Adaptive Enterprise Architecture for the Digital Healthcare Industry: A Digital Platform for Drug Development
Abstract
1. Introduction
2. Literature Review and Theoretical Development
2.1. Direction of Digital IT and Enterprise Architecture
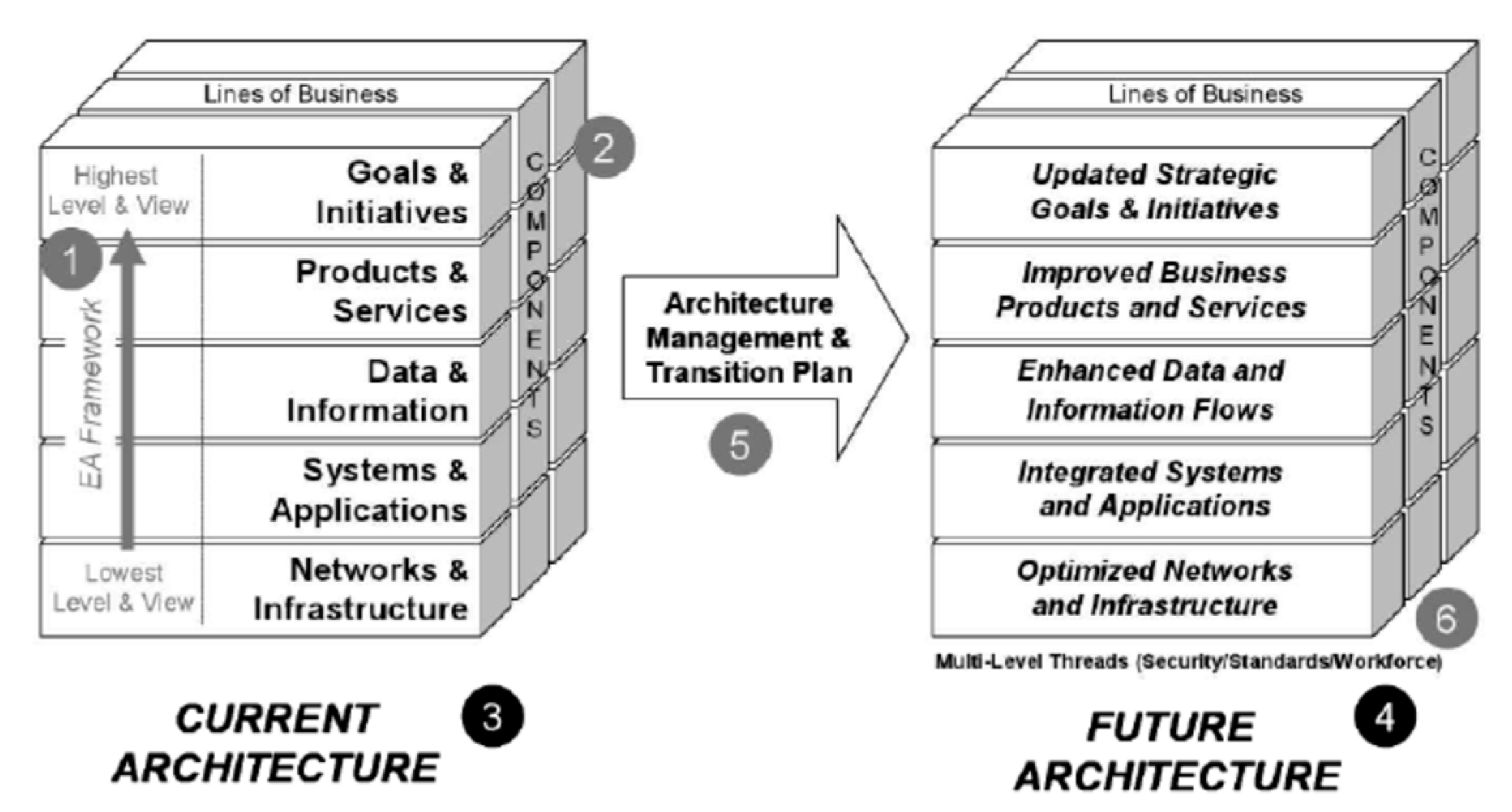
2.1.1. Concepts of Enterprise Architecture
2.1.2. Literature Review for Enterprise Architecture (EA) and Digital IT
2.2. Enterprise Architecture for Digital Transformation in Healthcare Industry
2.2.1. Digital Transformation
2.2.2. Adaptive Integrated Digital Architecture Framework (AIDAF) Framework
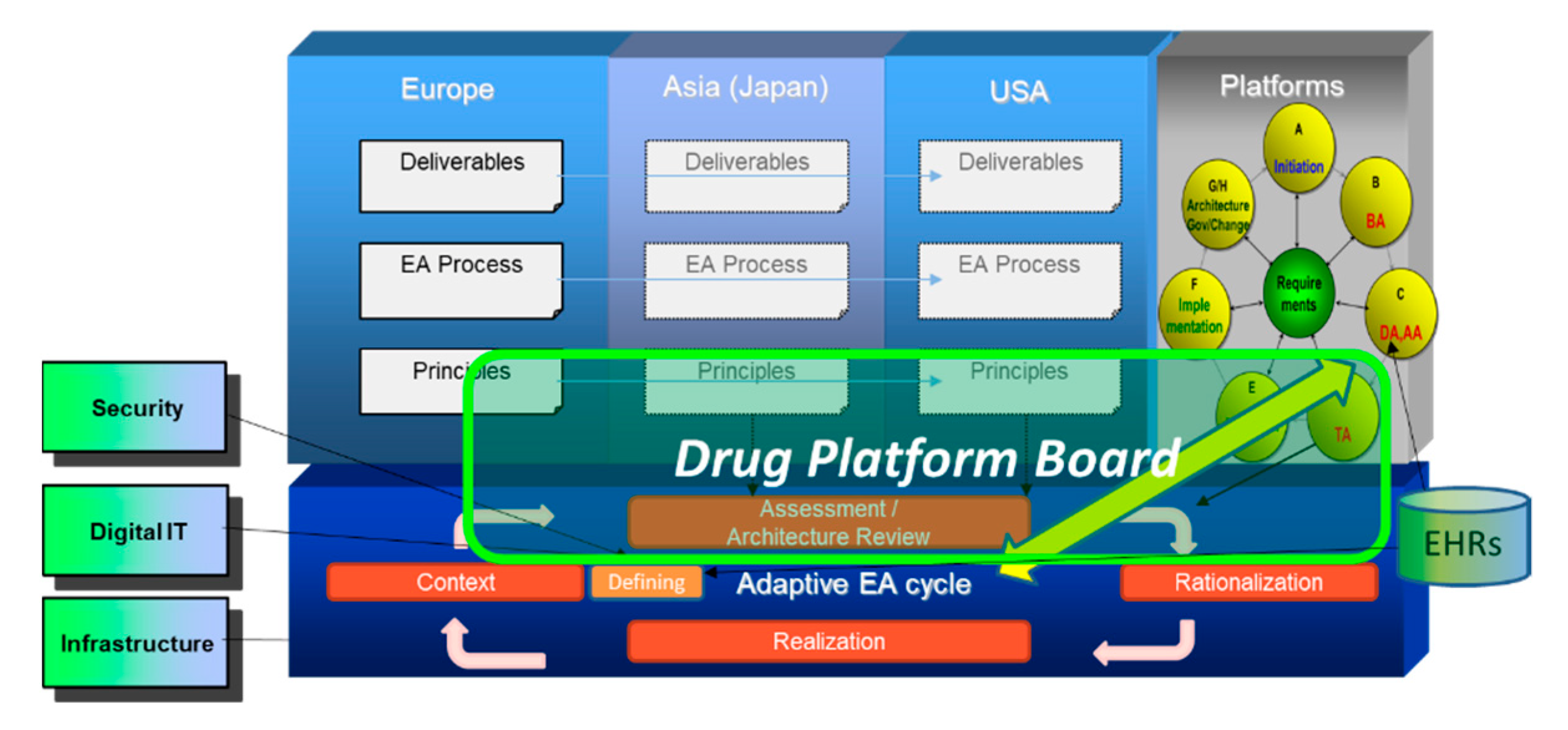
2.2.3. Architecture Board and Global Healthcare Company
2.2.4. Social Collaboration Model (SCM) in Architecture Board in AIDAF Framework
2.3. Direction of Drug Discovery
- Part 1: “Drug discovery” is processed to target the drug and synthesize drugs from natural sources and chemicals. It covers some assays to test the effectiveness.
- Part 2: The review of “drug design” can focus on how better to select the candidate drugs’ selectiveness and effectiveness.
- Part 3: “Drug development” includes preclinical development, clinical development, drug approval, and recall. It emphasizes better understanding about the drug, including potential benefits, safety concerns, best dose, best dosage form, and choice for the route of administration. It also involves experiments on human beings for effectiveness, safety, and other essential elements.
- Step 1: “Disease Pathology.” It answers high-level strategic questions regarding drug discovery, such as the specific therapeutic area and the disease domain.
- Step 2: “Target identification.” This step covers figuring out the exact processes in a specific disease area.
- Step 3: “Assay development and screen.” It will investigate what kind of assay to run. The primary classification is identifying whether it is target-based or phenotypic.
- Step 4: “Hit to lead.” In this step, you will have the hits and triage. Results are categorized into most interesting, possibly interesting, and non-factor.
- Step 5: “Lead optimization.” It involves traditional medicinal chemistry, and one will start to improve properties in potency, selectivity, toxic side effects, and pharmacokinetics. The candidates are narrowed down to up to three clinical candidates.
- Step 6: “Preclinical development.” The classic scale-up questions for each new medicine should be solved in this step.
- Step 7: “Clinical trial. Drug development” This is the step where the real money is invested. It is the leverage point for the whole process. One has to get everything correct before this step to prevent a waste of money and time.
