The Potential of Palynology with Regard to the Archaeology of Medieval Monastery Sites in Iceland
Abstract
:1. Introduction
2. Methodology
2.1. Study Sites
2.2. Pollen Sampling
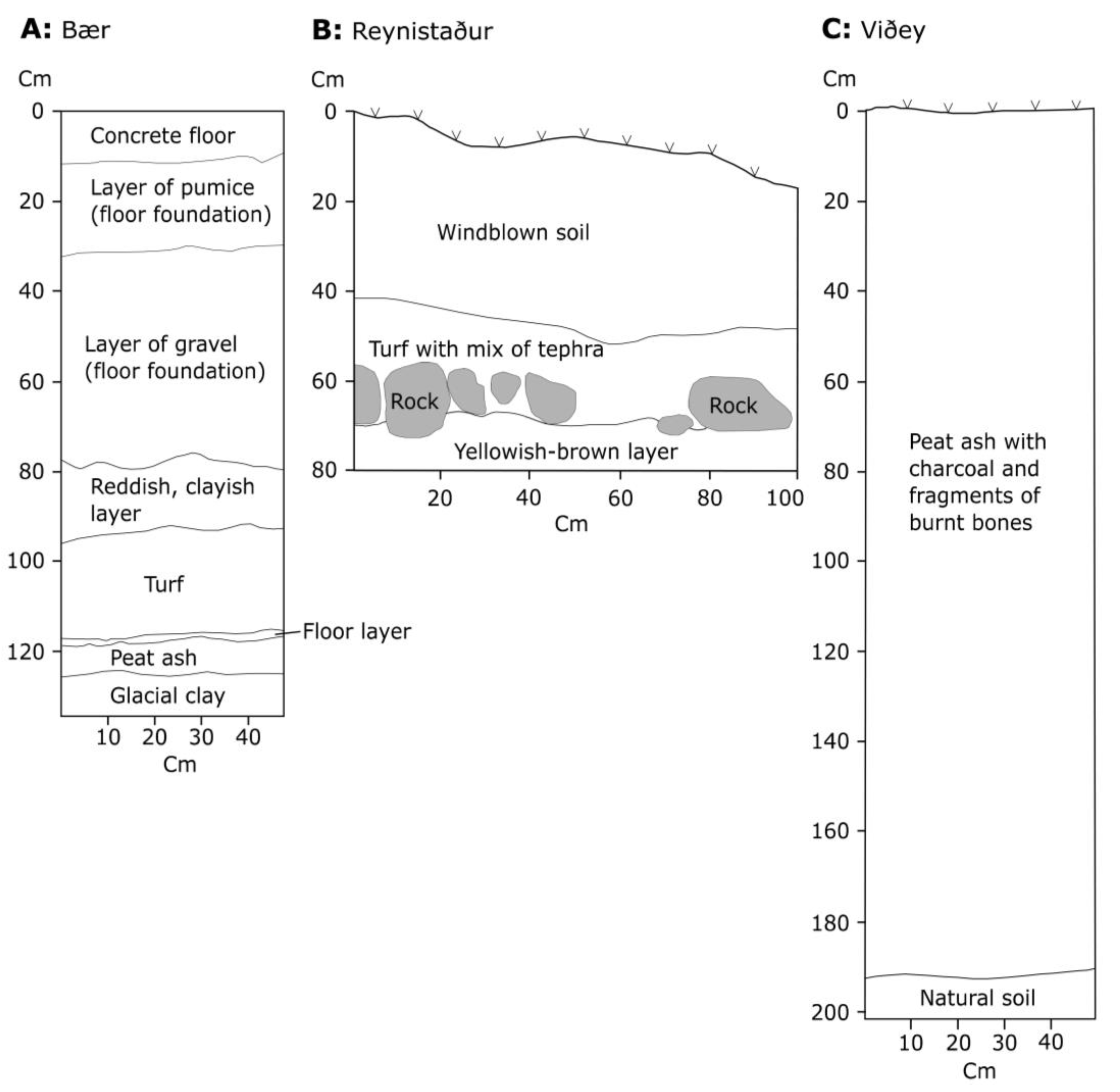
3. Results
4. Discussion
4.1. Land Use and Cereals at Bær
4.2. Land Use and Cereals at the Convent at Reynistaður
4.3. The Midden at Viðey
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abu-Rabia, Aref. 2012. Ethno-botanic treatments for paralysis (falij) in the Middle East. Chinese Medicine 3: 157–66. [Google Scholar] [CrossRef] [Green Version]
- Andersen, Svend Th. 1979. Identification of wild grass and cereal pollen. Danmarks Geologiske Undersøgelse Årbog 25: 491–518. [Google Scholar]
- Árnarson, M. 2002. Íslensk Orðabók. Reykjavík: Edda. [Google Scholar]
- Åsen, Per Arvid. 2021. Medieval Monastery Gardens in Iceland and Norway. Religions 12: 317. [Google Scholar] [CrossRef]
- Aston, Mick. 2000. Monasteries in the Landscape. Stroud: Tempus. [Google Scholar]
- Bakels, Corrie. 2020. Pollen and Archaeology. In Handbook for the Analysis of Micro-Particles in Archaeological Samples. Edited by Amanda Henry. Switzerland: Springer, pp. 203–24. [Google Scholar]
- Berglund, Björn E. 1985. Early agriculture in Scandinavia: Research problems related to pollen-analytical studies. Norwegian Archaeological Review 18: 77–90. [Google Scholar] [CrossRef]
- Bjarnadottir, Ragnheiđur Erla. 1997. Viðey Island Vegetation History on the Basis of Pollen Analysis and Documentary Search. Boston: Boston University. [Google Scholar]
- Björck, Svante, Ólafur Ingólfsson, Haflidi Haflidason, Margrét Hallsdóttir, and N. John Anderson. 1992. Lake Torfadalsvatn: A high resolution record of the North Atlantic ash zone I and the last glacial-interglacial environmental changes in Iceland. Boreas 21: 15–22. [Google Scholar] [CrossRef]
- Björck, Svante, Thomas Persson, and Ingrid Kristersson. 1978. Comparison of two concentration methods for pollen in minerogenic sediments. Geologiska Föreningen i Stockholm Förhandlingar 100: 107–11. [Google Scholar] [CrossRef]
- Bond, James. 2004. Monastic Landscapes. Stroud: Tempus. [Google Scholar]
- Bonny, Anne P. 1972. A method for determing absolute pollen frequencies in lake sediments. New Phytologist 71: 393–405. [Google Scholar] [CrossRef]
- Breitenlechner, Elisabeth, Marina Hilber, Joachim Lutz, Yvonne Kathrein, Alois Unterkircher, and Klaus Oeggl. 2010. The impact of mining activities on the environment reflected by pollen, charcoal and geochemical analyses. Journal of Archaeological Science 37: 1458–67. [Google Scholar] [CrossRef]
