Resonating with the World: Thinking Critically about Brain Criticality in Consciousness and Cognition
Abstract
:1. Introduction
1.1. Resonating with the World
1.2. Schumann Resonance and Living
1.3. Resonance in Cognitive–Motor Interaction
1.4. Criticality and Consciousness
1.5. Criticality in Consciousness and Cognition
2. Resonating with the Brain in Consciousness and Cognition
2.1. Thinking Critically about Brain Criticality in the Context of Resonance
2.2. Critical Biofield Systems and Information
2.3. Consciousness as a Function of Resonance
3. Resonating with Life (and Death): Gaps in Current Understanding
3.1. The Combination Problem in General Resonance Theory
3.2. Everything Has a Unique Resonance
3.3. What Is Resonance and Synchrony?
3.4. How Is Shared Resonance Attained in Resonating Structures?
4. Criticality of Resonating Consciousness
4.1. Interaction of Physical Systems and Consciousness
4.2. Connecting Biofield Resonance and Consciousness
4.3. Grossberg’s Input Regarding the Connection between Awareness and Biofield Resonance
4.4. Formulating a Theory Linking the Conscious Mind and Resonant Brain Dynamics
4.5. The Conscious Mind and Resonant Dynamics Are Linked by the Adaptive Resonance Theory
4.6. The Conflict between Stability and Adaptability and Lifelong Learning
4.7. Why Not AI Representations?
5. Coherence in Resonance
6. Integrating Consciousness and Cognition through Resonance
7. Cortical Activity Waves as a Vehicle for Consciousness and Cognition
7.1. Continuum Waves in Consciousness and Cognition
7.2. Continuum Analysis Applied to Consciousness and Cognition
7.3. The Wilson–Cowan Equations
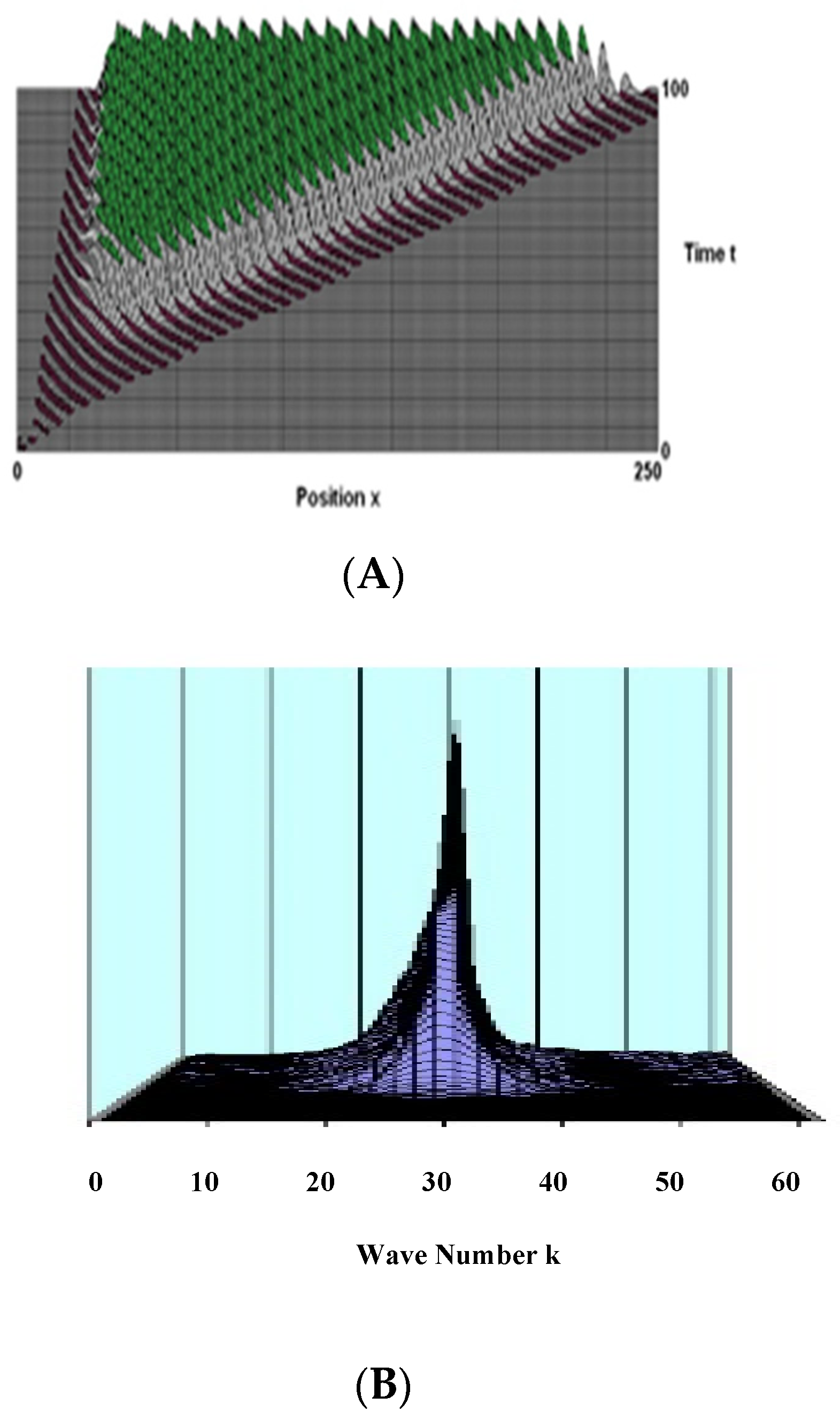
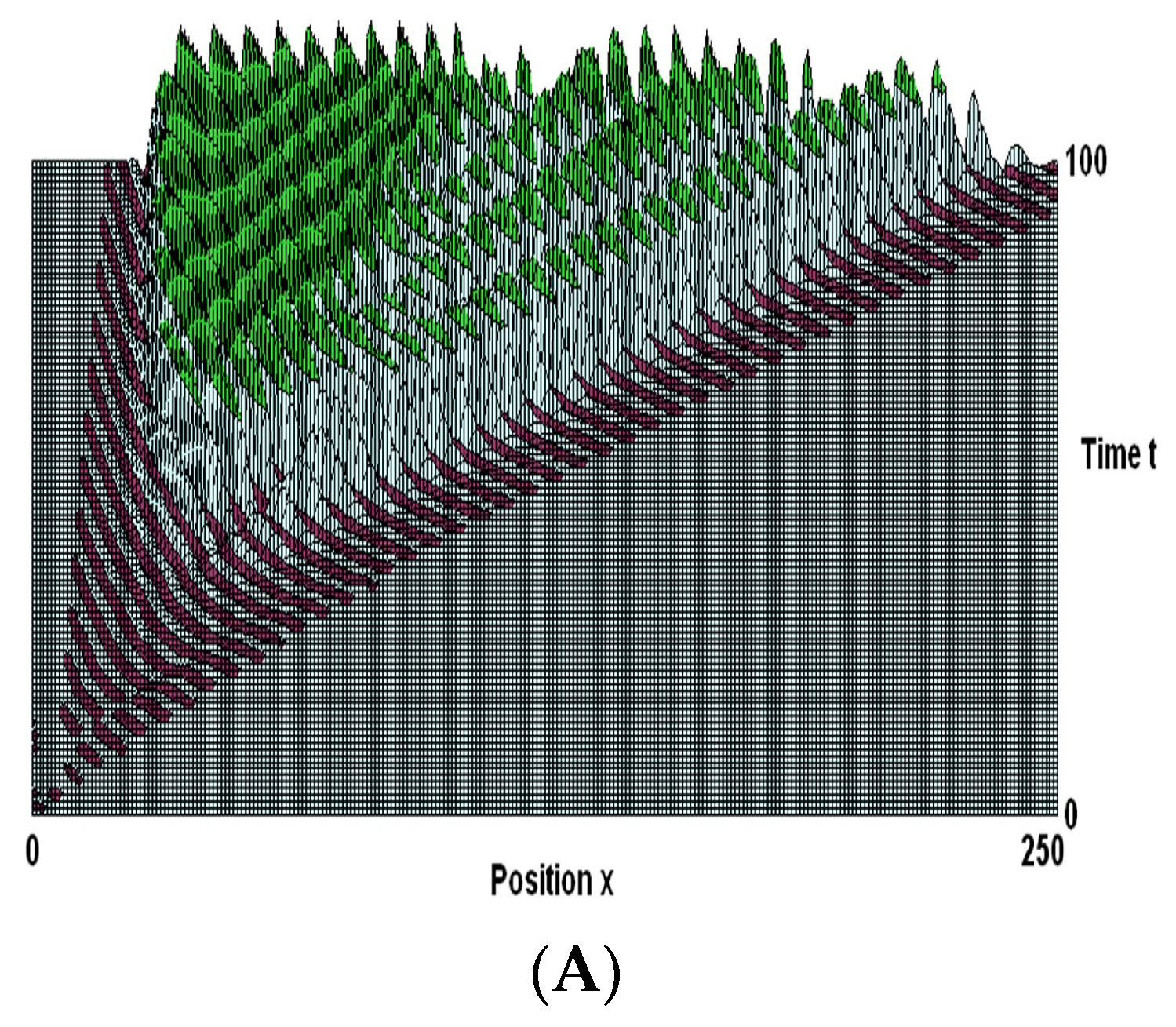
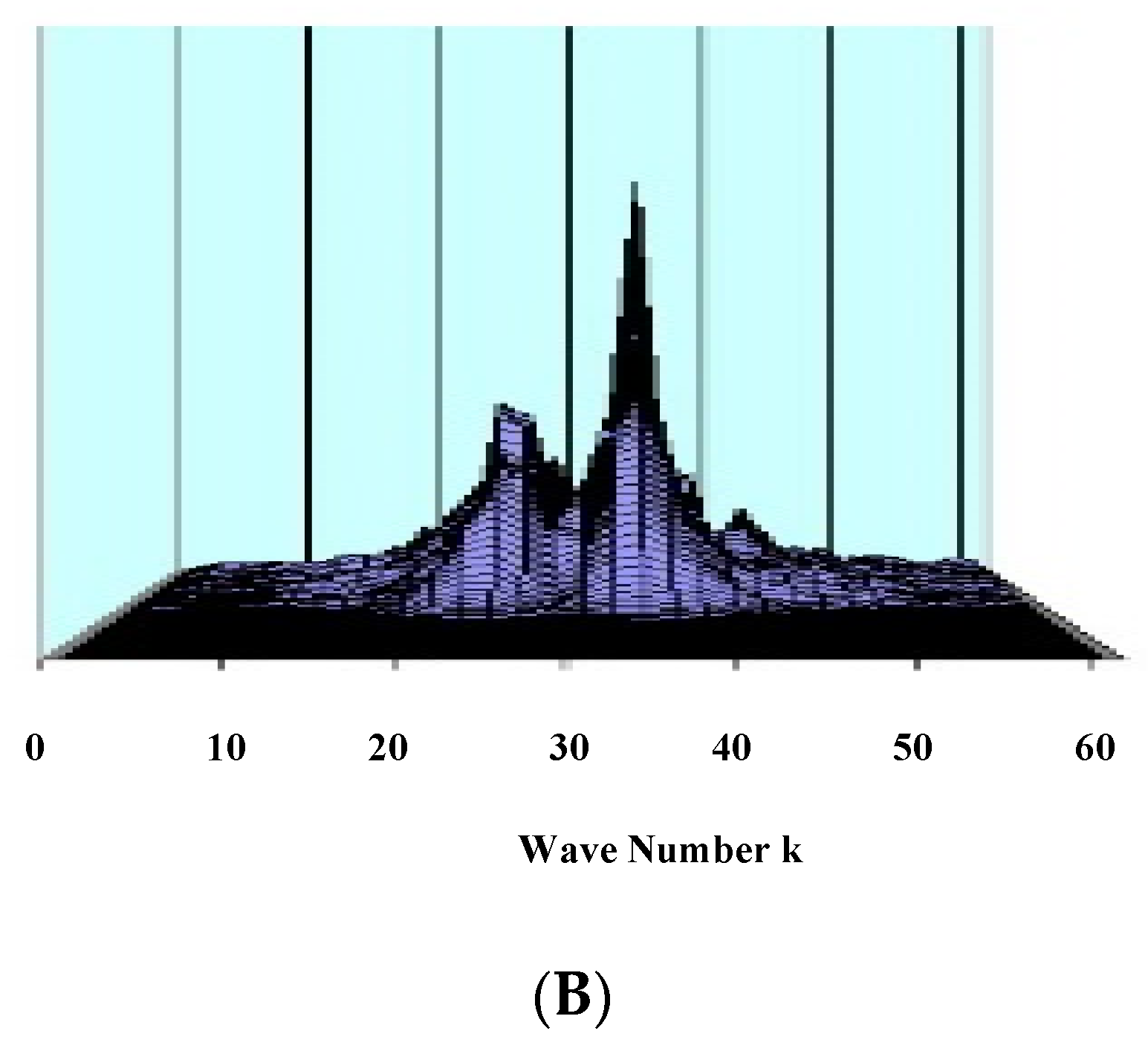
7.4. Numerical Results
8. Conclusions: Resonance Signatures Allow Us to Adapt and Effectively Interact with Our Environment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacoma Bridge. Available online: https://www.youtube.com/watch?v=3mclp9QmCGs (accessed on 18 March 2023).
- Bare, J.E. Resonant Frequency Therapy Device. US Patent 6,221,094, 24 April 2001. Available online: https://patents.google.com/patent/US5908441A/en (accessed on 16 May 2023).
- Bare, J.E. Resonant Frequency Therapy Device. US Patent 5,908,441, 1 June 1999. Available online: https://patents.google.com/patent/US6221094B1/en (accessed on 16 May 2023).
- Bare, J.E. Resonant Frequency Therapy Device. US Patent 8,652,184, 18 February 2014. Available online: https://patents.google.com/patent/US8652184B2/en (accessed on 16 May 2023).
- Pitt, W.G. Protein Adsorption on Polyurethanes (FTIR); University of Wisconsin Press: Madison, WI, USA, 1987. [Google Scholar]
- Mittelstein, D.R.; Ye, J.; Schibber, E.F.; Roychoudhury, A.; Martinez, L.T.; Fekrazad, M.H.; Ortiz, M.; Lee, P.P.; Shapiro, M.G.; Gharib, M. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl. Phys. Lett. 2020, 116, 013701. [Google Scholar] [CrossRef]
- Buckner, C.A.; Buckner, A.L.; Koren, S.A.; Persinger, M.A.; Lafrenie, R.M. Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS ONE 2015, 10, e0124136. [Google Scholar] [CrossRef]