2.4. Electronic Health Record (EHR) and Clinical Decision Support (CDS)
2.4.1. Case of a Healthcare Organization in Australian Government with Electronic Health Record (EHR)
2.4.2. The AIDAF Application with CDS System, a Platform in Healthcare and Medical Organizations
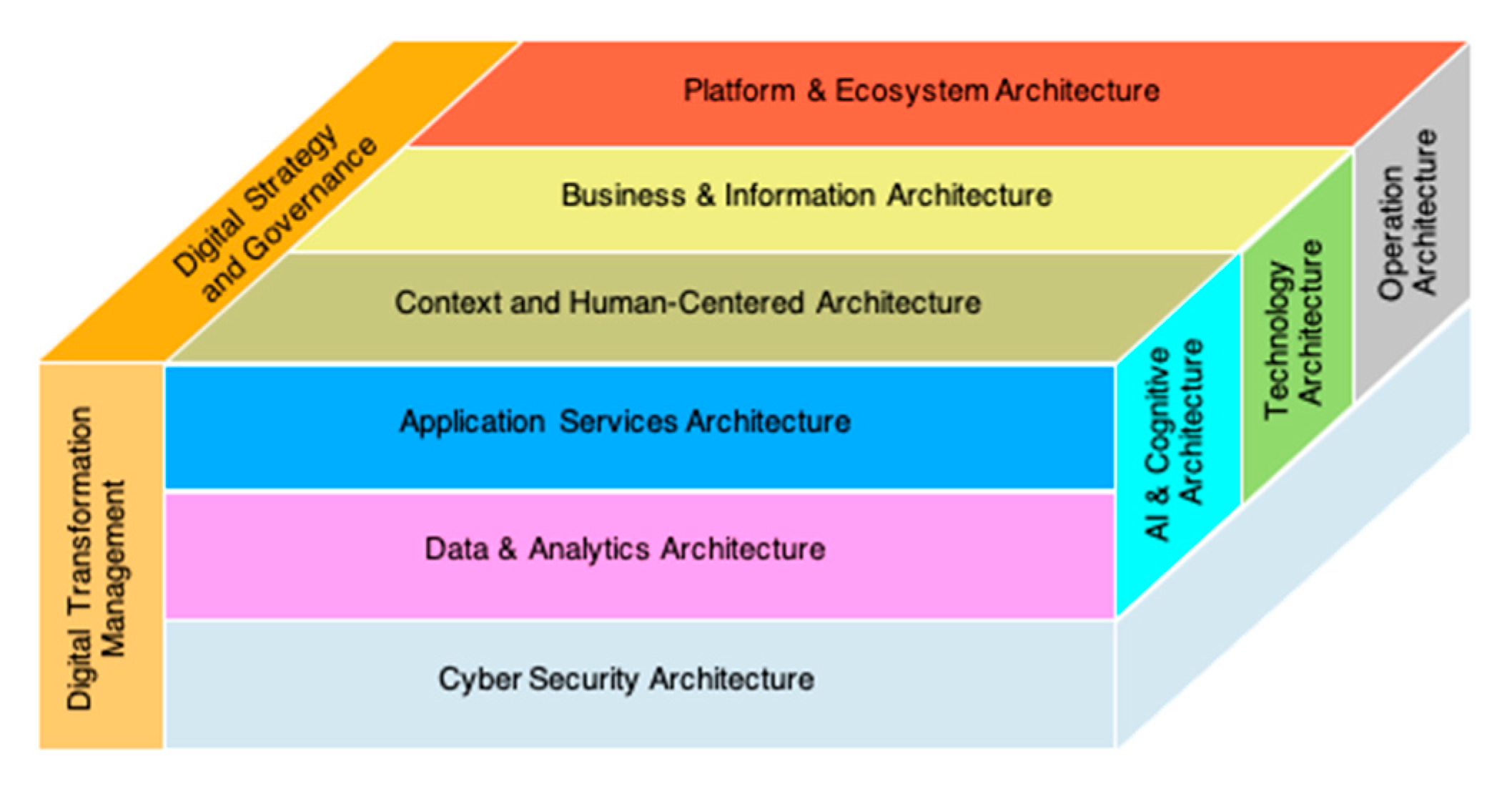
2.5. Big Data and Cloud Computing, Digital Healthcare with Enterprise Architecture
2.6. Artificial Intelligence, Intermediary Knowledge Model, and Global Digital Transformation Communication (GDTC) Model in Enterprise Architecture
2.7. Hybrid Database
3. Research Methodology
- RQ1:
- How can enterprise architecture with establishing digital platforms contribute to the digital transformation in the healthcare industry?
- RQ2:
- How can a use case of a digital platform (and services’ ecosystem) for drug development contribute to the application and benefits of a digital enterprise architecture for efficiency and effectiveness of reviews?
4. Digital Platform with Drug Discovery and Development in Enterprise Architecture
4.1. Drug Discovery Process on a Digital Platform
4.1.1. Proposed Process in the Part of “Drug Discovery”
4.1.2. Proposed Process in the Part of “New Drug Development”
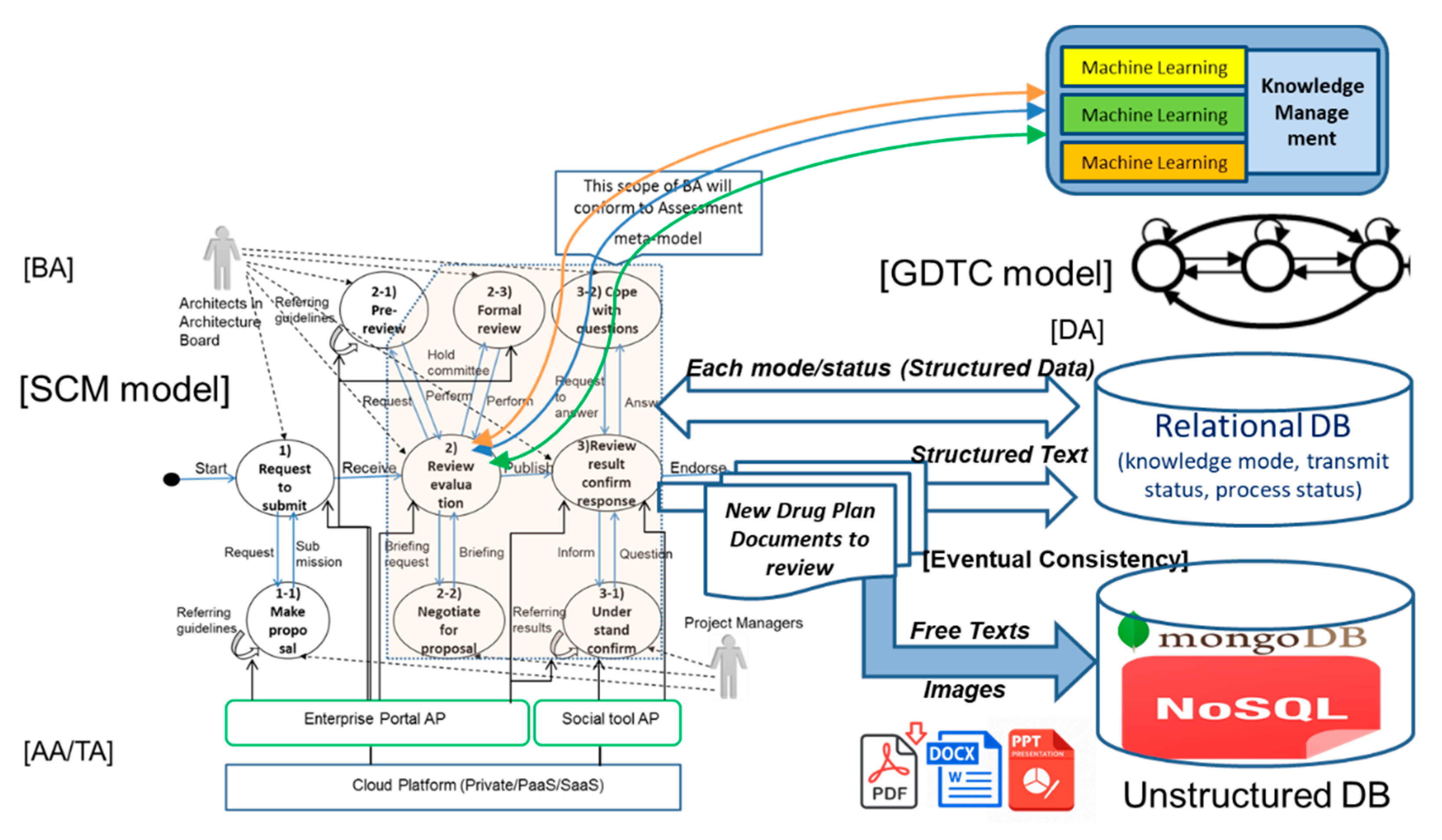
4.1.3. Database for Digital Platform Related to Drug Design and Development
4.2. Process for Drug Discovery Worldwide, and the Status of Digital Platform
4.3. Application of GDTC Model for Digital Platform Related to Drug Development
4.4. Overview of Digital Platform for Drug Discovery, Drug Development Process Using AIDAF
5. Discussion
5.1. Structure for Implementation of the New Drug Development Review System
5.2. Study Contribution to Research and Practice
5.2.1. Research Findings 1: Streamlined Processes through Digital Platforms in Organizations in the Healthcare Industry
5.2.2. Research Findings 2: Informal Knowledge Supply and Sharing among Organizational Members on Digital Platforms in Healthcare Communities
5.2.3. Research Findings 3: Improvement of Efficiency and Effectiveness in Planning Production and Business on Digital Platforms for Drug Development
5.3. Verifying Research Question 1
5.4. Verifying Research Question 2
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boardman, S.; Harrington, E. Open Group Snapshot—Open Platform 3.0™; The Open Group: San Francisco, CA, USA, 2015. [Google Scholar]
- Alwadain, A.; Fielt, E.; Korthaus, A.; Rosemann, M. A comparative analysis of the integration of SOA elements in widely-used enterprise architecture frameworks. Int. J. Intell. Inf. Technol. 2014, 9, 54–70. [Google Scholar] [CrossRef][Green Version]
- Buckl, S.; Matthes, F.; Schulz, C.; Schweda, C.M. Exemplifying a framework for interrelating enterprise architecture concerns. In Ontology, Conceptualization and Epistemology for Information Systems, Software Engineering and Service Science; Sicilia, M.A., Kop, C., Sartori, F., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2010; Volume 62, pp. 33–46. [Google Scholar]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S.; Hardjono, T. An Adaptive Enterprise architecture Framework and Implementation in the Era of Cloud/Mobile IT/ Digital IT in Global Enterprise. Int. J. Enterp. Inf. Syst. 2017, 13, 1–22. [Google Scholar] [CrossRef]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S.; Hardjono, T. Architecture Board Practices in Adaptive Enterprise architecture with Digital Platform: A Case of Global Healthcare Enterprise. Int. J. Enterp. Inf. Syst. 2018, 14, 1–20. [Google Scholar] [CrossRef]
