Exploring Determinants and Predictive Models of Latent Tuberculosis Infection Outcomes in Rural Areas of the Eastern Cape: A Pilot Comparative Analysis of Logistic Regression and Machine Learning Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Integration of Predictive Modeling and Knowledge Diffusion
2.2.1. Logistic Regression Model
2.2.2. Machine Learning Models
2.3. Data Analysis
2.4. Prediction Tools and Software
2.5. Application of the Machine Learning Algorithms
2.6. Evaluation of the Applied Models
2.7. Performance Measure of the Applied Model
2.8. Knowledge Diffusion Model
3. Results
3.1. Performance of Three Machine Learning Models
3.2. Logistic Regression Results
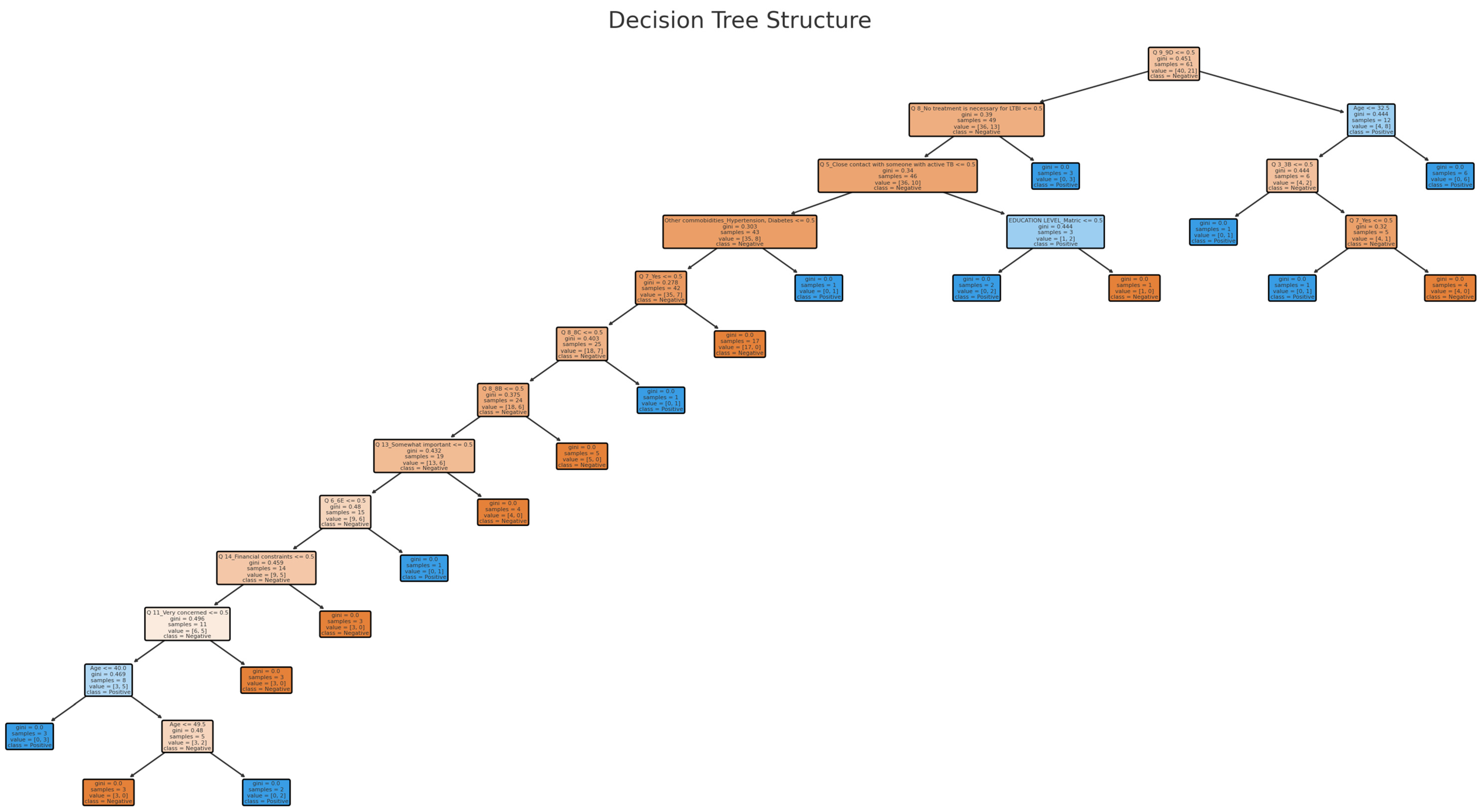
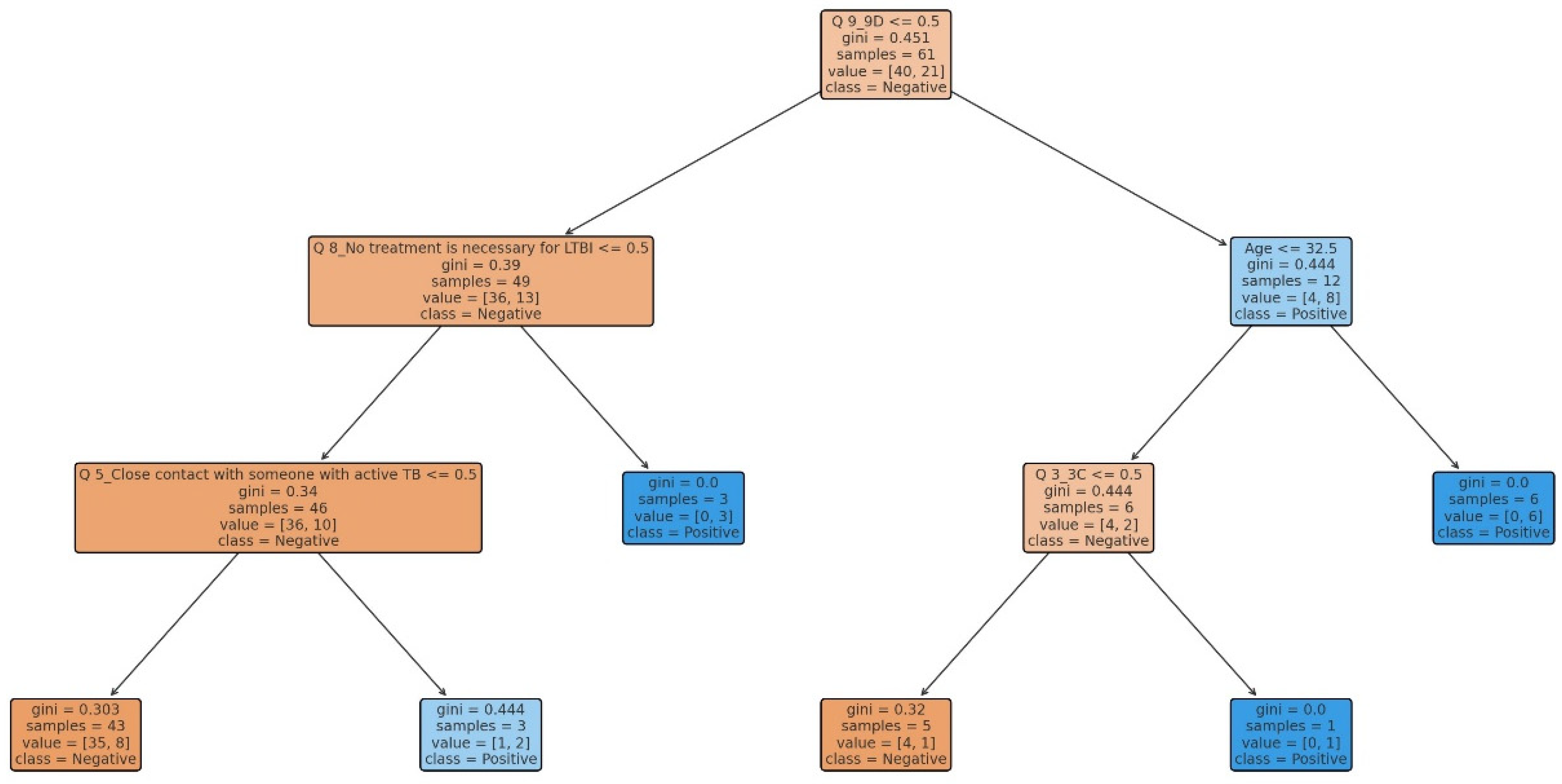
3.3. Decision Tree Model
3.4. Random Forest
3.5. Comparison of Logistic Regression, Random Forest, and Decision Tree Models
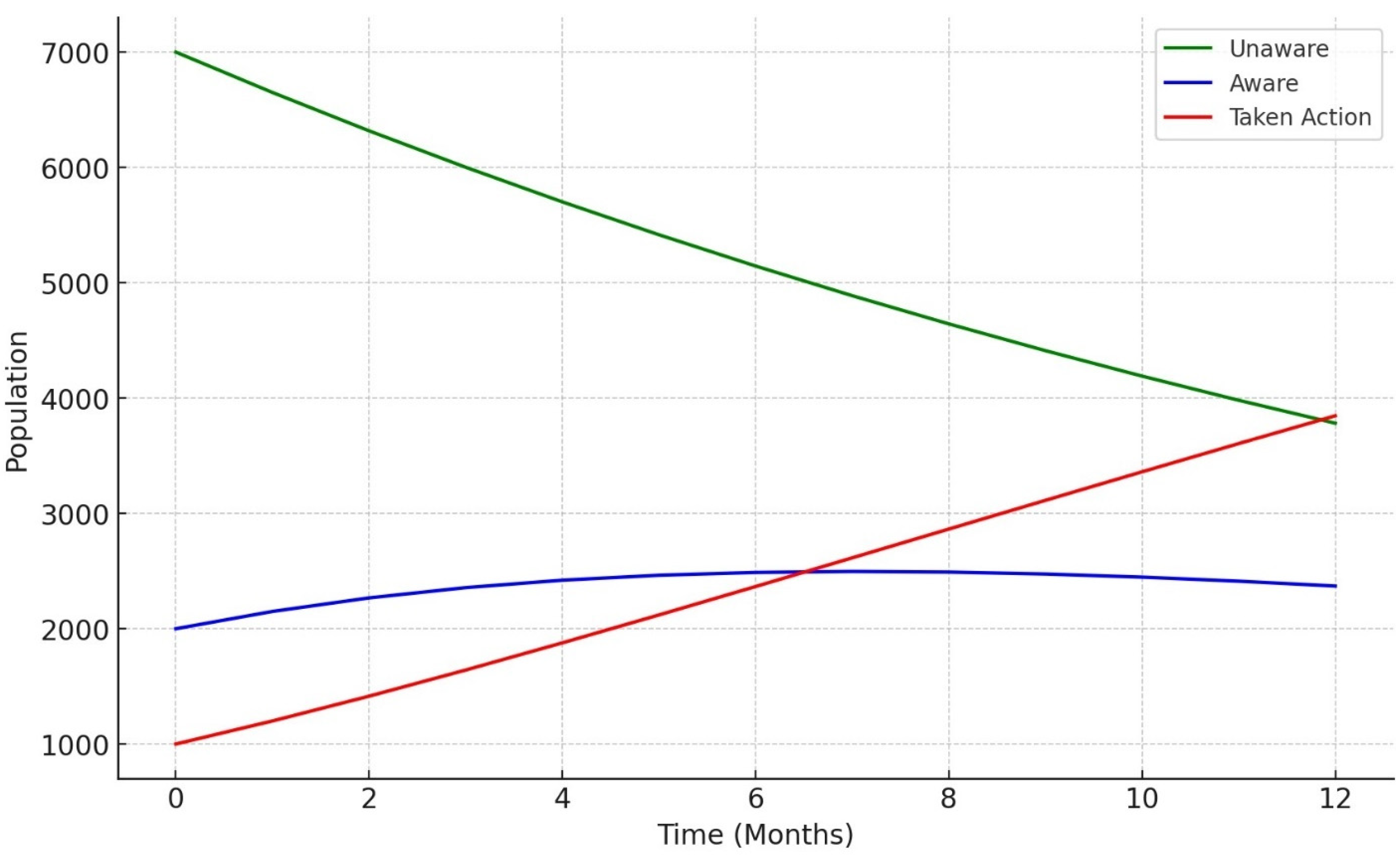
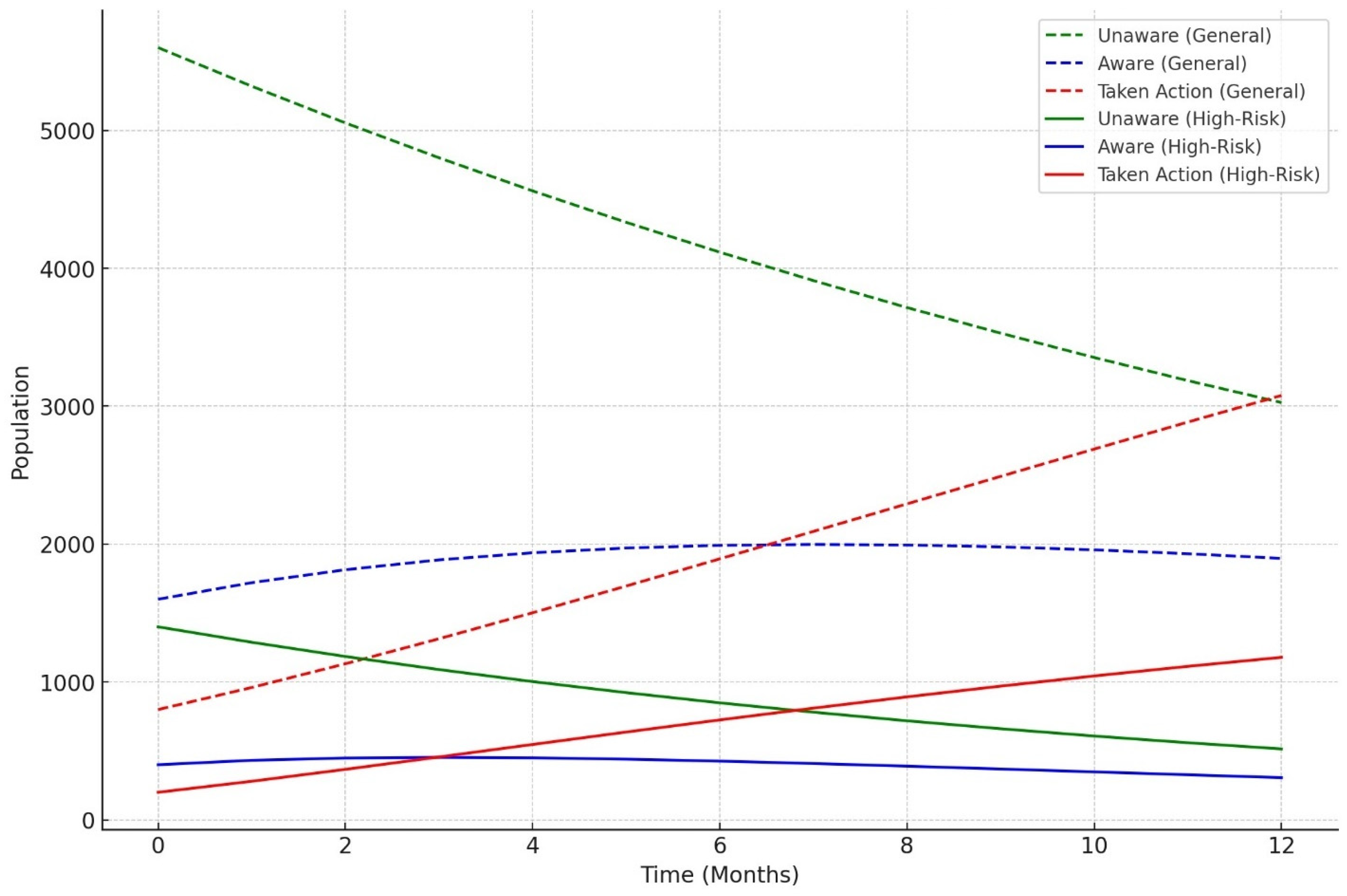
3.6. Knowledge Diffusion Model Outcomes
4. Discussion
4.1. Interpretation of Applied Model Performance
4.2. Feature Importance and Implications for LTBI Risk
4.3. Discussion of the Knowledge Diffusion Model
4.4. Public Health Implications
4.5. Model Suitability and Practical Applications
4.6. Limitations
4.7. Recommendation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, X.; Chen, J.; Jiang, Y.; Li, P.; Li, J.; Lu, L.; Zhao, Y.; Tang, L.; Zhang, T.; Wu, Z.; et al. Prevalence of latent tuberculosis infection and incidence of active tuberculosis in school close contacts in Shanghai, China: Baseline and follow-up results of a prospective cohort study. Front. Cell. Infect. Microbiol. 2022, 12, 1000633. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240062030 (accessed on 14 November 2024).
- Yoopetch, P.; Wu, O.; Jittikoon, J.; Thavorncharoensap, M.; Youngkong, S.; Praditsitthikorn, N.; Mahasirimongkol, S.; Anothaisintawee, T.; Udomsinprasert, W.; Chaikledkaew, U. Economic evaluation of diagnosis and treatment for latent tuberculosis infection among contacts of pulmonary tuberculosis patients in Thailand. Sci. Rep. 2024, 14, 17693. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 15 November 2024).
