Semantic Integration of BPMN Models and FHIR Data to Enable Personalized Decision Support for Malignant Melanoma
Abstract
:1. Introduction
2. Materials and Methods
3. Results
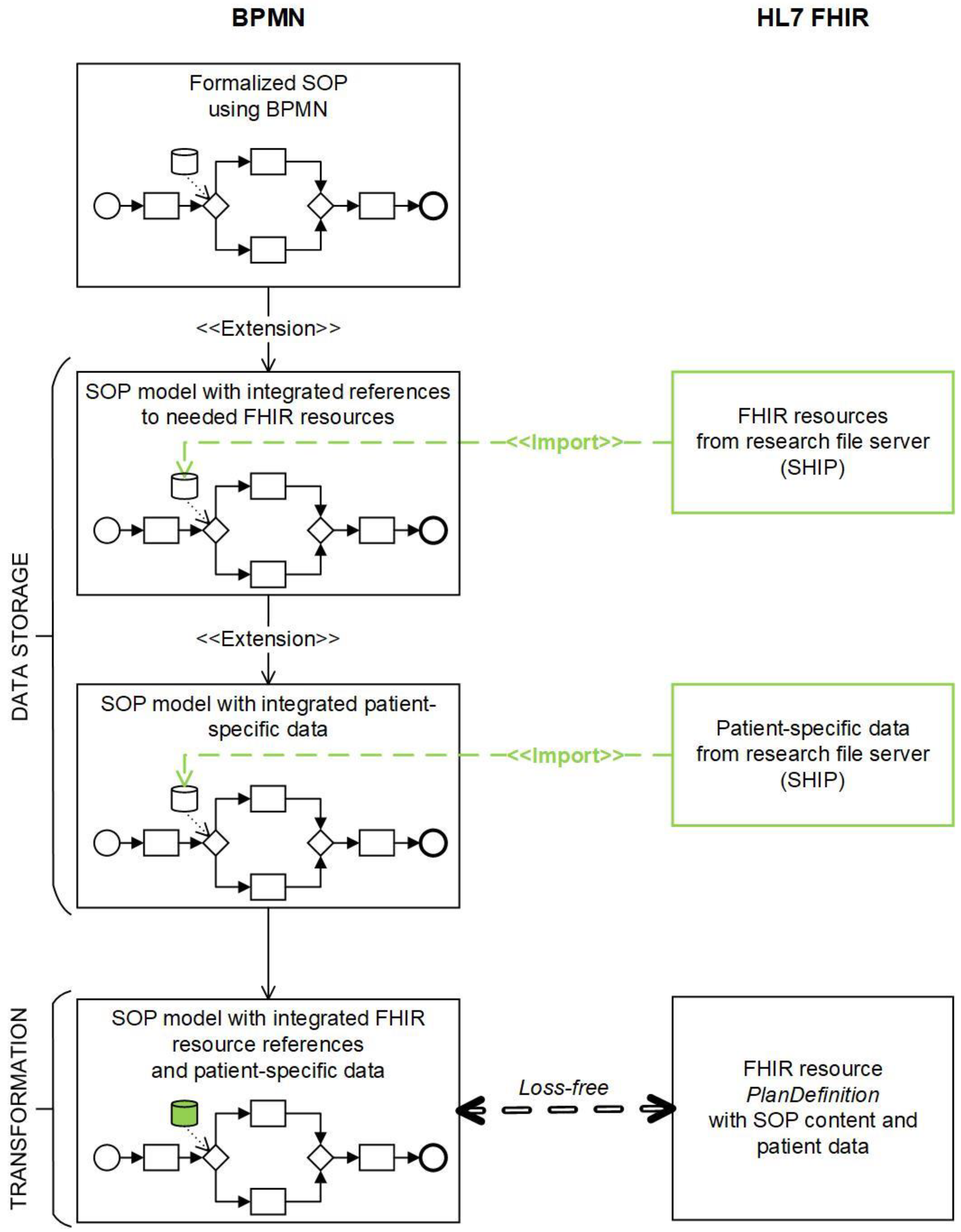
3.1. Loss-Free Mapping as a Foundation for the TRANSFORMATION Approach between BPMN and FHIR
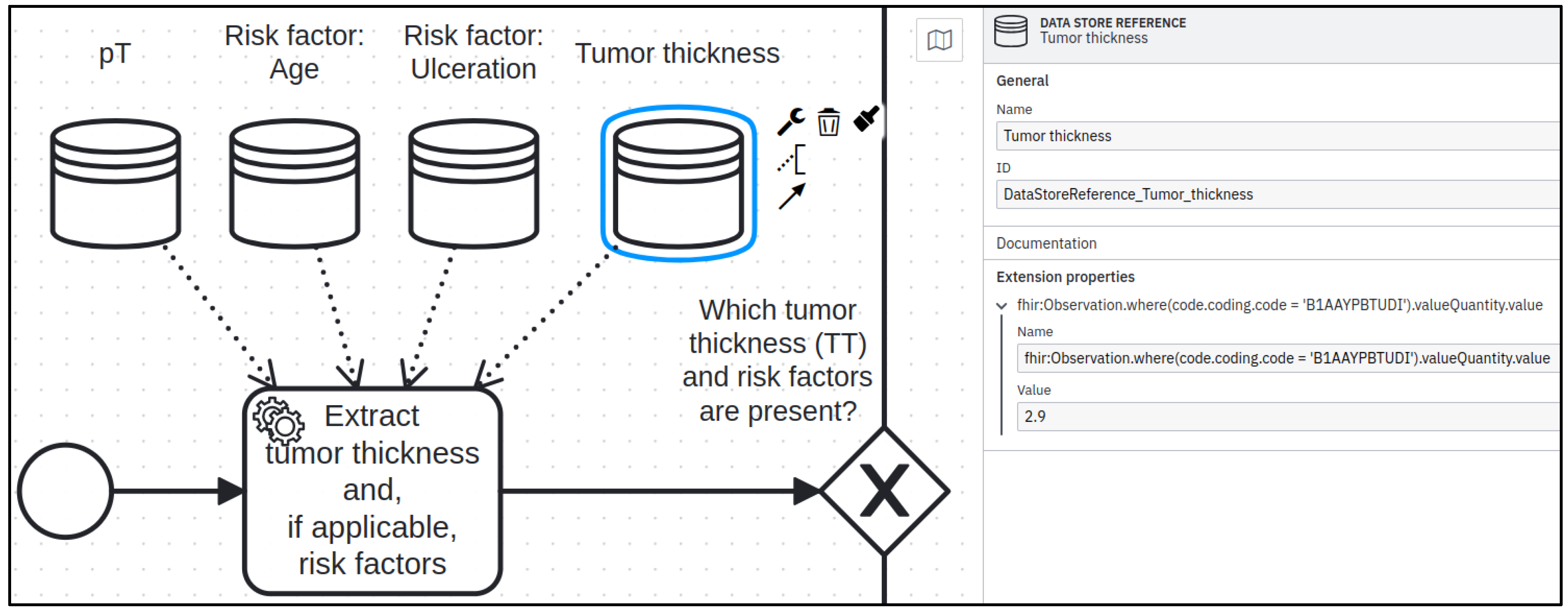
3.2. DATA STORAGE Aspect Realized in BPMN
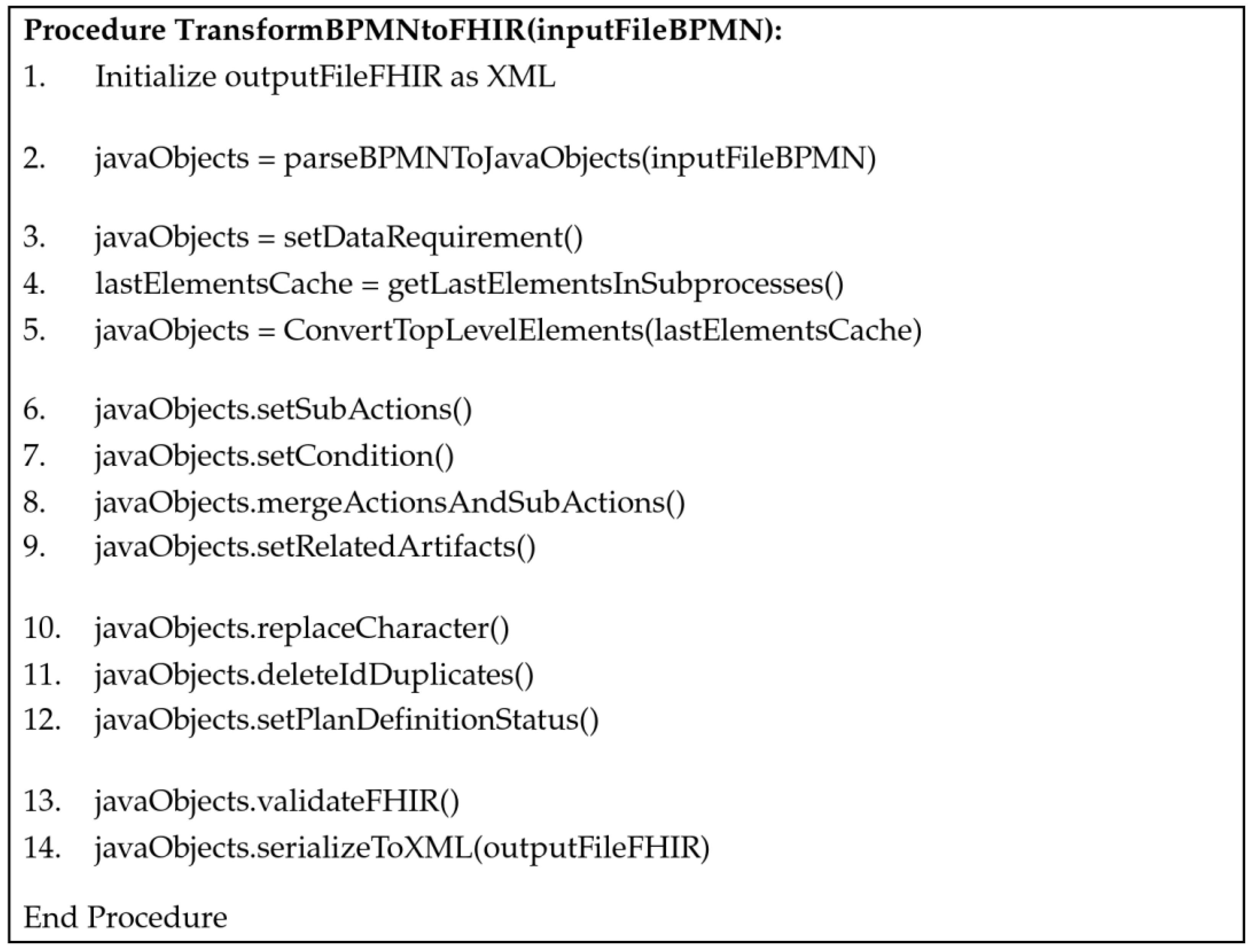
3.3. Generic Transformation Algorithm to Enable Personalized Decision Support
- BPMN elements with direct transformation: These elements, including tasks, events, gateways, as well as ‘DataStoreReferences’ existing at the top level (not within subprocesses), can directly be transformed into the corresponding FHIR fields (Figure 3, lines 3–5).