- Rouhani, B.D.; Mahrin, M.N.; Nikpay, F.; Ahmad, R.B. A systematic literature review on Enterprise Architecture Implementation Methodologies. Inf. Softw. Technol. 2015, 62, 1–20. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Luo, A. A systematic review of Business-IT Alignment Research with Enterprise Architecture. IEEE Access 2018, 6, 18933–18944. [Google Scholar] [CrossRef]
- Silva, N.; Sousa, P.; da Silva, M.M. Maintenance of Enterprise Architecture Models. In Business and Information Systems Engineering; Springer: Berlin, Germany, 2018; pp. 1–24. [Google Scholar]
- Zhou, Z.; Zhi, Q.; Morisaki, S.; Yamamoto, S. A systematic literature review on Enterprise Architecture Visualization Methodologies. IEEE Access 2020, 8, 96404–96427. [Google Scholar] [CrossRef]
- Hadaya, P.; Leshob, A.; Marchildon, P. Enterprise architecture framework evaluation criteria: A literature review and artifact development. Serv. Oriented Comput.Appl. 2020, 14, 203–222. [Google Scholar] [CrossRef]
- Bernard, S.A. An Introduction to Enterprise Architecture; AuthorHouse: Bloomington, IN, USA, 2012; pp. 31–45. [Google Scholar]
- Lankhorst, M. Enterprise Architecture at Work; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009. [Google Scholar]
- Simon, D.; Fischbach, K.; Schoder, D. Enterprise architecture management and its role in corporate strategic management. Inf. Syst. e-Bus. Manag. 2014, 12, 5–42. [Google Scholar] [CrossRef]
- Gama, N.; Sousa, P.; da Silva, M.M. Integrating enterprise architecture and IT service management. In Building Sustainable Information Systems; Springer: Boston, MA, USA, 2013; pp. 153–165. [Google Scholar]
- Iacob, M.E.; Meertens, L.O.; Jonkers, H.; Quartel, D.A.; Nieuwenhuis, L.J.; van Sinderen, M.J. From enterprise architecture to business models and back. Softw. Syst. Model. 2014, 13, 1059–1083. [Google Scholar] [CrossRef]
- Jahani, B.; Javadein, S.R.S.; Jafari, H.A. Measurement of enterprise architecture readiness within organizations. Bus. Strategy Ser. 2010, 11, 177–191. [Google Scholar] [CrossRef]
- Drews, P.; Schirmer, I. From Enterprise architecture to Business Ecosystem Architecture: Stages and Challenges for Extending Architectures beyond Organizational Boundaries. In Proceedings of the 18th International Enterprise Distributed Object Computing Conference Workshops and Demonstrations, Ulm, Germany, 1–2 September 2014; pp. 13–22. [Google Scholar]
- Tambo, T. Enterprise architecture beyond the Enterprise: Extended Enterprise architecture Revisited. In International Conference on Enterprise Information Systems; SCITEPRESS Digital Library: Setúbal, Portugal, 2017; pp. 381–390. [Google Scholar]
- Guédria, W.; Gaaloul, K.; Naudet, Y.; Proper, H.A. A Modelling Approach to Support Enterprise architecture Interoperability. In OTM Confederated International Conferences on the Move to Meaningful Internet Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 189–198. [Google Scholar]
- Tambo, T.; Bargholz, J.; Yde, L. Evaluation of TOGAF as a Management of Technology Framework. Int. Assoc. Manag. Technol. 2016, 25, 1–17. [Google Scholar]
- Rusli, D.; Bandung, Y. Designing an enterprise architecture (EA) based on TOGAF ADM and MIPI. In Proceedings of the International Conference on Information Technology Systems and Innovation, Bandung, Indonesia, 23–24 October 2017; pp. 38–43. [Google Scholar]
- Kearny, C.; Gerber, A.; van der Merwe, A. Data-driven enterprise architecture and the TOGAF ADM phases. In Proceedings of the International Conference on Systems, Man, and Cybernetics, Budapest, Hungary, 9–12 October 2016; pp. 4603–4608. [Google Scholar]
- Cabrera, A.; Abad, M.; Jaramillo, D.; Gómez, J.; Verdum, J.C. Definition and implementation of the Enterprise Business Layer through a Business Reference Model, using the architecture development method ADM-TOGAF. In Trends and Applications in Software Engineering; Springer: Berlin, Germany, 2016; pp. 111–121. [Google Scholar]
