and Field Experiments. Environ. Toxicol. Chem. 2020, 39, 2065–2075. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Song, X.; Cao, X. Effects of modified clay on the infant of Patinopecten yessoensis for HABs control. Mar. Environ. Sci. 2014, 33, 817–821. [Google Scholar]
- Gao, Y.; Yu, Z.; Song, X.; Cao, X. Impact of Modified Clays on the Infant Oyster(Crassostrea gigas). Mar. Sci. Bull. 2007, 26, 53–60. [Google Scholar]
- Wang, Z.; Song, X.; Zhang, Y.; Yu, Z.; Tang, X. Effects of modified clay on Mercenaria mercenaria. Oceanol. Limnol. Sin. 2019, 50, 692–699. [Google Scholar]
- Kim, B.M.; Kim, J.; Choi, I.Y.; Raisuddin, S.; Au, D.; Leung, K.; Wu, R.; Rhee, J.S.; Lee, J.S. Omics of the marine medaka (Oryzias melastigma) and its relevance to marine environmental research. Mar. Environ. Res. 2016, 113, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W. Relations Among Equilibrium and Nonequilibrium Aqueous Species of Aluminum Hydroxy Complexes. In Nonequilibrium Systems in Natural Water Chemistry; American Chemical Society: Washington, WA, USA, 1971; Volume 106, pp. 250–279. [Google Scholar]
- Bersillon, J.; Hsu, L.; Pa, H.; Fiessinger, F. Characterization of Hydroxy-Aluminum Solutions. Soil Sci. Soc. Am. J. 1980, 44, 630–634. [Google Scholar] [CrossRef]
- Winston, G.W.; Giulio, R. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Stegeman, J.J.; Brouwer, M.; Giulio, R.; Frlin, L.; Veld, P. Molecular responses to environmental contamination Enzyme and protein systems as indicators of chemical exposure and effect. Biomark. Biochem. Physiol. Histol. Mark. Anthropog. Stress 1992, 235–335. [Google Scholar]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Wang, X.Q. Effects of dimethylhydantoin and carbohydrate on growth and immunity of Litopenaeus vannamei. Adv. Mater. Res. 2012, 356–360, 146–151. [Google Scholar]
- Lee, C.K.; Kim, W.S.; Park, Y.T.; Jo, Q.T. Effect of Yellow Clay on the Oxygen Consumption Rate of Korean rockfish, Sebastes schlegelii. J. Korean Soc. Mar. Environ. Saf. 2013, 19, 241–247. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Song, X.; Cao, X.; Liu, K. Impact of modified clay on the growth of the infant Apostichopus japonicas Selenka in HABs controling. Oceanol. Limnol. Sin. 2014, 45, 233–238. [Google Scholar]
- Zhang, Y.; Song, X.; Yu, Z.; Zhang, P.; Cao, X.; Yuan, Y. Impact assessment of modified clay on embryo-larval stages of turbot Scophthalmus maximus L. Chin. J. Oceanol. Limnol. 2019, 037, 1051–1061. [Google Scholar] [CrossRef]
- Cao, X.H.; Yu, Z.-M.; Qiu, L.X. Field experiment and emergent application of modified clays for phaeocystis globosa blooms mitigation. Oceanol. Limnol. Sin. 2017, 48, 753–759. [Google Scholar]
- Wang, G.; Xie, J.; Yu, D.-G.; Wu, L.; Hu, Z.-Y. Physiological responses of abalone Haliotis divericolor to suspended sediment stress. J. Dalian Fish. Univ. 2007, 22, 352–356. [Google Scholar]
- Yuan, J.; Wang, X.; Gu, Z.; Zhang, Y.; Wang, Z. Activity and Transcriptional Responses of Hepatopancreatic Biotransformation and Antioxidant Enzymes in the Oriental River Prawn Macrobrachium nipponense Exposed to Microcystin-LR. Toxins 2015, 7, 4006–4022. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Xu, W.; Jia, L.; Zheng, D.; Zhang, B.; Ma, C. Effects of Suspended Substances on Antioxidant Enzyme Activities in Muscle of Paralichthys olivaceus and Related Gene Expressions. Adv. Mar. Sci. 2016, 34, 542–552. [Google Scholar]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of Cell Death in Oxidative Stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Assefa, Z.; An, V.L.; Garmyn, M.; Agostinis, P. Ultraviolet radiation-induced apoptosis in keratinocytes: On the role of cytosolic factors. Biochim. Biophys. Acta 2005, 1755, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Richier, S.; Sabourault, C.; Courtiade, J.; Zucchini, N.; Allemand, D.; Furla, P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J. 2010, 273, 4186–4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Au, D.W.T.; Anderson, D.M.; Lam, P.K.S.; Wu, R.S.S. Effects of nutrients, salinity, pH and light:dark cycle on the production of reactive oxygen species in the alga Chattonella marina. J. Exp. Mar. Biol. Ecol. 2007, 346, 76–86. [Google Scholar] [CrossRef]