- Kang, C.; Li, Y.; Novak, D.; Zhang, Y.; Zhou, Q.; Hu, Y. Brain networks of maintenance, inhibition and disinhibition during working memory. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G. On the Application of Developmental Cognitive Neuroscience in Educational Environments. Brain Sci. 2022, 12, 1501. [Google Scholar] [CrossRef]
- Leisman, G.; Melillo, R. Front and center: Maturational dysregulation of frontal lobe functional neuroanatomic connections in attention deficit hyperactivity disorder. Front. Neuroanat. 2022, 16, 936025. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Turrigiano, G.G.; Wessel, R.; Hengen, K.B. Cortical circuit dynamics are homeostatically tuned to criticality in vivo. Neuron 2019, 104, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Miller, I. The Schumann’s resonances and human psychobiology. Nexus Mag. 2003, 10, 43–49. [Google Scholar]
- McCraty, R.; Al Abdulgader, A. Consciousness, the human heart and the global energetic field environment. Cardiol. Vasc. Res 2021, 5, 1–19. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Li, Z.; Zhao, Z.; Zeren, Z.; Shen, X.; Wang, Q. Recent Advances and Challenges in Schumann Resonance Observations and Research. Remote Sens. 2023, 15, 3557. [Google Scholar] [CrossRef]
- Kruglov, A.G.; Kruglov, A.A.; Utkin, V.N. Resonant Interaction of the Psyche, Circadian Rhythms and External Electromagnetic Fields. Curr. J. Appl. Sci. Technol. 2023, 42, 23–30. [Google Scholar] [CrossRef]
- Leisman, G.; Machado, C. Many Paths to Consciousness or Just One? Life in a Bounded Continuum. J. Conscious. Stud. 2021, 28, 83–96. [Google Scholar]
- Young, A.; Hunt, T.; Ericson, M. The slowest shared resonance: A review of electromagnetic field oscillations between central and peripheral nervous systems. Front. Hum. Neurosci. 2022, 15, 796455. [Google Scholar] [CrossRef] [PubMed]
- Garvanova, M.; Garvanov, I.; Borissova, D. The influence of electromagnetic fields on human brain. In Proceedings of the 2020 21st International Symposium on Electrical Apparatus & Technologies (SIELA), Bourgas, Bulgaria, 3–6 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–4. [Google Scholar]
- Leisman, G.; Moustafa, A.A.; Shafir, T. Thinking, walking, talking: Integratory motor and cognitive brain function. Front. Public Health 2016, 4, 179575. [Google Scholar] [CrossRef]
- Signorelli, C.M.; Szczotka, J.; Prentner, R. Explanatory profiles of models of consciousness-towards a systematic classification. Neurosci. Conscious. 2021, 2021, niab021. [Google Scholar] [CrossRef] [PubMed]
- Toker, D.; Pappas, I.; Lendner, J.D.; Frohlich, J.; Mateos, D.M.; Muthukumaraswamy, S.; Carhart-Harris, R.; Paff, M.; Vespa, P.M.; Monti, M.M.; et al. Consciousness is supported by near-critical slow cortical electrodynamics. Proc. Natl. Acad. Sci. USA 2022, 119, e2024455119. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G.; Alfasi, R.; D’Angiulli, A. The development of fetal primary consciousness: An emergent transitions framework. Curr. Opin. Behav. Sci. 2024, in press. [Google Scholar]
- Hebb, D. The Organisation of Behavior: A Neuropsychological Theory; Wiley: New York, NY, USA, 1948. [Google Scholar]
- Koch, P.; Leisman, G. A continuum model of activity waves in layered neuronal networks: Computer models of brain-stem seizures. In Proceedings of the Third Annual IEEE Symposium on Computer-Based Medical Systems, Chapel Hill, NC, USA, 3–6 June 1990; IEEE: Piscataway, NJ, USA, 1990; pp. 525–531. [Google Scholar]