- Bullock, James M., Dan Chapman, Steffi Schafer, David Roy, Marco Girardello, Thomas Haynes, Stephen Beal, Belinda Wheeler, Ian Dickie, Zara Phang, and et al. 2013. Assessing and Controlling the Spread and the Effects of Common Ragweed in Europe. Wallingford: European Union. [Google Scholar]
- Caseldine, Chris, and Jackie Hatton. 1994. Interpretation of Holocene climatic change for the Eyjaförður area of northern Iceland from pollen-analytical data: Comments and preliminary results. Environmental Change in Iceland: Münchener Geographische Abhandlungen, Reihe B 12: 41–62. [Google Scholar]
- Clark, James G. 2021. The Making of Nordic Monasticism, c. 1076–c. 1350. Religions 12: 581. [Google Scholar] [CrossRef]
- Cugny, Carole, Florence Mazier, and Didier Galop. 2010. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Vegetation History and Archaeobotany 19: 391–408. [Google Scholar] [CrossRef] [Green Version]
- DI-VI. 1857–1986. Diplomatarium Islandicum: Íslenzkt fornbréfasafn. Kaupmannahöfn: Íslenzka bókmenntafélagið, 16 vols, vol. VI. [Google Scholar]
- DI-VII. 1857–1986. Diplomatarium Islandicum: Íslenzkt fornbréfasafn. Kaupmannahöfn: Íslenzka bókmenntafélagið, 16 vols, vol. VII. [Google Scholar]
- DI-VIII. 1857–1986. Diplomatarium Islandicum: Íslenzkt fornbréfasafn. Kaupmannahöfn: Íslenzka bókmenntafélagið, 16 vols, vol. VIII. [Google Scholar]
- DI-X. 1857–1986. Diplomatarium Islandicum: Íslenzkt fornbréfasafn. Kaupmannahöfn: Íslenzka bókmenntafélagið, 16 vols, vol. X. [Google Scholar]
- Drechsler, Stefan. 2021. Illuminated Manuscript Productionin Medieval Iceland: Literary and Artistic Activities of the Monastery at Helgafell in the Fourteenth Century. Turnhout: Brepols. [Google Scholar]
- Dugmore, Andrew J., Gudrún Gísladóttir, Ian A. Simpson, and Anthony Newton. 2009. Conceptual models of 1200 years of Icelandic soil erosion reconstructed using tephrochronology. Journal of the North Atlantic 2: 1–18. [Google Scholar] [CrossRef]
- Edwards, Kevin J., Egill Erlendsson, and J. Edward Schofield. 2011. Is there a Norse ‘footprint’ in North Atlantic pollen records? In Viking Settlements and Viking Society; Papers from Proceedings of the 16th Viking Congress, Reykjavík and Reykholt, 16th–23rd August 2009. Edited by Svavar Sigmundsson. Reykjavík: Hið íslenzka fornleifafélag & Háskóli Íslands, pp. 65–82. [Google Scholar]