- Tamm, T.; Seddon, P.B.; Shanks, G.; Reynolds, P. How does enterprise architecture add value to organizations? Commun. Assoc. Inf. Syst. 2011, 28, 10. [Google Scholar]
- Kitsios, F.; Kamariotou, M. Business strategy modelling based on enterprise architecture: A state of the art review. Bus. Process Manag. J. 2019, 25, 606–624. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, F.; Bustos, G. Integration of Business Process Architectures within Enterprise Architecture Approaches: A Literature Review. Eng. Manag. J. 2019, 31, 127–140. [Google Scholar] [CrossRef]
- Gellweiler, C. Connecting Enterprise Architecture and Project Portfolio Management: A Review and a Model for IT Project Alignment. Int. J. Inf. Technol. Proj. Manag. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Muhammad, K.; Khan, M.N.A. Augmenting mobile cloud computing through enterprise architecture: A survey paper. Int. J. Grid Distrib. Comput. 2015, 8, 323–336. [Google Scholar] [CrossRef]
- Richards, M. Microservices vs. Service-Oriented Architecture, 1st ed.; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2015. [Google Scholar]
- MacKenzie, C.M.; Laskey, K.; McCabe, F.; Brown, P.F.; Metz, R. Reference Model for Service-Oriented Architecture 1.0. In Advancing Open Standards for the Information Society; Technical Report; OASIS: Burlington, MA, USA, 2006. [Google Scholar]
- Chen, H.M.; Kazman, R.; Perry, O. From software architecture analysis to service engineering: An empirical study of methodology development for enterprise SOA implementation. IEEE Trans. Serv. Comput. 2014, 3, 145–160. [Google Scholar] [CrossRef]
- Newman, S. Building Microservices; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2015. [Google Scholar]
- Familiar, B. Microservices, IoT and Azure: Leveraging DevOps and Microservice Architecture to Deliver SaaS Solutions; Apress: Berkeley, CA, USA, 2015. [Google Scholar]
- Gill, A.Q. Adaptive Cloud Enterprise Architecture. Intelligent Information Systems 4; World Scientific Publishing Co.: Singapore, 2015. [Google Scholar]
- Khan, K.M.; Gangavarapu, N.M. Addressing cloud computing in enterprise architecture: Issues and challenges. Cut. IT J. 2009, 22, 27–33. [Google Scholar]
- Gill, A.Q.; Smith, S.; Beydoun, G.; Sugumaran, V. Agile enterprise architecture: A case of a cloud technology-enabled government enterprise transformation. In Proceedings of the 19th Pacific Asia Conference on Information Systems (PACIS 2014), Chengdu, China, 24–28 June 2014; pp. 1–11. [Google Scholar]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S. Integrating mobile IT/cloud into enterprise architecture: A comparative analysis. In Proceedings of the 21st Pacific Asia Conference on Information Systems (PACIS 2016), Chiayi, Taiwan, 6–9 July 2016; p. 4. [Google Scholar]
- Masuda, Y.; Viswanathan, M. Enterprise Architecture for Global Companies in a Digital IT Era: Adaptive Integrated Digital Architecture Framework (AIDAF); Springer: Singapore, 2019. [Google Scholar]
- Zimmermann, A.; Schmidt, R.; Sandkuhl, K.; Jugel, D.; Schweda, C.; Bogner, J. Architecting Digital Products and Services. In Architecting the Digital Transformation; Zimmermann, A., Schmidt, R., Jain, L., Eds.; Springer: Berlin, Germany, 2020; Volume 188, pp. 181–197. [Google Scholar]
- Rogers, D.L. The Digital Transformation Playbook; Columbia University Press: New York, NY, USA, 2016. [Google Scholar]
- Hamilton, E.R.; Rosenberg, J.M.; Akcaoglu, M. Examining the Substitution Augmentation Modification Redefinition (SAMR) Model for Technology Integration. Tech. Trends 2016, 60, 433–441. [Google Scholar] [CrossRef]