- Koch, P.; Leisman, G. Cortical Activity waves are the physical carriers of memory and thought. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 364–367. [Google Scholar]
- Leisman, G.; Koch, P. Networks of conscious experience: Computational neuroscience in understanding life, death, and consciousness. Rev. Neurosci. 2009, 20, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, R.; Jain, S.; Baldwin, A.L.; Gronowicz, G.; Lutgendorf, S.K.; Oschman, J.L.; Yount, G.L. Biofield research: A roundtable discussion of scientific and methodological issues. J. Altern. Complement. Med. 2012, 18, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, R.; Levin, M.; McCraty, R.; Bat, N.; Ives, J.A.; Lutgendorf, S.K.; Oschman, J.L. Biofield physiology: A framework for an emerging discipline. Glob. Adv. Health Med. 2015, 4 (Suppl. S1), gahmj-2015. [Google Scholar] [CrossRef]
- Ho, M.W. The Rainbow and the Worm: The Physics of Organisms; World Scientific: Singapore, 2008. [Google Scholar]
- Finger, A.M.; Kramer, A. Mammalian circadian systems: Organization and modern life challenges. Acta Physiol. 2021, 231, e13548. [Google Scholar] [CrossRef]
- Doelling, K.; Herbst, S.; Arnal, L.; van Wassenhove, V. Psychological and Neuroscientific Foundations of Rhythms and Timing; HAL Open Science: London, UK; Oxford, UK, 2023. [Google Scholar]
- Crick, F.; Koch, C. Towards a neurobiological theory of consciousness. Semin. Neurosci. 1990, 2, 263–275. [Google Scholar]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef]
- Koch, C. Qualia. Curr. Biol. 2004, 14, R496. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S. Consciousness and the Brain: Deciphering How the Brain Codes Our Thoughts; Viking Penguin: New York, NY, USA, 2014. [Google Scholar]
- Grossberg, S.T. Towards solving the hard problem of consciousness: The varieties of brain resonances and the conscious experiences that they support. Neural Netw. 2017, 87, 38–95. [Google Scholar] [CrossRef]
- Freeman, W.J.; Vitiello, G. Nonlinear brain dynamics as macroscopic manifestation of underlying many-body field dynamics. Phys. Life Rev. 2006, 3, 93–118. [Google Scholar] [CrossRef]
- Pockett, S. The Nature of Consciousness: A Hypothesis; Iuniverse: Bloomington, IN, USA, 2000. [Google Scholar]
- Pockett, S. The electromagnetic field theory of consciousness a testable hypothesis about the characteristics of conscious as opposed to non-conscious fields. J. Conscious. Stud. 2012, 19, 191–223. [Google Scholar]
- Bandyopadhyay, A. Resonance Chains and New Models of the Neuron. 2019. Available online: https://medium.com/@aramis720/resonance-chains-and-new-models-of-the-neuron-7dd82a5a7c3a (accessed on 15 May 2023).
- Sahu, S.; Ghosh, S.; Ghosh, B.; Aswani, K.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Atomic water channel controlling remarkable properties of a single brain microtubule: Correlating single protein to its supramolecular assembly. Biosens. Bioelectron. 2013, 47, 141–148. [Google Scholar] [CrossRef]
- Sahu, S.; Ghosh, S.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Multi-level memory-switching properties of a single brain microtubule. Appl. Phys. Lett. 2013, 102, 123701. [Google Scholar] [CrossRef]