- Stop TB Partnership. UNHLM on TB: Key Targets and Commitments; STOP, TB Partnership: Geneva, Switzerland, 2020; Available online: http://www.stoptb.org/global/advocacy/unhlm_targets.asp (accessed on 16 November 2024).
- Velleca, M.; Malekinejad, M.; Miller, C.; Abascal Miguel, L.; Reeves, H.; Hopewell, P.; Fair, E. The yield of tuberculosis contact investigation in low- and middle-income settings: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 1011. [Google Scholar] [CrossRef]
- Luo, Y.; Xue, Y.; Liu, W.; Song, H.; Huang, Y.; Tang, G.; Wang, F.; Wang, Q.; Cai, Y.; Sun, Z. Development of diagnostic algorithm using machine learning for distinguishing between active tuberculosis and latent tuberculosis infection. BMC Infect. Dis. 2022, 22, 965. [Google Scholar] [CrossRef]
- Nyachama, K. Effectiveness of recommender systems in knowledge discovery. Eur. J. Inf. Knowl. Manag. 2024, 3, 50–62. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, L.; Sun, T.; Liu, R.; Huang, Q.; Deng, S. The performance of vcs (volume, conductivity, light scatter) parameters in distinguishing latent tuberculosis and active tuberculosis by using a machine learning algorithm. BMC Infect. Dis. 2023, 23, 881. [Google Scholar] [CrossRef]
- Murri, R.; De Angelis, G.; Antenucci, L.; Fiori, B.; Rinaldi, R.; Fantoni, M.; Masciocchi, C. A machine learning predictive model of bloodstream infection in hospitalized patients. Diagnostics 2024, 14, 445. [Google Scholar] [CrossRef]
- Stoltzfus, J. Logistic regression: A brief primer. Acad. Emerg. Med. 2011, 18, 1099–1104. [Google Scholar] [CrossRef]
- Mercurio, G.; Gottardelli, B.; Lenkowicz, J.; Patarnello, S.; Bellavia, S.; Scala, I.; Frisullo, G. A novel risk score predicting 30-day hospital re-admission of patients with acute stroke by machine learning model. Eur. J. Neurol. 2023, 31, e16153. [Google Scholar] [CrossRef]
- Gong, W.; Wu, X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: A key to a successful tuberculosis control strategy. Front. Microb. 2021, 12, 745592. [Google Scholar] [CrossRef]
- Gichuhi, H.W.; Magumba, M.; Kumar, M.; Mayega, R.W. A machine learning approach to explore individual risk factors for tuberculosis treatment non-adherence in Mukono district. PLoS Glob. Public Health 2023, 3, e0001466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermes, L.; Kersten, J.; Nienhaus, A.; Schablon, A. Risk analysis of latent tuberculosis infection among health workers compared to employees in other sectors. Int. J. Environ. Res. Public Health 2020, 17, 4643. [Google Scholar] [CrossRef]
- Adams, S.; Ehrlich, R.; Baatjies, R.; Zyl-Smit, R.; Said-Hartley, Q.; Dawson, R.; Dheda, K. Incidence of occupational latent tuberculosis infection in South African healthcare workers. Eur. Respir. J. 2015, 45, 1364–1373. [Google Scholar] [CrossRef]
- Meregildo-Rodriguez, E. Latent Tuberculosis Infection (LTBI) in healthcare workers: A cross-sectional study at a northern Peruvian Hospital. Front. Med. 2023, 10, 1295299. [Google Scholar] [CrossRef]
- Apriani, L.; McAllister, S.; Sharples, K.; Alisjahbana, B.; Ruslami, R.; Hill, P.; Menzies, D. Latent tuberculosis infection in healthcare workers in low- and middle-income countries: An updated systematic review. Eur. Respir. J. 2019, 53, 1801789. [Google Scholar] [CrossRef]
- Kinikar, A.; Chandanwale, A.; Kadam, D.; Joshi, S.; Basavaraj, A.; Pardeshi, G.; Mave, V. High risk for latent tuberculosis infection among medical residents and nursing students in India. PLoS ONE 2019, 14, e0219131. [Google Scholar] [CrossRef]
- Nasreen, S.; Shokoohi, M.; Malvankar-Mehta, M. Prevalence of latent tuberculosis among health care workers in high burden countries: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0164034. [Google Scholar] [CrossRef]
- Stewart, R.; Tsang, C.; Pratt, R.; Price, S.; Langer, A. Tuberculosis—United States, 2017. MMWR Morb. Mortal. Wkl. Rep. 2018, 67, 317–323. [Google Scholar] [CrossRef]
- Wong, Y.J.; Ng, K.Y.; Lee, S.W.H. How can we improve latent tuberculosis infection management using behavior change wheel: A systematic review. J. Public Health 2023, 45, e447–e466. [Google Scholar] [CrossRef]
- Ayakaka, I.; Ackerman, S.; Ggita, J.M.; Kajubi, P.; Dowdy, D.; Haberer, J.E.; Fair, E.; Hopewell, P.; Handley, M.A.; Cattamanchi, A.; et al. Identifying barriers to and facilitators of tuberculosis contact investigation in Kampala, Uganda: A behavioral approach. Implement. Sci. 2017, 12, 33. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2022; Available online: https://cdn.who.int/media/docs/default-source/hq-tuberculosis/global-tuberculosis-report2022/global-tb-report-2022-factsheet.pdf.24 (accessed on 17 November 2024).
