1. Introduction
Nowadays, the more vulnerability of the aquatic environment to several sources of pollutants is well known. It can be considered to be a veritable tank for most of the environmental contaminants which can accumulate in several compartments of the aquatic ecosystem, such as living beings [
1,
2,
3]. Among these pollutants, there are polycyclic aromatic hydrocarbons (PAHs) which have several sources in the environment, such as anthropogenic sources, concerning the high temperature pyrolysis of fats and oils, or the combustion of organic compounds, e.g., tobacco, fossil fuel, grilled meat, waste, coal burning etc., often [
4,
5,
6] from industries, automobile exhaust fumes, houses heating, combustion of biomass etc. [
6,
7,
8]. Due to their relative chemical stability and non-biodegradability, PAHs are very persistent and ubiquitous in the environment and have a high tendency to accumulate in food chains, leading to human exposure. These characteristics classify them as very significant pollutants for environmental concern [
6,
9,
10].
During recent decades, PAHs which are mutagenic and carcinogenic have been considered hazardous environmental pollutants. Consequently, they have received much attention due to their potential adverse on human health and ecosystem impacts [
6,
11,
12]. Human exposure to these pollutants may cause toxic effects, such as mutagenesis, birth defects, and cancers etc. [
6,
13]. Malformations of embryo and larvae, growth reduction, DNA damage, endocrine alteration, and other toxic effects caused by PAHs have also been observed in marine organisms [
6,
14,
15,
16]. The major pathway of fish and other aquatic organisms’ exposure to PAHs would be the ingestion of contaminated food and diffusion phenomenon of the molecules present in the surrounding water through their gills and skin [
10,
17]. The fatty tissues of fish are the place of predilection for PAHs accumulation due to their lipophilic nature and high chemical stability [
6,
18]. Therefore, fishes are good indicators of pollution in aquatic ecosystems and have been widely used for environmental monitoring [
19,
20,
21].