- Edwards, Kevin J., Egill Erlendsson, and J. Edward Schofield. 2021. Landnám and the North Atlantic flora. In Biogeography in the Sub-Arctic: The Past and Future of North Atlantic Biota. Edited by Eva Panagiotakopulu and Jon P. Sadler. Oxford: Jon Wiley & Sons, pp. 185–214. [Google Scholar]
- Edwards, Kevin J., Ralph M. Fyfe, Chris O. Hunt, and J. Edward Schofield. 2015. Moving forwards? Palynology and the human dimension. Journal of Archaeological Science 56: 117–32. [Google Scholar] [CrossRef] [Green Version]
- Einarsson, Þorleifur. 1962. Vitnisburður frógreiningar um gróður, veðurfar og landnám á Íslandi. Saga 3: 442–69. [Google Scholar]
- Einarsson, Þorleifur. 1963. Pollen-analytical studies on the vegetation and climate history of Iceland in late and post-glacial times. In North Atlantic Biota and Their History. Edited by Áskell Löve and Doris Löve. Oxford: Pergamon, pp. 255–365. [Google Scholar]
- Erlendsson, Egill, Kevin J. Edwards, and Paul C. Buckland. 2009. Vegetational response to human colonisation of the coastal and volcanic environments of Ketilsstaðir, southern Iceland. Quaternary Research 72: 174–87. [Google Scholar] [CrossRef]
- Erlendsson, Egill. 2007. Environmental Change around the Time of the Norse settlement of Iceland. Aberdeen: University of Aberdeen. [Google Scholar]
- Gilchrist, Roberta. 2014. Monastic and church archaeology. Annual Review of Anthropology 43: 235–50. [Google Scholar] [CrossRef]
- Greipsson, Sigurdur, and Anthony J. Davy. 1994. Germination of Leymus arenarius and its significance for land reclamation in Iceland. Annals of Botany 73: 393–401. [Google Scholar] [CrossRef]
- Guðmundsson, Garðar. 1996. Gathering and processing of lyme-grass (Elymus arenarius L.) in Iceland: An ethnohistorical account. Vegetation History and Archaeobotany 5: 13–23. [Google Scholar] [CrossRef]
- Hald, Mette Marie, Jacob Mosekilde, Betina Magnussen, Martin Jensen Søe, Camilla Haarby Hansen, and Morten Fischer Mortensen. 2018. Tales from the barrels: Results from a multi-proxy analysis of a latrine from Renaissance Copenhagen, Denmark. Journal of Archaeological Science: Reports 20: 602–10. [Google Scholar] [CrossRef]
- Hallgrímsdóttir, Margrét. 1991. The excavation on Viðey, Reykjavík, 1987–1988. A preliminary report. Acta Archaeologica 61: 120–25. [Google Scholar]
- Hallsdóttir, Margrét. 1987. Pollen Analytical Studies of Human Influence on Vegetation in Relation to the Landnám Tephra Layer in Southwest Iceland. Lund: University of Lund. [Google Scholar]