- Brynjolfsson, E.; McAfee, A. Platform, Crowd. Harnessing Our Digital Future; W. W. Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- Vargo, S.L.; Akaka, M.A.; Vaughan, C.M. Conceptualizing Value: A Service-ecosystem View. J. Creat. Value 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Nils Olaya, F.; Ross, J.W. Building Business Agility: Cloud-Based Services and Digitized Platform Maturity, Research Briefing; MIT Center for Information Systems Research: Cambridge, MA, USA, 2015; Volume XV. [Google Scholar]
- Ross, J.W.; Beath, C.M.; Mocker, M. Designed for Digital. How to Architect Your Business for Sustained Success; The MIT Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Russel, S.; Norvig, P. Artificial Intelligence. A Modern Approach; Pearson: London, UK, 2015. [Google Scholar]
- Poole, D.L.; Mackworth, A.K. Artificial Intelligence. Foundations of Computational Agents; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Bones, C.; Hammersley, J.; Shaw, N. Optimizing Digital Strategy—How to Make Informed, Tactical Decisions That Deliver Growth; Kogan Page: London, UK, 2019. [Google Scholar]
- Ross, J.W.; Sebastian, I.M.; Beath, C.; Mocker, M.; Moloney, K.G.; Fonstad, N.O. Designing and Executing Digital Strategies. In Proceedings of the ICIS, Dublin, Ireland, 11–14 December 2016. [Google Scholar]
- Osterwalder, A.; Pigneur, Y. Business Model Generation; John Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Osterwalder, A.; Pigneur, Y.; Bernarda, G.; Smith, A.; Papadokos, T. Value Proposition Design; John Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Meertens, L.O.; Iacob, M.E.; Nieuwenhuis, L.J.M.; van Sinderen, M.J.; Jonkers, H.; Quertel, D. Mapping the Business Model Canvas to ArchiMate. In Proceedings of the 27th Annual ACM Symposium on Applied Computing, Trento, Italy, 26–30 March 2012. [Google Scholar]
- Open Group: ArchiMate 3.0 Specification; The Open Group: San Francisco, CA, USA, 2016.
- Gamma, E.; Helm, R.; Johnson, R.; Vlissides, J. Design Patterns; Addison Wesley: Boston, MA, USA, 1995. [Google Scholar]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S.; Hardjono, T. Risk Management for Digital transformation in Architecture Board: A Case Study on Global Enterprise. In Proceedings of the 2017 6th IIAI International Congress on Advanced Applied Informatics (IIAI-AAI), Hamamatsu, Japan, 9–13 July 2017; pp. 255–262. [Google Scholar]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S.; Hardjono, T. Risk Management for Digital transformation and Big Data in Architecture Board: A Case Study on Global Enterprise. Inf. Eng. Express 2018, 4, 33–51. [Google Scholar]
- Lowe, D. 7 Steps to Drug Discovery; ACS Webinars, American Chemical Society: Washington, DC, USA, 2014. [Google Scholar]
- United. States. Food and Drug Administration. The Drug Development Process. 2018. Available online: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process (accessed on 1 February 2021).
- Turk, M. Electronic Health Records: How to Suture the Gap between Privacy and Efficient Delivery of Healthcare. Brooklyn Law Rev. 2015, 80, 565–597. Available online: https://www.brooklaw.edu (accessed on 1 February 2021).
- Wulff, A.; Haarbrandt, B.; Tute, E.; Marschollek, M.; Beerbaum, P.; Jack, T. An interoperable clinical decision-support system for early detection of SIRS in pediatric intensive care using openEHR. Artif. Intell. Med. 2018, 89, 10–23. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Health Informatics-Electronic Health Record Definition, Scope and Context; ISO/TR 20514; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization. Management of Patient Information. November 2012. Available online: http://apps.who.int/iris/bitstream/10665/76794/1/9789241504645_eng.pdf (accessed on 1 February 2021).