- Pradipta, I.S.; Idrus, L.R.; Probandari, A.; Lestari, B.W.; Diantini, A.; Alffenaar, J.W.C.; Hak, E. Barriers and strategies to successful tuberculosis treatment in a high-burden tuberculosis setting: A qualitative study from the patient’s perspective. BMC Public Health 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Matakanye, H.; Tshitangano, T.G.; Mabunda, J.T.; Maluleke, T.X. Knowledge, Beliefs, and Perceptions of TB and Its Treatment amongst TB Patients in the Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 10404. [Google Scholar] [CrossRef]
- Kigozi, G.; Heunis, C.; Chikobvu, P.; Botha, S.; van Rensburg, D. Factors influencing treatment default among tuberculosis patients in a high burden province of South Africa. Int. J. Infect. Dis. 2017, 54, 95–102. [Google Scholar] [CrossRef]
- Shamputa, I.C.; Law, M.A.; Kelly, C.; Nguyen, D.T.K.; Burdo, T.; Umar, J.; Barker, K.; Webster, D. Tuberculosis related barriers and facilitators among immigrants in Atlantic Canada: A qualitative study. PLoS Glob. Public Health 2023, 3, e0001997. [Google Scholar] [CrossRef]
- Zawedde-Muyanja, S.; Manabe, Y.C.; Cattamanchi, A.; Castelnuovo, B.; Katamba, A. Patient and health system level barriers to and facilitators for tuberculosis treatment initiation in Uganda: A qualitative study. BMC Health Serv. Res. 2022, 22, 831. [Google Scholar] [CrossRef]
- Meaza, A.; Tola, H.H.; Eshetu, K.; Mindaye, T.; Medhin, G.; Gumi, B. Tuberculosis among refugees and migrant populations: Systematic review. PLoS ONE 2022, 17, e0268696. [Google Scholar] [CrossRef]
- Yousif, K.; Ei Maki, M.; Babikir, R.K.; Abuaisha, H. The effect of an educational intervention on awareness of various aspects of pulmonary tuberculosis in patients with the disease. East. Mediterr. Health J. 2021, 27, 287–292. [Google Scholar] [CrossRef]
- Wu, T.; He, H.; Wei, S.; Pan, J.; Yang, J.; Huang, S.; Gan, S.; Ye, C.; Huo, H.; Tang, Z.; et al. How to optimize tuberculosis health education in college under the new situation? Based on a cross-sectional study among freshmen of a medical college in Guangxi, China. Front. Public Health 2022, 10, 845822. [Google Scholar] [CrossRef]
- Subbaraman, R.; Nathavitharana, R.R.; Mayer, K.H.; Satyanarayana, S.; Chadha, V.K.; Arinaminpathy, N.; Pai, M. Constructing care cascades for active tuberculosis: A strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019, 16, e1002754. [Google Scholar] [CrossRef]
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.; Brey, Z.O.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J. Infect. Dis. 2017, 216, S702–S713. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Osberg, M.; Brown, J.; Durham, G.; Chin, D.P. Finding the Missing Patients with Tuberculosis: Lessons Learned from Patient-Pathway Analyses in 5 Countries. J. Infect. Dis. 2017, 216, S686–S695. [Google Scholar] [CrossRef]
- Mwangwa, F.; Chamie, G.; Kwarisiima, D.; Ayieko, J.; Owaraganise, A.; Ruel, T.D.; Plenty, A.; Tram, K.H.; Clark, T.D.; Cohen, C.R.; et al. Gaps in the Child Tuberculosis Care Cascade in 32 Rural Communities in Uganda and Kenya. J. Clin. Tuberc. Other Mycobact. Dis. 2017, 9, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Harries, A.D.; Lin, Y.; Kumar, A.M.V.; Satyanarayana, S.; Takarinda, K.C.; Dlodlo, R.A.; Zachariah, R.; Olliaro, P. What can National TB Control Programmes in low- and middle-income countries do to end tuberculosis by 2030? F1000Research 2018, 7, F1000–Faculty. [Google Scholar] [CrossRef]
- Spruijt, I.; Haile, D.T.; van den Hof, S.; Fiekert, K.; Jansen, N.; Jerene, D.; Klinkenberg, E.; Leimane, I.; Suurmond, J. Knowledge, attitudes, beliefs, and stigma related to latent tuberculosis infection: A qualitative study among Eritreans in the Netherlands. BMC Public Health 2020, 20, 1602. [Google Scholar] [CrossRef]
- Campbell, J.I.; Menzies, D. Testing and Scaling Interventions to Improve the Tuberculosis Infection Care Cascade. J. Pediatr. Infect. Dis. Soc. 2022, 11, S94–S100. [Google Scholar] [CrossRef]