- Hallsdóttir, Margrét. 1993. Frjórannsókn á mósniðum úr Viðey. Reykjavík: Raunvísindastofnun Háskólans. [Google Scholar]
- Hallsdóttir, Margrét. 1995. On the pre-settlement history of Icelandic vegetation. Búvísindi 9: 19–29. [Google Scholar]
- Hättestrand, Martina, Christin Jensen, Margrét Hallsdóttir, and Karl-Dag Vorren. 2008. Modern pollen accumulation rates at the north-western fringe of the European boreal forest. Review of Palaeobotany and Palynology 151: 90–109. [Google Scholar] [CrossRef]
- Hjelle, Kari Loe, Lene S. Halvorsen, and Anette Overland. 2010. Heathland development and relationship between humans and environment along the coast of western Norway through time. Quaternary International 220: 133–46. [Google Scholar] [CrossRef]
- Hjelle, Kari Loe. 1997. Relationships between pollen and plants in human-influenced vegetation types using presence-absence data in western Norway. Review of Palaeobotany and Palynology 99: 1–16. [Google Scholar] [CrossRef]
- Icelandic Meteorological Office. 2014. Vindatlas. Reykjavík: Veðurstofa Íslands. [Google Scholar]
- Jensson, Gottskálk. 2021. Þingeyrar Abbey in Northern Iceland: A Benedictine Powerhouse of Cultural Heritage. Religions 12: 423. [Google Scholar] [CrossRef]
- Júlíusson, Árni Daníel. 2014. Jarðeignir Kirkjunnar og Tekjur af þeim 1000–1550. Reykjavík: Center for Agrarian Historical Dynamics. [Google Scholar]
- Karlsdóttir, Lilja, Ægir Th. Thórsson, Margrét Hallsdóttir, Adalsteinn Sigurgeirsson, Thröstur Eysteinsson, and Kesara Anamthawat-Jónsson. 2007. Differentiating pollen of Betula species from Iceland. Grana 46: 78–84. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, Gunnar. 2000. Iceland’s 1100 years: The History of a Marginal Society. London: C. Hurst & Co. [Google Scholar]
- Kristinsson, Hörður. 1986. A Guide to Flowering Plants and Ferns of Iceland. Reykjavík: Mál og Menning. [Google Scholar]
- Kristjánsdóttir, Steinunn, and Vala Gunnarsdóttir. 2014a. Bær í Borgarfirði. Reykjavík: Kortlagning klaustra á Íslandi. [Google Scholar]
- Kristjánsdóttir, Steinunn, and Vala Gunnarsdóttir. 2014b. Reynistaður. Reykjavík: Kortlagning klaustra á Íslandi. [Google Scholar]
- Kristjánsdóttir, Steinunn, and Vala Gunnarsdóttir. 2014c. Viðey. Reykjavík: Kortlagning klaustra á Íslandi. [Google Scholar]
- Kristjánsdóttir, Steinunn, Inger Larsson, and Per Arvid Åsen. 2014. The Icelandic medieval monastic garden-did it exist? Scandinavian Journal of History 39: 560–79. [Google Scholar] [CrossRef]