- Murphy-Abdouch, K.; Biedermann, S. The electronic health record. In Introduction to Healthcare Informatics; Fenton, S.H., Biedermann, S., Eds.; AHIMA Press: Chicago, IL, USA, 2014; pp. 25–70. [Google Scholar]
- Singh, K.; Wright, A. Clinical decision support. In Clinical Informatics Study Guide; Finnell, J.T., Dixon, B.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 111–133. [Google Scholar]
- Shortliffe, E.H. Biomedical informatics: Defining the science and its role in health professional education. In Information Quality in e-Health. Lecture Notes in Computer Science; Hutchison, D., Kanade, T., Kittler, J., Kleinberg, J.M., Mattern, F., Mitchell, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 711–714. [Google Scholar]
- Greenes, R.A. (Ed.) Clinical Decision Support: The Road to Broad Adoption, 2nd ed.; Elsevier Science: Burlington, VT, USA, 2014. [Google Scholar]
- Masuda, Y.; Shepard, D.S.; Yamamoto, S. Adaptive Governance on Electronic Health Record in a Digital IT era. In Proceedings of the 25th Americas Conference on Information Systems, Cancún, Mexico, 15–17 August 2019. [Google Scholar]
- Grubb, B. My Health Record’s privacy chief quits amid claims agency not listening. The Sydney Morning Herald, 9 November 2018. [Google Scholar]
- Masuda, Y.; Shepard, D.S.; Yamamoto, S.; Toma, T. Clinical Decision-Support System with Electronic Health Record: Digitization of Research in Pharma. In Proceedings of the 7th International KES Conference on Innovation in Medicine & Healthcare, St. Julians, Malta, 17–19 June 2019; Springer: Singapore; Volume 145, pp. 47–57. [Google Scholar]
- Aceto, G.; Persico, V.; Pescapéa, A. The role of Information and Communication Technologies in healthcare: Taxonomies, perspectives, and challenges. J. Netw. Comput. Appl. 2018, 107, 125–154. [Google Scholar] [CrossRef]
- Calabrese, B.; Cannataro, M. Cloud computing in healthcare and biomedicine. Scalable Comput. Pract. Exp. 2015, 16, 1–18. [Google Scholar]
- Chawla, N.V.; Davis, D.A. Bringing big data to personalized healthcare: A patient-centered framework. J. Gen. Intern. Med. 2013, 28, 660–665. [Google Scholar] [CrossRef]
- Archenaa, J.; Anita, E.M. A survey of big data analytics in healthcare and government. Procedia Comput. Sci. 2015, 50, 408–413. [Google Scholar] [CrossRef]
- Chang, H.; Choi, M. Big data and healthcare: Building an augmented world. Health Inf. Res. 2016, 22, 153–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costa, F.F. Big data in biomedicine. Drug Discov. Today 2014, 19, 433–440. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kanbe, M. Knowledge Creation by Enterprise SNS. Int. J. Knowl. Cult. Chang. Manag. 2008, 8, 255–264. [Google Scholar]
- Kanbe, M.; Yamamoto, S. An Analysis of Computer Mediated Communication Patterns. Int. J. Knowl. Cult. Chang. Manag. 2009, 9, 35–47. [Google Scholar] [CrossRef]
- Kanbe, M.; Yamamoto, S.; Ohta, T. A Proposal of TIE Model for Communication in Software Development Process. In JSAI-Is AI, LNAI 6284; Nakakoji, K., Murakami, Y., McCready, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 104–115. [Google Scholar]
- Masuda, Y.; Shirasaka, S.; Yamamoto, S.; Hardjono, T. Proposal of GDTC model for global communication on enterprise portal. Procedia Comput. Sci. 2017, 112, 1178–1188. [Google Scholar] [CrossRef]
- Novartis. Machine Learning Poised to Accelerate Drug Discovery | Novartis. 2018. Available online: https://www.novartis.com/stories/discovery/machine-learning-poised-accelerate-drug-discovey (accessed on 1 February 2021).
- Morrison, C. AI developers tout revolution, drugmakers talk evolution. Nat. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Smalley, E. AI-powered drug discovery captures pharma interest. Nature 2017, 35, 604–605. [Google Scholar] [CrossRef]
- Davenport, T.H.; Ronanki, R. Article Technology: Artificial intelligence for the real world. Harv. Bus. Rev. 2018, 96, 108–116. [Google Scholar]
- Waxer, N.; Ninan, D.; Ma, A.; Dominguez, N. How cloud computing and social media are changing the face of health care. Phys. Exec. 2013, 39, 58–60. [Google Scholar]
- Aleksovska-Stojkovska, L.; Loskovska, S. Clinical Decision Support Systems: Medical knowledge acquisition and representation methods. In Proceedings of the 2010 IEEE International Conference on Electro/Information Technology, Normal, IL, USA, 20–22 May 2010; p. 1. [Google Scholar]
- Razzaque, A.; Karolak, M. Knowledge Management and Electronic Health Record Facilitate Clinical Support to Improve Healthcare Quality. In Proceedings of the International Conference on E-business, Management and Economics IPEDR, Hong Kong, China, 28–30 December 2010. [Google Scholar]
- Blei, D.M. Probabilistic topic models. Commun. ACM 2012, 55, 77–84. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009; pp. 501–528. [Google Scholar]






| Research Categories | Highlights | References |
|---|---|---|
| EA general | Definitions of an architecture framework | [2] A. Alwadain, E. Fielt, A. Korthaus, and M. Rosemann, 2014; Van der Raadt et al., 2008, 2010; Hjort-Madsen, 2006; Martin, 2012 |
| Benefits of EA | [24] T. Tamm, P. B. Seddon, G. Shanks, and P. Reynolds, 2011; Roeleven, 2008; Gregor et al., 2007 | |