- Singh, P.; Ray, K.; Fujita, D.; Bandyopadhyay, A. Complete dielectric resonator model of human brain from MRI data: A journey from connectome neural branching to single protein. In Engineering Vibration, Communication and Information Processing; Springer: New York, NY, USA, 2019; pp. 717–733. [Google Scholar]
- Strogatz, S.H. Sync: How Order Emerges from Chaos in the Universe, Nature, and Daily Life; Hachette: London, UK, 2012. [Google Scholar]
- Craddock, J.A.; Hameroff, T.R.; Ayoub, S.T.; Klobukowski, M.; Tuszynski, A. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr. Top. Med. Chem. 2015, 15, 523–533. [Google Scholar] [CrossRef]
- Keppler, J. A new perspective on the functioning of the brain and the mechanisms behind conscious processes. Front. Psychol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T. Kicking the psychophysical laws into Gear a new approach to the combination problem. J. Conscious. Stud. 2011, 18, 96–134. [Google Scholar]
- Schooler, J.W.; Hunt, T.; Schooler, J.N. Reconsidering the metaphysics of science from the inside out. In Neuroscience, Consciousness and Spirituality; Springer: Dordrecht, The Netherlands, 2011; pp. 157–194. [Google Scholar]
- Hunt, T. Eco, Ego, Eros: Essays in Philosophy, Spirituality and Science; Aramis Press: Arcueil, France, 2014. [Google Scholar]
- Goff, P. Consciousness and Fundamental Reality; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Koch, C. Ubiquitous minds. Sci. Am. Mind 2014, 25, 26–29. [Google Scholar] [CrossRef]
- Tononi, G.; Koch, C. Consciousness: Here, there and everywhere? Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140167. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T. Calculating the boundaries of consciousness in general resonance theory. J. Conscious. Stud. 2020, 27, 55–80. [Google Scholar]
- Griffin, D.R. Unsnarling the World-Knot: Consciousness, Freedom, and the Mind-Body Problem; University of California Press: Berkeley, CA, USA, 1998; ISBN 0-520-20944-3. [Google Scholar]
- Hameroff, S. The “conscious pilot”—Dendritic synchrony moves through the brain to mediate consciousness. J. Biol. Phys. 2010, 36, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Jones, M. Electromagnetic-field theories of mind. J. Conscious. Stud. 2013, 20, 124–149. [Google Scholar]
- McFadden, J. Synchronous Firing and its influence on the brain’s electromagnetic field. J. Conscious. Stud. 2002, 9, 23–50. [Google Scholar]
- McFadden, J. The conscious electromagnetic information (Cemi) field theory: The hard problem made easy? J. Conscious. Stud. 2002, 9, 45–60. [Google Scholar]
- John, E.R. A field theory of consciousness. Conscious. Cogn. 2001, 10, 184–213. [Google Scholar] [CrossRef]
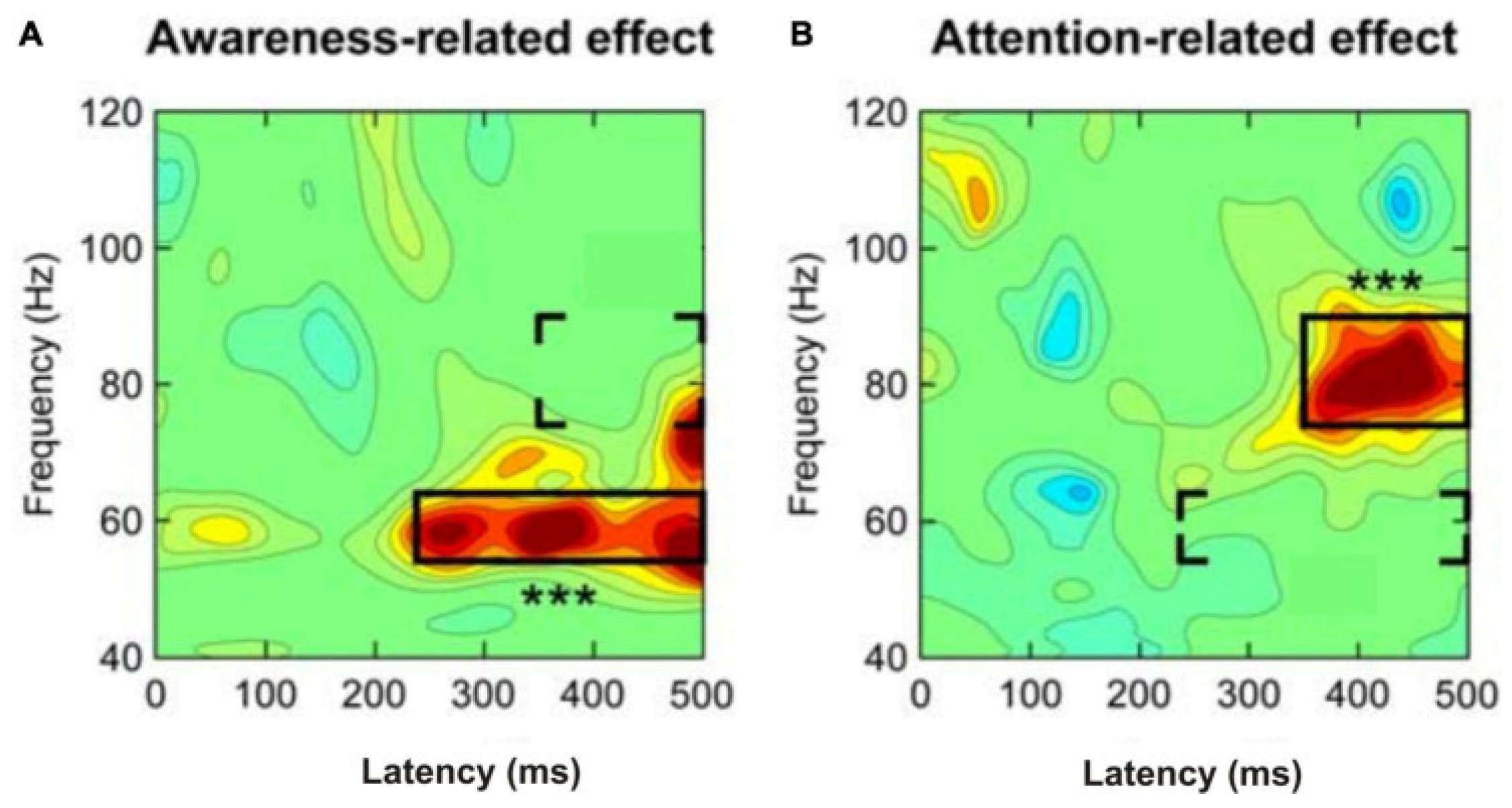
- Wyart, V.; Tallon-Baudry, C. Neural dissociation between visual awareness and spatial attention. J. Neurosci. 2008, 28, 2667–2679. [Google Scholar] [CrossRef]
- Whitehead, C. Cultural distortions of self-and reality-perception. J. Conscious. Stud. 2010, 17, 95–118. [Google Scholar]