- BPMN elements requiring adjustments: This category contains elements like subprocesses, elements following gateways, as well as text annotations needing adjustments during transformation (Figure 3, lines 6–9).
- Mandatory FHIR requirements: Specific requirements outlined by the FHIR standard, including certain characters required for the FHIR server, unique IDs, and the mandatory field “fhir:PlanDefinition.status” (Figure 3, lines 10–12).
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, S.; de Jong, E.E.C.; van Timmeren, J.E.; Ibrahim, A.; Compter, I.; Peerlings, J.; Sanduleanu, S.; Refaee, T.; Keek, S.; Larue, R.T.H.M.; et al. Decision Support Systems in Oncology. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Zeng, J.; Bailey, A.M.; Holla, V.; Litzenburger, B.; Lara-Guerra, H.; Mills, G.B.; Mendelsohn, J.; Shaw, K.R.; Meric-Bernstam, F. The right drugs at the right time for the right patient: The MD Anderson precision oncology decision support platform. Drug Discov. Today 2015, 20, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Lödel, S.; Ostgathe, C.; Heckel, M.; Oechsle, K.; Gahr, S. Standard Operating Procedures (SOPs) for Palliative Care in German Comprehensive Cancer Centers—An evaluation of the implementation status. BMC Palliat. Care 2020, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Object Management Group. Business Process Model and Notation (BPMN). Version 2.0.2. Available online: https://www.omg.org/spec/BPMN/2.0.2/PDF (accessed on 23 October 2023).