- Khan, A.A.; Awan, M.S. Barriers to tuberculosis screening: A qualitative study in a low-income setting. Int. J. Tuberc. Lung Dis. 2019, 23, 579–586. [Google Scholar] [CrossRef]
- Baker, M.G.; Firth, M. The impact of educational interventions on tuberculosis awareness and testing: A knowledge diffusion model simulation. BMC Public Health 2018, 18, 1234. [Google Scholar]
- Pérez, A.; Martínez, M. Community health worker-led interventions to improve tuberculosis knowledge and testing rates in underserved populations. J. Community Health 2021, 46, 345–353. [Google Scholar]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lönnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef]
- Fenta, M.D.; Ogundijo, O.A.; Warsame, A.A.A.; Belay, A.G. Facilitators and barriers to tuberculosis active case findings in low- and middle-income countries: A systematic review of qualitative research. BMC Infect. Dis. 2023, 23, 515. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Zhou, L.; Wang, F.; Chen, B.; Qu, Y. The necessity for enhancing awareness of tuberculosis starting from the early college semesters: Empirical evidence from a cross-sectional research. Front Public Health 2023, 11, 1272494. [Google Scholar] [CrossRef]



| Question Code | Question | Choice of Responses |
|---|---|---|
| Q1 | Have you ever heard of LTBI before? | 1A: Yes 1B: No |
| Q2 | Have you ever received health education on LTBI and TB? | 2A: Yes 2B: No |
| Q3 | What do you understand by the term “Latent tuberculosis infection”? | 3A: A form of tuberculosis that is highly contagious and easily spread through the air 3B: Tuberculosis infection that remains dormant in the body without causing symptoms or spreading to others 3C: An advanced stage of tuberculosis where the an infection has spread to multiple organs |
| Q4 | How is LTBI different from active TB? | 3D: Tuberculosis infection that is resistant to standard treatments and requires specialized medications 3E: A condition where the tuberculosis bacteria have been completely eradicated from the body 4A: LTBI is a condition where the tuberculosis bacteria are actively multiplying in the body, causing symptoms such as cough, fever, and weight loss, while active TB is a dormant infection that does not cause symptoms 4B: LTBI is a contagious form of tuberculosis that can Be easily transmitted to others through respiratory droplets, while active TB is not contagious 4C: LTBI is characterized by the presence of tuberculosis bacteria in the body without causing symptoms or making the person sick, whereas active TB manifests with symptoms and can make the person sick 4D: LTBI is a more severe form of tuberculosis infection that requires intensive treatment with multiple medications, whereas active TB can be managed with a single antibiotic 4E: LTBI is a temporary condition that resolves on its own without treatment, while active TB requires long-term treatment to prevent complications and transmission to others |
| Q5 | What are the risk factors for developing LTBI? | 5A: Age 5B: Close contact with someone with active TB 5C: Immunocompromised condition 5D: Living or working in crowded environments 5E: All of the above |
| Q6 | What are the possible consequences of having untreated LTBI? | 6A: Development of active tuberculosis (TB) disease 6B: Increased risk of transmitting tuberculosis to others 6C: Progression of TB infection to more severe forms affecting multiple organs 6D: Complications such as meningitis, bone, or joint infection, or respiratory failure 6E: All of the above |
| Q7 | Can LTBI progress to active TB? | 7A: Yes 7B: No 7C: Not sure |
| Q8 | What are the recommended treatments for LTBI? | 8A: High-dose antibiotics for a short duration 8B: Combination therapy with multiple antibiotics 8C: Isoniazid (INH) monotherapy for 6 to 9 months 8D: Surgical removal of infected tissues 8E: No treatment is necessary for LTBI |