- Olsen, L.F.; Degn, H. Chaos in biological systems. Q. Rev. Biophys. 1985, 18, 165–225. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Cortassa, S.; Lloyd, D. Chaos in biochemistry and physiology. Encycl. Biochem. Mol. Cell Biol. Mol. Med. Syst. Biol. 2012, 239–276. [Google Scholar]
- Teuscher, C. Revisiting the edge of chaos: Again? Biosystems 2022, 218, 104693. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Gotta, M. Order from chaos: Cellular asymmetries explained with modelling. Trends Cell Biol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, B.; Noble, D.; Hatta, A. (Eds.) The Language of Symmetry; Chapman & Hall/CRC: Boca Raton, FL, USA, 2023. [Google Scholar]
- Gutjahr, N.; Hövel, P.; Viol, A. Controlling extended criticality via modular connectivity. J. Phys. Complex. 2021, 2, 035023. [Google Scholar] [CrossRef]
- Dunham, C.S.; Lilak, S.; Hochstetter, J.; Loeffler, A.; Zhu, R.; Chase, C.; Stieg, A.Z.; Kuncic, Z.; Gimzewski, J.K. Nanoscale neuromorphic networks and criticality: A perspective. J. Phys. Complex. 2021, 2, 042001. [Google Scholar] [CrossRef]
- O’Byrne, J.; Jerbi, K. How critical is brain criticality? Trends Neurosci. 2022, 45, 820–837. [Google Scholar] [CrossRef]
- Korchinski, D.J.; Orlandi, J.G.; Son, S.W.; Davidsen, J. Criticality in spreading processes without timescale separation and the critical brain hypothesis. Phys. Rev. X 2021, 11, 021059. [Google Scholar] [CrossRef]
- Beggs, J.M. Addressing skepticism of the critical brain hypothesis. Front. Comput. Neurosci. 2022, 16, 703865. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ohse, S.; Turalska, M.; West, B.J.; Garcia-Ojalvo, J. Structural determinants of criticality in biological networks. Front. Physiol. 2015, 6, 141017. [Google Scholar] [CrossRef] [PubMed]
- Calderon, D.P.; Kilinc, M.; Maritan, A.; Banavar, J.R.; Pfaff, D. Generalized CNS arousal: An elementary force within the vertebrate nervous system. Neurosci. Biobehav. Rev. 2016, 68, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Del Papa, B.; Priesemann, V.; Triesch, J. Fading memory, plasticity, and criticality in recurrent networks. Funct. Role Crit. Dyn. Neural Syst. 2019, 95–115. [Google Scholar] [CrossRef]
- Cocchi, L.; Gollo, L.L.; Zalesky, A.; Breakspear, M. Criticality in the brain: A synthesis of neurobiology, models and cognition. Prog. Neurobiol. 2017, 158, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Marinazzo, D.; Pellicoro, M.; Wu, G.; Angelini, L.; Cortés, J.M.; Stramaglia, S. Information transfer and criticality in the Ising model on the human connectome. PLoS ONE 2014, 9, e93616. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P. The ising model: Brief introduction and its application. In Solid State Physics-Metastable, Spintronics Materials and Mechanics of Deformable Bodies-Recent Progress; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/71210 (accessed on 17 March 2024).
- Popiel, N.J.; Khajehabdollahi, S.; Abeyasinghe, P.M.; Riganello, F.; Nichols, E.S.; Owen, A.M.; Soddu, A. The emergence of integrated information, complexity, and ‘consciousness’ at criticality. Entropy 2020, 22, 339. [Google Scholar] [CrossRef]
- Hidalgo, J.; Grilli, J.; Suweis, S.; Munoz, M.A.; Banavar, J.R.; Maritan, A. Information-based fitness and the emergence of criticality in living systems. Proc. Natl. Acad. Sci. USA 2014, 111, 10095–10100. [Google Scholar] [CrossRef]