A large part of the world’s population depends on seafood, especially fish, to satisfy their nutritional requirements. Indeed, they are widely consumed as an important source of protein, energy, vitamins, polyunsaturated fatty acids, and minerals, which are known for their health benefits [
1,
22,
23,
24]. However, polluted aquatic organisms may pose significant risk to human health [
1,
25,
26,
27]. Food consumption has been identified as an important pathway for human exposure to many contaminants including PAHs. Thus, PAHs contamination of widely consumed fish species may have serious public health issues.
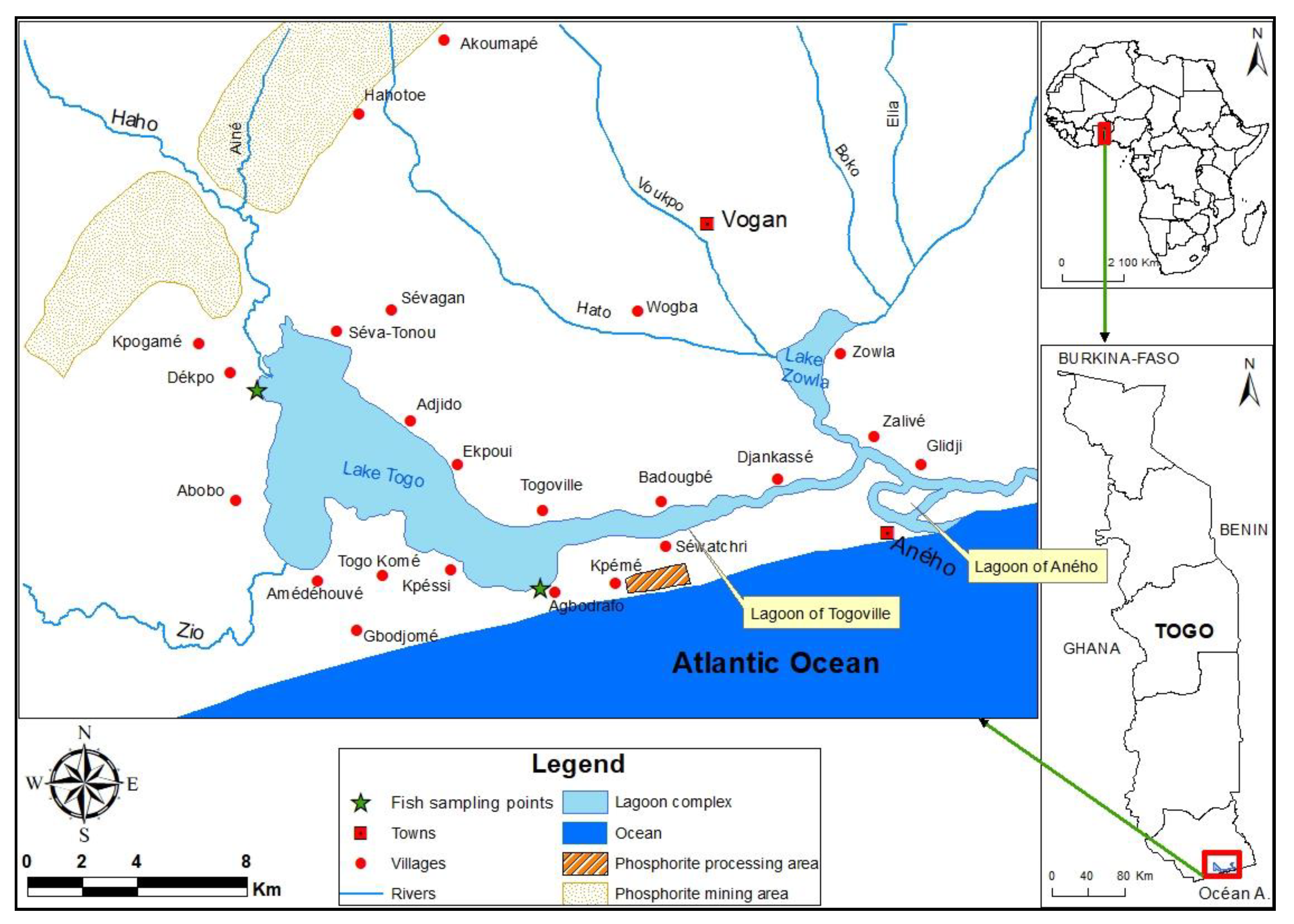
In Togo, the Lake Togo-Lagoon of Aného complex is a coastal located hydrosystem and in the phosphorite mining and treatment area. These activities discharge several kinds of waste into the coastal zone without any prior treatment. Thus, uncontrolled rejection of waste, such as the mismanagement of used oil from machinery maintenance and fuel used for furnace heating, can be noted. Furthermore, the hydrosystem receives runoff and river inputs after leaching from urban, agricultural, and mining soils as well as atmospheric deposition of particles from automobile exhaust gases. The species
Chrysichthys nigrodigitatus is potentially exposed to the bioaccumulation of several kinds of pollutants due to its high trophic level, diet composition, and demersal habitat especially on the muddy bottom [
28,
29]. However, it has an important ecological role and presents valuable economic, nutritional and aquaculture interest in West African countries [
30,
31,
32,
33]. Like in other Sub-Saharan African countries, this species is very appreciated in Togo, where it contributes to the socio-economic well-being and food security for local populations who are mainly fishermen. However, there is no study regarding fish contamination by PAHs in Togo. Accordingly, there is no information on the health risks associated with the consumption of fish contaminated by PAHs in the country. The present study aims to determine PAH concentrations in commercially consumed fish species (
Chrysichthys nigrodigitatus) from the Lake Togo-Lagoon of the Aného hydrosystem and to assess the associated human health risks for consumers.