- Weske, M. Business Process Management; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Martínez-Salvador, B.; Marcos, M.; Palau, P.; Domínguez Mafé, E. A model-driven transformation approach for the modelling of processes in clinical practice guidelines. Artif. Intell. Med. 2023, 137, 102495. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, F.; Oliboni, B.; Combi, C.; Campos, M.; Juarez, J.M. BPMN-Based Representation and Comparison of Clinical Pathways for Catheter-Related Bloodstream Infections. In Proceedings of the 2015 International Conference on Healthcare Informatics, Dallas, TX, USA, 21–23 October 2015; pp. 346–355. [Google Scholar] [CrossRef]
- De Ramón Fernández, A.; Ruiz Fernández, D.; Sabuco García, Y. Business Process Management for optimizing clinical processes: A systematic literature review. Health Inform. J. 2020, 26, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.; Sartipi, K. HL7 FHIR: An Agile and RESTful approach to healthcare information exchange. In Proceedings of the 26th IEEE International Symposium on Computer-Based Medical Systems, Porto, Portugal, 20–22 June 2013; pp. 326–331. [Google Scholar] [CrossRef]
- FHIR Clinical Guidelines. Available online: http://hl7.org/fhir/uv/cpg/index.html (accessed on 14 July 2023).
- Lichtner, G.; Alper, B.S.; Jurth, C.; Spies, C.; Boeker, M.; Meerpohl, J.J.; von Dincklage, F. Representation of evidence-based clinical practice guideline recommendations on FHIR. J. Biomed. Inform. 2023, 139, 104305. [Google Scholar] [CrossRef] [PubMed]
- CODEX+ CELIDA—COVID-19 Clinical Guidelines to Data Mapper. Available online: https://github.com/CODEX-CELIDA/ (accessed on 17 July 2023).
- Guideline Recommendation Evaluator (GREvaluator). Available online: https://github.com/CEOsys/grevaluator (accessed on 17 July 2023).
- HL7 International. Using Resources with Services and Service-Oriented Architecture. Available online: https://build.fhir.org/services.html (accessed on 7 September 2023).
- Krauss, O.; Holzer, K.; Schuler, A.; Egelkraut, R.; Franz, B. Challenges and Approaches to Make Multidisciplinary Team Meetings Interoperable—The KIMBo Project. Stud. Health Technol. Inform. 2017, 236, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Trisotech. How BPM+ Health Complements FHIR. Available online: https://www.trisotech.com/how-bpm-health-complements-fhir/ (accessed on 7 September 2023).
- Hund, H.; Wettstein, R.; Hampf, C.; Bialke, M.; Kurscheidt, M.; Schweizer, S.T.; Zilske, C.; Mödinger, S.; Fegeler, C. No Transfer Without Validation: A Data Sharing Framework Use Case. Stud. Health Technol. Inform. 2023, 302, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Hund, H.; Wettstein, R.; Heidt, C.M.; Fegeler, C. HiGHmed Data Sharing Framework (HiGHmed DSF). 2020. Available online: https://github.com/highmed/highmed-dsf (accessed on 7 September 2023).
- Hund, H.; Wettstein, R.; Heidt, C.M.; Fegeler, C. Executing Distributed Healthcare and Research Processes—The HiGHmed Data Sharing Framework. Stud. Health Technol. Inform. 2021, 278, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, R.; Hund, H.; Fegeler, C.; Heinze, O. Data Sharing in Distributed Architectures—Concept and Implementation in HiGHmed. Stud. Health Technol. Inform. 2021, 283, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, R.; Hund, H.; Kobylinski, I.; Fegeler, C.; Heinze, O. Feasibility Queries in Distributed Architectures—Concept and Implementation in HiGHmed. Stud. Health Technol. Inform. 2021, 278, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, R.; Kussel, T.; Hund, H.; Fegeler, C.; Dugas, M.; Hamacher, K. Secure Multi-Party Computation Based Distributed Feasibility Queries—A HiGHmed Use Case. Stud. Health Technol. Inform. 2022, 296, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kober, G.; Robaldo, L.; Paschke, A. Using BPMN for medical guidelines that integrate with FHIR-RDF. In Proceedings of the 14th International Conference on Semantic Web Applications and Tools for Health Care and Life Sciences (SWAT4HCLS 2023), Basel, Switzerland, 13–16 February 2023; pp. 1–10. [Google Scholar]
- Scheuerlein, H.; Rauchfuss, F.; Dittmar, Y.; Molle, R.; Lehmann, T.; Pienkos, N.; Settmacher, U. New methods for clinical pathways-Business Process Modeling Notation (BPMN) and Tangible Business Process Modeling (t.BPM). Langenbeck’s Arch. Surg. 2012, 397, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, C.L.; Lodde, G.; Livingstone, E.; Schadendorf, D.; Böckmann, B. Guideline-Based Context-Sensitive Decision Modeling for Melanoma Patients. Stud. Health Technol. Inform. 2022, 296, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Haug, P.; Huff, S.; Bledsoe, J. Diagnosing and Treating Pulmonary Embolus Using HL7 FHIR and the OMG’s Business Process Modeling Standards. Available online: https://www.bpm-plus.org/pdf/intermountain-fhir-bpm-6-25-20.pdf (accessed on 7 September 2023).