- Vilone, D.; Realpe-Gomez, J.; Andrighetto, G. Evolutionary advantages of turning points in human cooperative behavior. PLoS ONE 2021, 16, e0246278. [Google Scholar] [CrossRef]
- Merker, B.; Williford, K.; Rudrauf, D. The integrated information theory of consciousness: A case of mistaken identity. Behav. Brain Sci. 2022, 45, e41. [Google Scholar] [CrossRef]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Zilio, F. From shorter to longer timescales: Converging integrated information theory (IIT) with the temporo-spatial theory of consciousness (TTC). Entropy 2022, 24, 270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, U. Criticality as a determinant of integrated information Φ in human brain networks. Entropy 2019, 21, 981. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.; Mashour, G.A.; Lee, U. Relationship of topology, multiscale phase synchronization, and state transitions in human brain networks. Front. Comput. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Golkowski, D.; Jordan, D.; Berger, S.; Ilg, R.; Lee, J.; Mashour, G.A.; The ReCCognition Study Group. Relationship of critical dynamics, functional connectivity, and states of consciousness in large-scale human brain networks. Neuroimage 2019, 188, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Pepić, S.; Shriki, O.; Tkačik, G.; De Martino, D. Statistical modeling of adaptive neural networks explains co-existence of avalanches and oscillations in resting human brain. Nat. Comput. Sci. 2023, 3, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Beggs, J.M.; Timme, N. Being critical of criticality in the brain. Front. Physiol. 2012, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Kloucek, M.B.; Machon, T.; Kajimura, S.; Royall, C.P.; Masuda, N.; Turci, F. Biases in inverse Ising estimates of near-critical behavior. Phys. Rev. E 2023, 108, 014109. [Google Scholar] [CrossRef] [PubMed]
- Girardi-Schappo, M. Brain criticality beyond avalanches: Open problems and how to approach them. J. Phys. Complex. 2021, 2, 031003. [Google Scholar] [CrossRef]
- Liu, X.; Fei, X.; Liu, J. The Cognitive Critical Brain: Modulation of Criticality in Task-Engaged Regions. bioRxiv 2023. [Google Scholar] [CrossRef]
- Del Papa, B.; Priesemann, V.; Triesch, J. Criticality meets learning: Criticality signatures in. a self-organizing recurrent neural network. PLoS ONE 2017, 12, e0178683. [Google Scholar] [CrossRef] [PubMed]
- Grigolini, P. Emergence of biological complexity: Criticality, renewal and memory. Chaos Solitons Fractals 2015, 81, 575–588. [Google Scholar] [CrossRef]
- Leisman, G.; Koch, P. Continuum model of mnemonic and amnesic phenomena. J. Int. Neuropsychol. Soc. 2000, 6, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, S. Adaptive Resonance Theory: How a brain learns to consciously attend, learn, and recognize a changing world. Neural Netw. 2013, 37, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Mormann, F.; Koch, C. Neural correlates of consciousness. Scholarpedia 2007, 2, 1740. [Google Scholar] [CrossRef]
- Logothetis, N.K. Single units and conscious vision. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998, 353, 1801–1818. [Google Scholar]