| Q9 | Are there any preventive measures individuals with LTBI should take to avoid developing active TB? | 9A: Regular exercise and a healthy diet 9B: Avoiding close contact with individuals diagnosed with active TB 9C: Taking vitamin supplements 9D: Completing a full course of treatments for LTBI as Prescribed by a healthcare provider 9E: Using herbal remedies and alternative therapies |
| Q10 | Do you think LTBI is a significant public health concern? | 10A: Strongly agree 10B: Agree 10C: Neutral 10D: Disagree 10E: Strongly |
| Q11 | How concerned are you about the possibility of progressing from LTBI to active TB? | 11A: Very concerned 11B: Somewhat concerned 11C: Neutral 11D: Not very concerned 11E: Not concerned at all |
| Q12 | Do you believe that LTBI treatment is necessary, even if you do not have symptoms? | 12A: Yes 12B: No 12C: Not sure |
| Q13 | How do you perceive the importance of LTBI screening programs? | 13A: Vey important 13B: Somewhat important 13C: Neutral 13D: Not very important 13E: Not important at all |
| Q14 | What barriers do you think may prevent individuals from seeking LTBI testing or treatment? | 14A: Lack of awareness 14B: Fear of side effects from medication 14C: Stigma associated with TB 14D: Financial constraints 14E: Other (please specify) |
| Q15 | Have you ever been screened for LTBI? | 15A: Yes, and I tested positive 15B: Yes, and I tested negative 15C: No |
| Q16 | If you tested for LTBI, did you receive treatment? | 16A: Yes 16B: No |
| Q17 | If you received treatment for LTBI, did you complete the entire course of medication? | 17A: Yes 17B: No |
| Q18 | Have you ever been in close contact with someone diagnosed with active TB? | 18A: Yes 18B: No |
| Q19 | If yes, did you seek medical evaluation or testing for LTBI? | 19A: Yes 19B: No |
| Model | Strengths | Weaknesses |
|---|---|---|
| Logistic Regression | - High interpretability, easy to explain. | - Low recall, misses many LTBI-positive cases. |
| - Good precision. | - Limited in capturing complex, non-linear interactions. | |
| Decision Tree | - Simple, interpretable structure. | Prone to overfitting, leading to lower generalizability. |
| - Captures non-linear relationships. | - Lower precision, higher false positives. | |
| - Better recall than logistic regression. | ||
| Random Forest | - Better overall accuracy and F1-score. | - Less interpretable due to ensemble structure. |
| - Handles complex interactions well. | - Struggled with recall for LTBI-positive cases. | |
| - Provides insights into feature importance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faye, L.M.; Magwaza, C.; Dlatu, N.; Apalata, T. Exploring Determinants and Predictive Models of Latent Tuberculosis Infection Outcomes in Rural Areas of the Eastern Cape: A Pilot Comparative Analysis of Logistic Regression and Machine Learning Approaches. Information 2025, 16, 239. https://doi.org/10.3390/info16030239
Faye LM, Magwaza C, Dlatu N, Apalata T. Exploring Determinants and Predictive Models of Latent Tuberculosis Infection Outcomes in Rural Areas of the Eastern Cape: A Pilot Comparative Analysis of Logistic Regression and Machine Learning Approaches. Information. 2025; 16(3):239. https://doi.org/10.3390/info16030239
Chicago/Turabian StyleFaye, Lindiwe Modest, Cebo Magwaza, Ntandazo Dlatu, and Teke Apalata. 2025. "Exploring Determinants and Predictive Models of Latent Tuberculosis Infection Outcomes in Rural Areas of the Eastern Cape: A Pilot Comparative Analysis of Logistic Regression and Machine Learning Approaches" Information 16, no. 3: 239. https://doi.org/10.3390/info16030239
APA StyleFaye, L. M., Magwaza, C., Dlatu, N., & Apalata, T. (2025). Exploring Determinants and Predictive Models of Latent Tuberculosis Infection Outcomes in Rural Areas of the Eastern Cape: A Pilot Comparative Analysis of Logistic Regression and Machine Learning Approaches. Information, 16(3), 239. https://doi.org/10.3390/info16030239