| Business Strategy, Process and Portfolio Management on EA | [25] F. Kitsios, M Kamariotou; 2019 [26] F. Gonzalez-Lopez, & G. Bustos: 2019 [27] C. Gellweiler; 2020 | |
| Systematic Literature Reviews on EA | [6] BD. Rouhani, MN. Mahrin, F. Nikpay, RB. Ahmad, 2015; [7] M. Zhang, H. Chen, A. Luo, 2018; [8] N. Silva, P. Sousa, MM. da Silva, 2020; [9] Z Zhou, Q. Zhi, S. Morisaki, S. Yamamoto; 2020 [10] P. Hadaya, A. Leshob, P. Marchildon; 2020 | |
| Mobile IT | Concepts of Mobile IT | [28] K. Muhammad, M. N. A. Khan, 2015 |
| Analysis of EA and Mobile IT | [37] Y. Masuda, S. Shirasaka, and S. Yamamoto, 2016 | |
| Cloud computing | Definitions of Cloud computing | [34] A. Q. Gill, 2015 |
| Relationships between EA and Cloud computing | [35] K. M. Khan and N. M. Gangavarapu, 2009 [36] A. Q. Gill, S. Smith, G. Beydoun, and V. Sugumaran, 2014 | |
| Analysis of EA and Cloud | [37] Y. Masuda, S. Shirasaka, and S. Yamamoto, 2016 | |
| SOA and Microservices | Definitions of SOA | [29] M. Richards, 2015; |
| Reference model of SOA | [30] C. M. MacKenzie, K. Laskey, F. McCabe, P. F. Brown, and R. Metz, 2006 | |
| Benefits of SOA | [31] H. Chen, R. Kazman, and O. Perry, 2014 | |
| Characteristics of Microservices | [32] S. Newman, 2015 [29] M. Richards, 2015 [33] B. Familiar, 2015 | |
| AIDAF framework | Case Study in Global Enterprise | [4] Y. Masuda, S. Shirasaka, and S. Yamamoto, 2017 |
| Architecture Board with Digital Platform | [5] Y. Masuda, S. Shirasaka, and S. Yamamoto, 2018 | |
| Overall AIDAF framework | [38] Y. Masuda, M. Viswanathan, 2019 | |
| DEA reference cube | Supporting Digital Transformation | [40] D. L. Rogers, 2016 [41] E. R. Hamilton, J. M. Rosenberg, M. Akcaoglu, 2016 |
| Digital platform | [42] A. McAfee, Brynjolfsson, E. Machine, 2017 | |
| Ecosystem with value co-creation | [43] S. L. Vargo, M. A. Akaka, C. M. Vaughan, 2017 |
| Knowledge Group | Group Node | Media | Documentation | Examples of Deliverables |
|---|---|---|---|---|
| Tacit knowledge group | Human | Telephone, web conference | No documentation | Discussions, web meetings |
| Intermediary knowledge group | Portal contents | Portal, SNS, e-mail | Just in time documentation | Drug platform board logs, SNS logs, e-mail logs |
| Explicit knowledge group | Document | Document management services | Full documentation | Drug platform board results, drug development guidelines |
| Category | DB Access Flow No. | Each Activity of the Equivalent DB Access Flow |
|---|---|---|
| (a) Topic Modeling | a-1 | PDFs, MS Word files, ppt files of new drug plan documents are requested from machine learning programs to NoSQL DB. |
| a-2 | Get PDFs, MS Word files, ppt files of new drug plan documents. | |
| a-3 | Selected topics are input into NoSQL DB (unstructured data). | |
| a-4 | Selected topics are input into Relational DB. | |
| (b) Clustering | b-1 | Topics’ group is read from NoSQL DB (unstructured). |
| b-2 | Topics’ group is read from Relational DB. | |
| b-3 | Proportionated topics and documents are input into NoSQL DB. | |
| b-4 | Proportionated topics and attributes of documents are input into Relational DB. | |
| (c) Performing Reviews | c-1 | Each topic related documents are read from NoSQL DB (unstructured data). |
| c-2 | Each topic related documents’ attributes are read from Relational DB. | |
| c-3 | A file of review’s result in a category is input into NoSQL DB. | |
| c-4 | A file’s attributes of review’s result are input into Relational DB. | |
| c-5 | Integrated result of reviews in all categories is input into NoSQL DB (unstructured data). | |
| c-6 | Three elements (knowledge mode, transmit status, process status) are updated into Relational DB. | |
| c-7 | While the integrated review result’s report is uploaded and published on web portal, necessary attributes of the above report are input into Relational DB. | |
| c-8 | While the integrated review result’s report is uploaded and published on web portal, a file of the above review result’s report is input into NoSQL DB (unstructured data). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, Y.; Zimmermann, A.; Viswanathan, M.; Bass, M.; Nakamura, O.; Yamamoto, S. Adaptive Enterprise Architecture for the Digital Healthcare Industry: A Digital Platform for Drug Development. Information 2021, 12, 67. https://doi.org/10.3390/info12020067
Masuda Y, Zimmermann A, Viswanathan M, Bass M, Nakamura O, Yamamoto S. Adaptive Enterprise Architecture for the Digital Healthcare Industry: A Digital Platform for Drug Development. Information. 2021; 12(2):67. https://doi.org/10.3390/info12020067
Chicago/Turabian StyleMasuda, Yoshimasa, Alfred Zimmermann, Murlikrishna Viswanathan, Matt Bass, Osamu Nakamura, and Shuichiro Yamamoto. 2021. "Adaptive Enterprise Architecture for the Digital Healthcare Industry: A Digital Platform for Drug Development" Information 12, no. 2: 67. https://doi.org/10.3390/info12020067
APA StyleMasuda, Y., Zimmermann, A., Viswanathan, M., Bass, M., Nakamura, O., & Yamamoto, S. (2021). Adaptive Enterprise Architecture for the Digital Healthcare Industry: A Digital Platform for Drug Development. Information, 12(2), 67. https://doi.org/10.3390/info12020067