- Grossberg, S.; Yazdanbakhsh, A.; Cao, Y.; Swaminathan, G. How does binocular rivalry emerge from cortical mechanisms of 3-D vision? Vis. Res. 2008, 48, 2232–2250. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, S. Human and computer rules and representations are not equivalent. Behav. Brain Sci. 1980, 3, 136–138. [Google Scholar] [CrossRef]
- Grossberg, S. Conscious Mind, Resonant Brain: How Each Brain Makes a Mind; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Bhatt, R.; Carpenter, G.A.; Grossberg, S. Texture segregation by visual cortex: Perceptual grouping, attention, and learning. Vis. Res. 2007, 47, 3173–3211. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Heeger, D.J. The normalization model of attention. Neuron 2009, 61, 168–185. [Google Scholar] [CrossRef]
- Crisan, M. Adaptive Resonance Theory Neural Network for Phoneme Perception and Production. In Proceedings of the 2019 2nd International Conference on Mathematics, Modeling and Simulation Technologies and Applications (MMSTA 2019), Xiamen, China, 27–28 October 2019; Atlantis Press: Dordrecht, The Netherlands, 2019; pp. 213–216. [Google Scholar]
- Dresp-Langley, B. Seven properties of self-organization in the human brain. Big Data Cogn. Comput. 2020, 4, 10. [Google Scholar] [CrossRef]
- Freriks, L.W.; Cluitmans, P.J.M.; van Gils, M.J. The Adaptive Resonance Theory Network:(Clustering-) Behaviour in Relation with Brainstem Auditory Evoked Potential Patterns; Technische Universiteit Eindhoven: Eindhoven, The Netherlands, 1992. [Google Scholar]
- LeCun, Y.; Chopra, S.; Hadsell, R.; Ranzato, M.; Huang, F.-J. A Tutorial on Energy-Based Learning. 2006. Available online: http://yann.lecun.com/exdb/publis/orig/lecun-06.pdf (accessed on 12 April 2024).
- Leisman, G. Enigma Variations: Elegy for Neural Coding in Understanding Cognition. J. Integr. Neurosci. 2024, 23, 104. [Google Scholar]
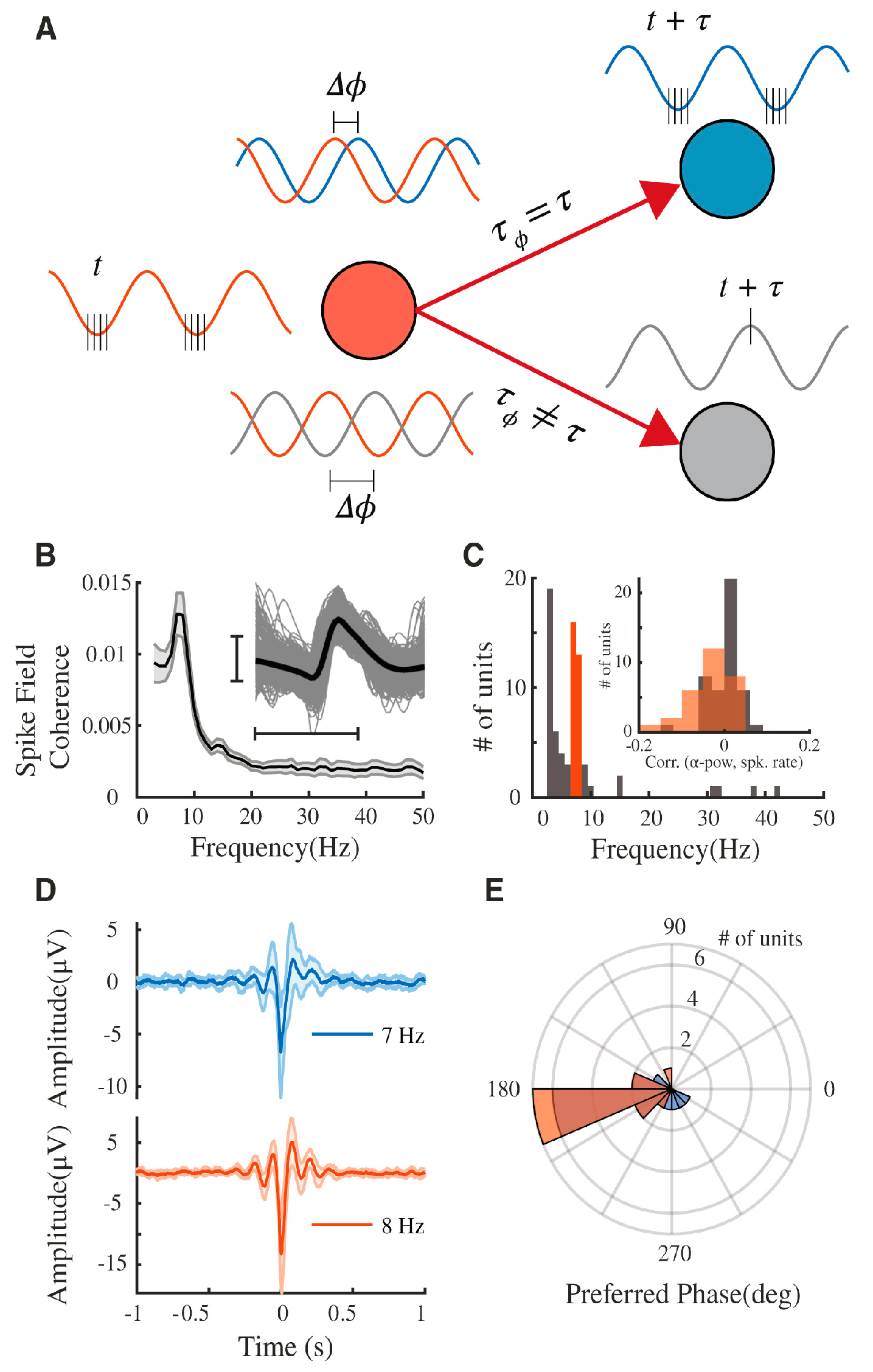
- Chapeton, J.I.; Haque, R.; Wittig, J.H.; Inati, S.K.; Zaghloul, K.A. Large-scale communication in the human brain is rhythmically modulated through alpha coherence. Curr. Biol. 2019, 29, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L.; Sagan, D. Origins of Sex: Three Billion Years of Genetic Recombination; Yale University Press: New Haven, CT, USA, 1990. [Google Scholar]
- Zeki, S.; Bartels, A. Toward a Theory of Visual Consciousness. Conscious. Cogn. 1999, 8, 225–259. [Google Scholar] [CrossRef] [PubMed]
- Zeki, S. The disunity of consciousness. Trends Cogn. Sci. 2003, 7, 214. [Google Scholar] [CrossRef]
- Steinke, G.K.; Galán, R.F. Brain rhythms reveal a hierarchical network organization. PLoS Comput. Biol. 2011, 7, e1002207. [Google Scholar] [CrossRef] [PubMed]
- Hilgetag, C.C.; Goulas, A. ‘Hierarchy’ in the organization of brain networks. Philos. Trans. R. Soc. B 2020, 375, 20190319. [Google Scholar] [CrossRef] [PubMed]
- Jiruska, P.; De Curtis, M.; Jefferys, J.G.; Schevon, C.A.; Schiff, S.J.; Schindler, K. Synchronization and desynchronization in epilepsy: Controversies and hypotheses. J. Physiol. 2013, 591, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, S. Studies of Mind and Brain: Neural Principles of Learning, Perception, Development, Cognition, and Motor Control; Springer: New York, NY, USA, 2012; Volume 70. [Google Scholar]