- Kristjánsdóttir, Steinunn. 2017. Leitin að klaustrunum—klausturhald á Íslandi í fimm aldir. Reykjavík: Forlagið. [Google Scholar]
- Kristjánsdóttir, Steinunn. 2023. Monastic Iceland. Abingdon: Routledge. [Google Scholar]
- Larsen, Henning. 1927. An Old Icelandic Medical Manuscript. Annals of Medical History 9: 60. [Google Scholar]
- Lechterbeck, Jutta, and Christin E. Jensen. 2020. Exploring the potential of palynology in archaeological contexts: Proceedings of the session held at the 24th Annual Meeting of the European Association of Archaeologists in Barcelona 2018. Vegetation History and Archaeobotany 29: 111–12. [Google Scholar] [CrossRef] [Green Version]
- Loftsdóttir, Kristín. 2019. Crisis and Coloniality at Europe’s Margins: Creating Exotic Iceland. Abingdon: Routledge. [Google Scholar]
- Lomas-Clarke, Sarah H., and Keith E. Barber. 2004. Palaeoecology of human impact during the historic period: Palynology and geochemistry of a peat deposit at Abbeyknockmoy, Co. Galway, Ireland. Holocene 14: 721–31. [Google Scholar] [CrossRef]
- Martin, Michael D., Marceloa Chamecki, Grace S. Brush, Charles Meneveau, and Marc B. Parlange. 2009. Pollen clumping and wind dispersal in an invasive angiosperm. American Journal of Botany 96: 1703–11. [Google Scholar] [CrossRef] [Green Version]
- Mooney, Dawn Elise, and Lísabet Guðmundsdóttir. 2020. Barley cultivation in Viking Age Iceland in light of evidence from Lækjargata 10–12, Reykjavík. Archaeobotanical Studies of Past Plant Cultivation in Northern Europe 5: 5. [Google Scholar]
- Moore, Peter D., Judith A. Webb, and Margaret E. Collison. 1991. Pollen Analysis. Oxford: Blackwell Scientific Publications. [Google Scholar]
- Morgan, Ruth M., J. Flynn, V. Sena, and Peter A. Bull. 2014. Experimental forensic studies of the preservation of pollen in vehicle fires. Science & Justice 54: 141–45. [Google Scholar]
- Noël, Hervé, Emmanuel Garbolino, Achim Brauer, Elisabeth Lallier-Vergès, Jacques-Louis De Beaulieu, and Jean-Robert Disnar. 2001. Human impact and soil erosion during the last 5000 yrs as recorded in lacustrine sedimentary organic matter at Lac d’Annecy, the French Alps. Journal of Paleolimnology 25: 229–44. [Google Scholar] [CrossRef]
- Ólsen, Björn Magnússon. 1910. Um kornirkju á Íslandi að fornu. Búnaðaritt 24: 81–168. [Google Scholar]
- Ottósson, Jón Gunnar, Anna Sveinsdóttir, and María Harðardóttir. 2016. Vistgerðir á Íslandi. Available online: http://vistgerdakort.ni.is/ (accessed on 1 April 2023).