- Kiourtis, A.; Mavrogiorgou, A.; Kyriazis, D. FHIR Ontology Mapper (FOM): Aggregating Structural and Semantic Similarities of Ontologies towards their Alignment to HL7 FHIR. In Proceedings of the 2018 IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Gißke, C.; Liu, J.; Gand, K. Applying Goal-Oriented Modelling for Machine Learning Based Rehabilitation Care. Stud. Health Technol. Inform. 2022, 294, 342–346. [Google Scholar] [CrossRef] [PubMed]
- HAPI FHIR Library. Class Plan Definition. Plan Definition Action Input Component. Available online: https://hapifhir.io/hapi-fhir/apidocs/hapi-fhir-structures-r5/org/hl7/fhir/r5/model/PlanDefinition.PlanDefinitionActionInputComponent.html (accessed on 7 September 2023).
- Health Level Seven International. FHIR Workflow Minutes CC 20201116. Available online: https://confluence.hl7.org/display/FHIRI/FHIR+Workflow+Minutes+CC+20201116 (accessed on 7 September 2023).
- IBM. Modeling Cancer Treatment Processes with BPMN and HL7 FHIR: A National Comprehensive Cancer Network (NCCN) Case Study. Available online: https://docplayer.net/64810938-Modeling-cancer-treatment-processes-with-bpmn-and-hl7-fhir.html (accessed on 23 October 2023).
- Van der Heijden, J.J.M.J. Standardized Software Solution for Guidance of Clinical Workflows; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 2021; Available online: https://research.tue.nl/en/publications/standardized-software-solution-for-guidance-of-clinical-workflows (accessed on 7 September 2023).
- vCare Project. Available online: https://vcare-project.eu/ (accessed on 7 September 2023).
- Helm, E.; Pointner, A.; Krauss, O.; Schuler, A.; Traxler, B.; Arthofer, K.; Halmerbauer, G. FHIR2BPMN: Delivering Actionable Knowledge by Transforming between Clinical Pathways and Executable Models. Healthc. Future 2022, 292, 9–14. [Google Scholar] [CrossRef]
- Tewes, R.; Matzke, U.C. Innovative Personalentwicklung im In- und Ausland: Für Einrichtungen im Gesundheitswesen; Springer: Berlin/Heidelberg, Germany, 2021; p. 294. [Google Scholar] [CrossRef]
- camunda-bpmn-model-7.17.0-alpha3.jar, and camunda-xml-model-7.17.0-alpha3.jar. Available online: https://jar-download.com/download-handling.php (accessed on 20 January 2022).
- hapi-fhir-base-6.2.1.jar. Available online: https://mvnrepository.com/artifact/ca.uhn.hapi.fhir/hapi-fhir-base (accessed on 17 January 2023).
- Hosch, R.; Baldini, G.; Parmar, V.; Borys, K.; Koitka, S.; Engelke, M.; Arzideh, K.; Ulrich, M.; Nensa, F. FHIR-PYrate: A data science friendly Python package to query FHIR servers. BMC Health Serv. Res. 2023, 23, 734. [Google Scholar] [CrossRef] [PubMed]
- bpmnlint. Available online: https://github.com/bpmn-io/bpmnlint (accessed on 2 June 2023).
- Russell, N.; ter Hofstede, A.; van der Aalst, W.; Mulyar, N. Workflow Control-Flow Patterns: A Revised View. Available online: http://www.workflowpatterns.com/documentation/documents/BPM-06-22.pdf (accessed on 8 September 2023).
- Van der Aalst, W.; ter Hofstede, A.; Kiepuszewski, B.; Barros, A.P. Workflow Patterns: Distributed and Parallel Databases. Available online: http://www.workflowpatterns.com/documentation/documents/wfs-pat-2002.pdf (accessed on 8 September 2023).