- Aboitiz, F.; Montiel, J.; Morales, D.; Concha, M. Evolutionary divergence of the reptilian and the mammalian brains: Considerations on connectivity and development. Brain Res. Rev. 2002, 39, 141–153. [Google Scholar] [CrossRef]
- Wilson, H.R.; Cowan, J.D. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 1973, 13, 55–80. [Google Scholar] [CrossRef]
- Destexhe, A.; Sejnowski, T.J. The Wilson–Cowan model, 36 years later. Biol. Cybern. 2009, 101, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.; Raimondo, F.; Hirsch, L.; Huang, Y.; Denis-Valente, M.; Pérez, P.; Engemann, D.; Faugeras, F.; Weiss, N.; Demeret, S.; et al. Combined behavioral and electrophysiological evidence for a direct cortical effect of prefrontal tDCS on disorders of consciousness. Sci. Rep. 2020, 10, 4323. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Wang, Y.; Ouyang, G.; Guo, Y. Long-term HD-tDCS modulates dynamic changes of brain activity on patients with disorders of consciousness: A resting-state EEG study. Comput. Biol. Med. 2024, 170, 108084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Du, J.; Huo, S.; Shan, G.; Li, R. Transcranial direct current stimulation in patients with prolonged disorders of consciousness: Combined behavioral and event-related potential evidence. Front. Neurol. 2017, 8, 620. [Google Scholar] [CrossRef]
- Koch, P.; Leisman, G. Wave theory of large-scale organization of cortical activity. Int. J. Neurosci. 1996, 86, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Golomb, D.; Amitai, Y. Propagating neuronal discharges in neocortical slices: Computational and experimental study. J. Neurophysiol. 1997, 78, 1199–1211. [Google Scholar] [CrossRef]
- Ermentrout, B. The analysis of synaptically generated traveling waves. J. Comput. Neurosci. 1998, 5, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Leisman, G. Effect of local synaptic strengthening on global activity-wave growth in the hippocampus. Int. J. Neurosci. 2001, 108, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.; Leisman, G. Typology of nonlinear activity waves in a layered neural continuum. Int. J. Neurosci. 2006, 116, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Neymotin, S.A.; Lee, H.; Park, E.; Fenton, A.A.; Lytton, W.W. Emergence of physiological oscillation frequencies in a computer model of neocortex. Front. Comput. Neurosci. 2011, 5, 19. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leisman, G.; Koch, P. Resonating with the World: Thinking Critically about Brain Criticality in Consciousness and Cognition. Information 2024, 15, 284. https://doi.org/10.3390/info15050284
Leisman G, Koch P. Resonating with the World: Thinking Critically about Brain Criticality in Consciousness and Cognition. Information. 2024; 15(5):284. https://doi.org/10.3390/info15050284
Chicago/Turabian StyleLeisman, Gerry, and Paul Koch. 2024. "Resonating with the World: Thinking Critically about Brain Criticality in Consciousness and Cognition" Information 15, no. 5: 284. https://doi.org/10.3390/info15050284
APA StyleLeisman, G., & Koch, P. (2024). Resonating with the World: Thinking Critically about Brain Criticality in Consciousness and Cognition. Information, 15(5), 284. https://doi.org/10.3390/info15050284