- Pluenneke, Molly. 2017. Shift in Icelandic Plant Populations Due to Climate Change: Through the Lens of Natural Dyes. In Independent Study Project. Vermont: School for International Training. [Google Scholar]
- Riddell, Scott J., Egill Erlendsson, Sigrún D. Eddudóttir, Guðrún Gísladóttir, and Steinunn Kristjánsdóttir. 2022. Pollen, Plague & Protestants: The Medieval Monastery of Þingeyrar (Þingeyraklaustur) in Northern Iceland. Environmental Archaeology 27: 193–210. [Google Scholar] [CrossRef]
- Riddell, Scott, Egill Erlendsson, Guðrún Gísladóttir, Kevin J. Edwards, Jesse Byock, and Davide Zori. 2018. Cereal cultivation as a correlate of high social status in medieval Iceland. Vegetation History and Archaeobotany 27: 679–96. [Google Scholar] [CrossRef]
- Samuelsen, Anne Berit. 2000. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. Journal of Ethnopharmacology 71: 1–21. [Google Scholar] [CrossRef]
- Stockmarr, Jens. 1971. Tablets with spores used in absolute pollen analysis. Pollen et Spores 13: 614–21. [Google Scholar]
- Stolz, Christian, and Jörg Grunert. 2010. Late Pleistocene and Holocene landscape history of the central Palatinate forest (Pfälzerwald, south-western Germany). Quaternary International 222: 129–42. [Google Scholar] [CrossRef]
- Sveinbjarnardóttir, Guðrun, Egill Erlendsson, Kim Vickers, Tom H. McGovern, Karen B. Milek, Kevin J. Edwards, Ian A. Simpson, and Gordon Cook. 2007. The palaeoecology of a high status Icelandic farm. Environmental Archaeology 12: 187–206. [Google Scholar] [CrossRef] [Green Version]
- Thorarinsson, Sigurdur, Trausti Einarsson, and Guðmundur Kjartansson. 1959. On the geology and geomorphology of Iceland. Geografiska Annaler 41: 135–69. [Google Scholar] [CrossRef]
- Trigg, Heather, Douglas Bolender, Katharine M. Johnson, Marisa D. Patalano, and John M. Steinberg. 2009. Note on barley found in dung in the lowest levels of the farm mound midden at Reynistaður, Skagafjörður, Iceland. Archaeologia Islandica 7: 64–73. [Google Scholar]
- Tweddle, John C., Kevin J. Edwards, and Nick R. J. Fieller. 2005. Multivariate statistical and other approaches for the separation of cereal from wild Poaceae pollen using a large Holocene dataset. Vegetation History and Archaeobotany 14: 15–30. [Google Scholar] [CrossRef]
- van Amerongen, Yvonne F. 2020. All’s well? Comparing on-and off-site pollen samples and exploring the potential of pollen from man-made contexts. Vegetation History and Archaeobotany 29: 125–31. [Google Scholar] [CrossRef] [Green Version]
- van Geel, Bas, Janneke Buurman, Otto Brinkkemper, Jaap Schelvis, André Aptroot, Guido van Reenen, and Tom Hakbijl. 2003. Environmental reconstruction of a Roman Period settlement site in Uitgeest (The Netherlands), with special reference to coprophilous fungi. Journal of Archaeological Science 30: 873–83. [Google Scholar] [CrossRef]
- Varga, György, Pavla Dagsson-Walhauserová, Fruzsina Gresina, and Agusta Helgadottir. 2021. Saharan dust and giant quartz particle transport towards Iceland. Scientific Reports 11: 1–12. [Google Scholar] [CrossRef]