- HL7 International. CPG Home. Available online: https://build.fhir.org/ig/HL7/cqf-recommendations/ (accessed on 23 October 2023).
- BPMN2FHIR: BPMN and FHIR Transformation with Integrated Patient FHIR Data. Available online: https://git.mi.fh-dortmund.de/guide2treat/bpmn2fhir/ (accessed on 24 October 2023).
- Camunda Platform 7.15. BPMN 2.0 Specification. Available online: https://camunda.com/de/download/modeler/ (accessed on 3 August 2023).

| BPMN Elements | HL7 FHIR ‘PlanDefinition’ |
|---|---|
| Flow Objects | |
| Activities | |
| Task | action |
| User Task | action.description=’userTask’ |
| Service Task | action.description=’serviceTask’ |
| Subprocess | The preceding BPMN element references the start element of the subprocess and all subsequent elements of the subprocess are subactions of it in FHIR. The end element of the subprocess references the subsequent BPMN element. |
| Events | action |
| Start | action which has no actions refers to it |
| End | action with no related action |
| Gateways | action |
| Parallel Gateway | action.selectionBehavior=’all’ |
| Exclusive Gateway | action.selectionBehavior=’exactly-one’ |
| Connecting Objects | |
| Sequence Flow | action.relatedAction |
| Data Input Association, Data Output Association | Not needed (Comment: These elements point to ‘DataStoreReference’, and because the ‘DataStoreReference’ in FHIR is directly included in action, no association is needed.) |
| Artifacts | |
| Annotation | action.relatedArtifact |
| Data | |
| DataStoreReference | action.input.requirement/ action.output.requirement |
| Name | DataRequirement.codeFilter.path |
| Value | DataRequirement.codeFilter.searchParam |
| BPMN | <serviceTask id=“Activity_1” name=“Extract tumor thickness”> <incoming>Flow_1</incoming> <outgoing>Flow_2</outgoing> <property id=“Property_1” name=“__targetRef_placeholder”/> <dataInputAssociation id=“DataInputAssociation_1”> <sourceRef>DataStoreRef_1</sourceRef> <targetRef>Property_1</targetRef> </dataInputAssociation> </serviceTask> <dataStoreReference id=“DataStoreRef_1” name=“Tumor thickness”> <extensionElements> <properties> <property name=“fhir:Observation.where(code.coding. code=‘B1AAYPBTUDI’).valueQuantity.value” value=“2.9”/> </properties> </extensionElements> </dataStoreReference> |
| HL7 FHIR ‘PlanDefinition’ | <action id=“Activity-1”> <title value=“Extract tumor thickness”></title> <description value=“serviceTask”></description> <input> <type value=“DataRequirement”></type> <mustSupport value=“Tumor thickness”></mustSupport> <codeFilter> <path value=“fhir:Observation.where(code.coding.code= ‘B1AAYPBTUDI’).valueQuantity.value”> </path> <searchParam value=“2.9”></searchParam> </codeFilter> </input> <relatedAction> <actionId value=“Gateway-1”></actionId> <relationship value=“before”></relationship> </relatedAction> </action> |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckmann, C.L.; Keuchel, D.; Soleman, W.O.I.A.; Nürnberg, S.; Böckmann, B. Semantic Integration of BPMN Models and FHIR Data to Enable Personalized Decision Support for Malignant Melanoma. Information 2023, 14, 649. https://doi.org/10.3390/info14120649
Beckmann CL, Keuchel D, Soleman WOIA, Nürnberg S, Böckmann B. Semantic Integration of BPMN Models and FHIR Data to Enable Personalized Decision Support for Malignant Melanoma. Information. 2023; 14(12):649. https://doi.org/10.3390/info14120649
Chicago/Turabian StyleBeckmann, Catharina Lena, Daniel Keuchel, Wa Ode Iin Arliani Soleman, Sylvia Nürnberg, and Britta Böckmann. 2023. "Semantic Integration of BPMN Models and FHIR Data to Enable Personalized Decision Support for Malignant Melanoma" Information 14, no. 12: 649. https://doi.org/10.3390/info14120649
APA StyleBeckmann, C. L., Keuchel, D., Soleman, W. O. I. A., Nürnberg, S., & Böckmann, B. (2023). Semantic Integration of BPMN Models and FHIR Data to Enable Personalized Decision Support for Malignant Melanoma. Information, 14(12), 649. https://doi.org/10.3390/info14120649