2. Materials and Methods
2.1. Study Area
The Hydrosystem Lake Togo-Lagoon of Aného located between latitudes North 6°17′37″ and 6°14′38″ and longitudes East 1°23′33″ and 1°37′38″. It is composed of Lake Togo with an area of 46 km
2, the lagoon of Togoville whose length and width are respectively 13 km and 150–900 m, and the Aného lagoon consisting of a narrow channel. The hydrosystem mainly receives inputs from Zio and Haho River [
34]. The hydrosystem communicates with the sea at Aného (
Figure 1). Phosphorite mining take place in its watershed with the discharge of several kinds of untreated mining waste. This watershed enjoys a subequatorial climate with two main seasons (rainy and dry) alternated by two small seasons (rainy and dry). The main economic activities of the populations around the hydrosystem are dominated by fishery. Other activities, e.g., agriculture and livestock cultivation, can be noted in the study area.
2.2. Sample Collection, Extraction and Cleanup
The fish samples were collected during the dry season in the hydrosystem at Dékpo and Agbodrafo in collaboration with the fishermen using passive collection method according to [
35]. Indeed, fishes were cut using gillnets, single lines, longlines, and traps. They were individually wrapped with aluminum foil and put in polyethylene bags because of the photo-degradability of PAHs. These samples were then transported to the laboratory, in a cooler containing ice cubes, where they were washed with tap water and then rinsed with distilled water. After that, they were measured (total length), weighted (total weight) and dissected. The muscles, gills and livers that were taken and stored in the freezer at −20 °C [
1,
36]. A total of 30 composites of each fish organ (muscle, liver, gills) were made from groups of 4–6 fishes according to the method of Pascal et al. [
37] which states that the smallest fish size in each group must not be less than 75% of the biggest one. The number of fish in each group varied according to the amount of organ necessary for analyses. Each composite sample of fish organs was ground and homogenized using a grinder (Retsch Grindomix GM 200), labelled, placed in amber glass vials, and stored at −20 °C.
The quick, effective, cheap, easy, rugged, safe (QuEChERS) methodology was used to extract PAHs from fish samples as it has been previously used by other authors for the analysis of PAHs [
8,
38]. Indeed, 10 g of ground and homogenized fresh samples were mixed with distilled water (15 mL) in QuEChERS tubes. After that, 15 mL of acetonitrile (CH
3CN) and 0.1 mL of surrogate standard containing p-terphenyl-d14 (100 μg/mL), were added to each extraction tube and shaken for one-minute. Following that, 8 g of magnesium sulfate (MgSO
4) and 2 g of sodium chloride (NaCl) were added to the QuEChERS tubes containing the previous mixtures. They were shaken vigorously for 1 min before centrifuging the extracts at 3500 rpm for 10 min in order to remove the upper layers.
One (1) mL of the CH3CN layers were transferred into the clean-up tubes containing 50 mg of primary-secondary amine and 150 mg of MgSO4. These tubes were shaken for 5 min and were centrifuged (8000 rpm) for 10 min. Portions of each tube upper layer (0.6 mL) were placed in vials for further analysis and 0.2 mL of deuterated internal standard mixture containing acenaphthene d10, chrysene, naphthalene, pyrene, and phenanthrene at 80 µg/mL were added. The reagents used for PAH extraction were from Sigma Aldrich and Merck. Twelve (12) PAHs from 16 priority PAHs (Naphthalene (Nap), Acenaphthylene (Acy), Acenaphthene (Ace), Anthracene (Ant), Phenanthrene (Phe), Fluoranthene (Flu), Pyrene (Pyr), Benzo(a)anthracene (BaA), Chrysene (Chr), Benzo(a)pyrene (BaP), Benzo(b)fluoranthene (BbF) and Benzo(k)fluoranthene (BkF)) were analyzed using a gas chromatograph-mass spectrometer (GC-MS); all of them from Agilent Technologies.