- Vésteinsson, Orri. 2009. A medieval merchants’ church in Gásir, North Iceland. Hikuin 36: 159–59. [Google Scholar]
- Vuorela, Irmeli. 1973. Relative pollen rain around cultivated fields. Acta Botanica Fennica 102: 25–27. [Google Scholar]
- Wasowicz, Pawel. 2018. The first attempt to list the archaeophytes of Iceland. Acta Societatis Botanicorum Poloniae 87: 4. [Google Scholar] [CrossRef]
- Wąsowicz, Paweł. 2020. Annotated Checklist of Vascular Plants of Iceland (Fjölrit Náttúrufræðistofnunar nr. 57). Garðabær: Náttúrufræðistofnun Íslands. [Google Scholar]
- Wimble, Guy, Colin E. Wells, and David Hodgkinson. 2000. Human impact on mid-and late Holocene vegetation in south Cumbria, UK. Vegetation History and Archaeobotany 9: 17–30. [Google Scholar] [CrossRef]
- Zori, Davide, Jesse Byock, Egill Erlendsson, Steve Martin, Thomas Wake, and Kevin J. Edwards. 2013. Feasting in Viking Age Iceland: Sustaining a chiefly political economy in a marginal environment. Antiquity 87: 150–65. [Google Scholar] [CrossRef]
- Zutter, Cynthia. 1999. Congruence or concordance in archaeobotany: Assessing micro-and macro-botanical data sets from Icelandic middens. Journal of Archaeological Science 26: 833–44. [Google Scholar] [CrossRef]


| Taxa/Monastery | Bær | Reynistaður | Viðey |
|---|---|---|---|
| Pollen | |||
| Anthemis-type | 9 (1.8) | - | - |
| Betula (undiff.) | 329 (68) | 5 (1.2) | 45 (4.4) |
| Brassicaceae | - | - | - |
| Caryophyllaceae | - | - | - |
| Cerastium-type | 1 (0.2) | 15 (3.6) | 6 (0.6) |
| Cyperaceae | 58 (12) | 22 (5.2) | 123 (12) |
| Drosera-type | - | 1 (0.2) | - |
| Empetrum nigrum | 3 (0.6) | - | 3 (0.3) |
| Ericales | 1 (0.2) | - | - |
| Filipendula ulmaria | 1 (0.2) | - | 5 (0.5) |
| Galium | - | - | 3 (0.3) |
| Hordeum-type | 2 (0.4) | - | 1 (0.1) |
| Lactuceae | 1 (0.2) | 8 (1.9) | 123 (12) |
| Montia fontana | - | 24 (5.7) | - |
| Plantago maritima | - | - | 1 (0.1) |
| Poaceae | 43 (8.9) | 250 (59.4) | 583 (57) |
| Polygonum aviculare | 2 (0.4) | - | - |
| Potentilla-type | - | 2 (0.5) | 1 (0.1) |
| Ranunculus acris-type | - | 3 (0.7) | 8 (0.8) |
| Rumex acetosa | 1 (0.2) | 1 (0.2) | 16 (1.5) |
| Rumex longifolius | - | - | 3 (0.3) |
| Salix | - | - | - |
| Thalictrum alpinum | 1 (0.2) | 7 (1.7) | 7 (0.7) |
| Indeterminate pollen | 12 (2.5) | 76 (18) | 18 (1.7) |
| Total | 464 | 414 | 946 |
| Spores | |||
| Botrychium | - | 1 (0.2 | - |
| Diphasiastrum alpinum | - | 2 (0.5) | - |
| Equisetum | 5 (1) | - | 29 (2.8) |
| Lycopodium annotinum | 3 (0.6) | - | 1 (0.1) |
| Pteropsida (monolete) indet. | 5 (1) | 1 (0.2) | 6 (0.6) |
| Selaginella selaginoides | - | 2 (0.5) | 2 (0.2) |
| Sphagnum | 8 (1.6) | 1 (0.2) | 44 (4.3) |
| Total | 21 | 7 | 82 |
| CFS | |||
| Sordaria-type (HdV-55a) | - | 4 | 70 |
| Sporormiella-type (HdV-113) | - | - | 1 |
| Podospora (HdV-368) | - | 1 | |
| Total | - | 4 | 72 |
| Taxa/Monastery | Bær | Reynistaður | Viðey |
|---|---|---|---|
| Pollen | |||
| Ambrosia-type | - | 2 | - |
| Angelica-undiff. | ● | - | - |
| Artemisia-type | - | - | - |
| Avena-type | 3 | - | - |
| Brassicaceae | ● | ● | - |
| Calluna vulgaris | ● | - | - |
| Caltha palustris | ● | - | - |