- Fan, Z.; Yang, A.; Liu, Z.; Dai, J.; Dong, Y.; Ren, J. Effect of pH on the immune factors of Chlamys farreri. J. Fish. Sci. China 2006, 13, 650–654. [Google Scholar]
- Wen, C.; Zhang, L.; Hu, B.; Rao, Y.; Chen, S.; Wang, F. Effect of pH on the Five Immune Factors of Anodonta Woodiana. J. Nanchang Univ. (Nat. Sci.) 2009, 33, 172–176. [Google Scholar]
- Cornwell, D.A.; Burmaster, J.W.; Francis, J.L.; Friedline, J.C.J.; Houck, C.; King, P.H.; San Giacomo, R. Committee report: Research needs for alum sludge discharge. J. Am. Water Work. Assoc. 1987, 79, 99–104. [Google Scholar]
- Yang, X.; Zhang, F.; Wang, X.; Gan, N.; Zou, G.; Bi, S. Novel Analytical Techniques for Fractionation and Speciation of Aluminum(Ⅲ) in Environmental and Biological Systems. Chin. J. Anal. Chem. 2003, 31, 1131–1138. [Google Scholar]
- Yan, M.; Wang, D.; Yu, J.; Ni, J.; Edwards, M.; Qu, J. Enhanced coagulation with polyaluminum chlorides: Role of pH/Alkalinity and speciation. Chemosphere 2008, 71, 1665–1673. [Google Scholar] [CrossRef]
- Dong, X.; Cao, X.; Jiang, W.; Song, X.; Yu, Z. Profiles of and variations in aluminum species in PAC-MC used for the removal of blooming microalgae. J. Environ. Sci. 2021, 106, 76–82. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, S.; Clift, P.D.; Huang, J.; Yu, Z.; Zhang, K.; Mei, X.; Liu, J.; Han, Z.; Nan, Q.; et al. History of Yellow River and Yangtze River delivering sediment to the Yellow Sea since 3.5Ma: Tectonic or climate forcing? Quat. Sci. Rev. 2019, 216, 74–88. [Google Scholar] [CrossRef]
- Wan, S.; Li, A.; Clift, P.D.; Stuur, J.-B.W. Development of the East Asian monsoon: Mineralogical and sedimentologic records in the South China Sea since 20 Ma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 254, 561–582. [Google Scholar] [CrossRef]


| Concentration of PAC-MC (g/L) | Survival Rate at 3 h (%) | Survival Rate at 12 h (%) | Survival Rate at 48 h (%) | Survival Rate at 96 h (%) |
|---|---|---|---|---|
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 0.2 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 0.5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 2 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 3 | 100 ± 0 | 100 ± 0 | 96.3 ± 6.4 | 84.3 ± 13.7 |
| 4 | 100 ± 0 | 100 ± 0 | 93.3 ± 11.6 | 83.3 ± 5.8 |
| 5 | 100 ± 0 | 88.9 ± 9.6 | 88.9 ± 9.6 | 83.3 ± 0 |
| 6 | 100 ± 0 | 64.4 ± 17.1 | 11.1 ± 9.6 | 11.1 ± 9.6 |
| 7 | 74.2 ± 22.4 | 32.5 ± 30.3 | 0 ± 0 | 0 ± 0 |
| MDA | SOD | CAT | POD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | P | F | P | F | P | F | P |
| Time | 6 | 1.523 | 0.187 | 1.429 | 0.220 | 5.378 | 0.000 | 2.960 | 0.014 |
| MC | 3 | 30.996 | 0.000 | 31.861 | 0.000 | 32.727 | 0.000 | 25.704 | 0.000 |
| Time × MC | 18 | 0.479 | 0.957 | 0.493 | 0.951 | 1.367 | 0.185 | 0.903 | 0.578 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Song, X.; Zhang, Y.; Shen, H.; Dong, X.; Li, J.; Yu, Z. Effect of Modified Clay on the Growth Dynamics and Physio—Biochemical Response of Newly Hatched Larvae of the Marine Medaka (Oryzias melastigma). J. Mar. Sci. Eng. 2021, 9, 822. https://doi.org/10.3390/jmse9080822
Zhang P, Song X, Zhang Y, Shen H, Dong X, Li J, Yu Z. Effect of Modified Clay on the Growth Dynamics and Physio—Biochemical Response of Newly Hatched Larvae of the Marine Medaka (Oryzias melastigma). Journal of Marine Science and Engineering. 2021; 9(8):822. https://doi.org/10.3390/jmse9080822
Chicago/Turabian StyleZhang, Peipei, Xiuxian Song, Yue Zhang, Huihui Shen, Xueyi Dong, Jing Li, and Zhiming Yu. 2021. "Effect of Modified Clay on the Growth Dynamics and Physio—Biochemical Response of Newly Hatched Larvae of the Marine Medaka (Oryzias melastigma)" Journal of Marine Science and Engineering 9, no. 8: 822. https://doi.org/10.3390/jmse9080822