The gas chromatograph system was used in the selected ion monitoring mode based on the use of one ion. The identification of compounds was based on their qualifier ions’ times of retention. Chromatographic separations were conducted using a HP-5MS (5% Phenyl Methyl Siloxane) fused silica capillary column (30 m long × 0.25 mm internal diameter × 0.25μm film thickness). The operation temperature program of the GC oven started at 70 °C, which was held for 3 min and was increased to 240 °C with 20 °C/min, and then to 310 °C with 5 °C/min. The injector temperature was 300 °C while the transfer line temperature was 280 °C. Hence, 2.5 μL of acetonitrile extracts were injected in splitless mode. The carrier gas was helium (1.0 mL/min of flow rate). Ionization voltage of 70 eV, acquisition mass range of 40–560 and scan time of 0.32 s were the mass spectrometer conditions. Quantitative analysis was based on the corresponding quantifier ions of each PAH molecule and previously recorded retention times. The PAH calibration standards were prepared using a certified reference standard of 2000 µg/mL, containing the 12 analyzed PAHs.
2.3. Quality and Accuracy Control
The method’s quality and accuracy were checked by analyzing duplicates of selected sample and procedural blank reagent. These solutions were analyzed for each batch of 15 samples. The coefficients of variation of the average values of the duplicate sample were used to assess the accuracy and repeatability. The calculated coefficients of variation for analyzed pollutants were <5% and ranged between 0.11% for Pyr to 4.49% for Ant. The blank was analyzed to detect possible contamination during extraction. The PAHs molecules were not detectable in blank solutions. In addition, certified reference materials (IAEA-435; IAEA-406) were analyzed to verify the quality of the extraction procedure and reading precision. The recoveries of the 12 analyzed PAH concentrations ranged between 86.96% for Chrysene (Chr) and 107.14% for Benzo(k)fluoranthène (BkF).
2.4. Determination of Probable Sources of PAHs in Fish Organs
The determination of the potential origins of PAHs was carried out through several relationships between the concentrations of PAH molecules: Phe/Ant, Flu/Pyr, Ant/(Ant+Phe), Flu/(Flu+Pyr), BaA/(BaA+Chr), low molecular weight PAHs (LMW PAHs)/high molecular weight PAHs (HMW PAHs). The results are interpreted as follows: Phe/Ant and Flu/Pyr (>1: pyrogenic and <1: petrogenic), Ant/(Ant+Phe) (>0.1 pyrogenic and <0.1: petrogenic), Flu/(Flu+Pyr) (<0.4: petrogenic, 0.4–0.5: petrogenic + pyrogenic and >0.5: pyrogenic), BaA/(BaA+Chr) (<0.2 petrogenic, 0.2–0.35 petrogenic + pyrogenic and >0.35: pyrogenic) and LMW PAHs/HMW PAHs (<1: pyrogenic and >1: petrogenic) [
39,
40,
41,
42]. The LMW PAHs detected are composed of Nap, Ant, and Phe and the HMW PAHs are composed of Flu, Pyr, BaA, and Chr.
2.5. Statistical Analysis
Analysis of variance followed by the Newman–Keuls test were applied to the data to determine the variability in mean PAH levels between fish organs. Mean values are significantly different when p < 0.05. Spearman’s correlation test was performed to determine the relationship between total lengths and total weights of fish. In addition, Student’s t test was performed to compare the average contents of PAHs having low molecular weight (LMW PAHs) and high molecular weight (HMW PAHs). Principal component analysis (PCA) was performed to determine the overall interrelationships between individual PAHs and their distribution in organs. Statistical analyses were performed using STATISTICA 6.1 software.
2.6. Human Health Risk Assessment
The dietary PAHs exposures for consumers were calculated using mean concentration of each PAHs in fish’s muscles and in all organs analyzed (muscle, gill, and liver). In addition, two types of peoples were considered (adults and children). The estimated daily intake (EDI) was determined according to the following equation [
43,
44]:
where EDI = estimated daily intake (mg/kg/day); C = concentration of PAHs in fish’s tissues (mg/kg); Q = daily quantity of fish ingested (kg/day). These quantities are 166.75 g/day of wet weight (g/d ww) for adults and 110.25 g/d ww for children [
45]; F = exposure frequency (day/year); BW = body weight (kg). The average body weights of the local population were 67.64 kg for adults of 22–60 years old and 29.40 kg for children of 3–16 years old [
46,
47]. The fish consumption frequency is considered as equal to one (365 days/year).