| Caryophyllaceae | ● | ● | - |
| Cornus suecica | - | - | - |
| Drosera-type | - | ● | ● |
| Empetrum nigrum | ● | ● | ● |
| Filipendula ulmaria | ● | ● | ● |
| Galium | ● | ● | ● |
| Hordeum-type | 70 | 10 | 1 |
| Lychnis viscaria-type | ● | ● | - |
| Parnassia palustris | - | - | - |
| Pinus | 1 | - | - |
| Plantago major | - | - | 1 |
| Plantago maritima | ● | - | ● |
| Polygonum viviparum | - | - | - |
| Potentilla-type | ● | ● | ● |
| Rumex acetosella | ● | - | - |
| Sagina | - | ● | - |
| Salix | ● | - | - |
| Saxifraga stellaris/nivalis | - | - | ● |
| Sorbus aucuparia | ● | - | - |
| Vaccinium-type | ● | - | - |
| Valeriana officinalis | - | - | 1 |
| Spores | |||
| Diphasiastrum alpinum | ● | ● | - |
| Lycopodium annotinum | - | ● | ● |
| Polypodium vulgare | - | - | - |
| Selaginella selaginoides | ● | ● | ● |
| Pollen-Type | Annulus Size (µm) | Grain Size (µm) | Pollen-Type | Annulus Size (µm) | Grain Size (µm) |
|---|---|---|---|---|---|
| Hordeum-type | ≥8 and ≤10 | ≥37 and ≤45 | Hordeum-type | ≥8 and ≤10 | ≥37 and ≤45 |
| 9 | 38 | 9 | 40 | ||
| 8 | 37 | 9 | 38 | ||
| 8 | 45 | 8 | 42 | ||
| 8 | 38 | 9 | 38 | ||
| 8 | 42 | 10 | 45 | ||
| 9 | 38 | 9 | 38 | ||
| 8 | 43 | 8 | 40 | ||
| 8 | 38 | 9 | 42 | ||
| 9 | 41 | 10 | 38 | ||
| 8 | 38 | 9 | 44 | ||
| 9 | 45 | 8 | 39 | ||
| 9 | 40 | 8 | 41 | ||
| 9 | 43 | Average | 8.5 | 40 | |
| 8 | 39 | ||||
| 8 | 37 | cf. Avena-type | >10 | >45 | |
| 8 | 42 | 12 | 46 | ||
| 10 | 41 | 10 | 49 | ||
| 8 | 39 | 10 | 50 | ||
| 9 | 40 | Average | 10 | 48 | |
| 8 | 43 | ||||
| 9 | 39 | Hordeum-type and Avena-type traits | |||
| 8 | 38 | 9 | 47 | ||
| 8 | 37 | 9 | 46 | ||
| 8 | 40 | 11 | 37 | ||
| 8 | 38 | 8 | 46 | ||
| 8 | 42 | 11 | 43 | ||
| 8 | 39 | 8 | 46 | ||
| 8 | 45 | 9 | 49 | ||
| 8 | 38 | 9 | 50 | ||
| 8 | 38 | 11 | 44 | ||
| 9 | 38 | 8 | 46 | ||
| 10 | 44 | Average | 9.3 | 45.4 | |
| 10 | 38 | ||||
| 8 | 37 | ||||
| 8 | 38 | ||||
| 9 | 45 | Summary | |||
| 8 | 40 | Hordeum-type | 52 | 80% | |
| 8 | 42 | cf. Avena-type | 3 | 5% | |
| 8 | 37 | Shared traits | 10 | 15% | |
| 10 | 38 | Total | 65 | - | |
| Hordeum-Type | Annulus (≥8 and ≤10 µm) | Grain Size (≥37 and ≤45 µm) |
|---|---|---|
| 9 | 45 | |
| 8 | 38 | |
| 8 | 38 | |
| 8 | 41 | |
| 8 | 37.5 | |
| 9 | 37 | |
| 10 | 38.5 | |
| 10 | 37 | |
| 8 | 37 | |
| 8 | 37 | |
| Average | 8.6 | 38.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddell, S.J.; Kristjánsdóttir, S.; Gísladóttir, G.; Erlendsson, E. The Potential of Palynology with Regard to the Archaeology of Medieval Monastery Sites in Iceland. Religions 2023, 14, 586. https://doi.org/10.3390/rel14050586
Riddell SJ, Kristjánsdóttir S, Gísladóttir G, Erlendsson E. The Potential of Palynology with Regard to the Archaeology of Medieval Monastery Sites in Iceland. Religions. 2023; 14(5):586. https://doi.org/10.3390/rel14050586
Chicago/Turabian StyleRiddell, Scott J., Steinunn Kristjánsdóttir, Guðrún Gísladóttir, and Egill Erlendsson. 2023. "The Potential of Palynology with Regard to the Archaeology of Medieval Monastery Sites in Iceland" Religions 14, no. 5: 586. https://doi.org/10.3390/rel14050586