The risk of non-carcinogenic effects was expressed by the calculation of the hazard quotient (HQ). The HQ were calculated using the following equations [
48,
49]:
where RfD
o is the oral reference dose (mg/kg/day). ED = exposure duration (year); T
m = total life duration (year). For non-carcinogenic effects, the ED is equal to T
m. If HQ < 1, toxic effect is less probable; if HQ > 1, toxic effect cannot be excluded. In order to determine the additive and/or iterative effects of PAHs, total hazard quotient (THQ) was calculated using the following equation [
50,
51]:
The cancer risk (CR) is the probability of an individual to develop cancer, due to the exposure over a lifetime [
52]. The cancer risk was calculated by the following formula [
35,
50]:
where CSF
o is the oral cancer slope factor (mg/kg/day)
−1.
For carcinogen effects, the ED is defined as equal to 30 years and the Tm is 70 years according to USEPA (USEPA, 1991). The CR interpretation is as follow: CR < 10
−6 is negligible; 10
−6 < CR < 10
−4 is acceptable et CR > 10
−4 is unacceptable [
50,
53,
54]. Oral reference doses (RfDo) and oral cancer slope factor (CSFo) are depicted in
Table 1.
4. Discussion
The average length (25.87 cm) obtained indicates that the
C. nigrodigitatus individuals studied are mostly mature [
61,
62,
63]. These sizes are broadly similar to those observed in
C. nogrodigitatus individuals in the West African sub-region [
64,
65,
66,
67]. However, these sizes are larger than those reported by Atobatele and Ugwumba [
68] in Aiba Reservoir in Nigeria (9.8–25.6 cm) while longer individuals have been observed by Andem et al. [
69] under the Itu bridge in Nigeria (9–109 cm). In addition, the strong and positive correlation obtained between the lengths and the weights of the fish is in agreement with the results obtained by Lawal et al. [
67] in the same species in the Epe lagoon in Nigeria (r = 0.868) and in
Chrysichthys furcatus from the Cross River in Nigeria (r = 0.97) by Irom et al. [
70].
The different routes of exposure of fish to contaminants in aquatic environments are mainly the direct bioconcentration of molecules dissolved in water through their gills and skin, as well as the ingestion of contaminated food and sediment particles. The rate of bioaccumulation may depend on the food preferences, habitats, and the trophic level of fish [
10,
71,
72]. The comparison of the results with other work is presented in
Table 7. Indeed, the concentrations of tPAHs observed in this study are lower than those obtained in fish from the Ghanaian coast [
73] and from Ogun and Eleyele Rivers in Nigeria [
19]. In addition, the PAH concentrations recorded in the muscles of fish from the coastal waters of Benin are generally higher than those of the present study [
74]. However, the results from this study are higher than those obtained in
Tilapia guineensis and
Liza falcipinnis in Nigeria [
72] and in catfish from Hong Kong markets [
71] (
Table 7).
The maximal levels of PAHs set and reviewed by European Commission (Commission Regulation 1255/2020) in fish concern individual BaP molecule (5 µg/kg) as the main marker for PAHs in food and the sum of four PAHs molecules (PAH4) composed of BaP, BaA, BbF, and Chr (30 µg/kg) [
77]. In the present study, BaP molecules were not detected in all of the samples analyzed. However, 67% of the analyzed liver samples presented PAH4 concentrations higher than the permissible level.
The highest average concentrations of PAHs having low molecular weight (LMW PAHs) recorded in the three fish organs are in agreement with other studies [
1,
26,
74,
76,
78]. This strong accumulation of LMW PAHs may be due to their high solubility in water, their high bioavailability, and a high metabolism of HMW PAHs [
1,
26]. The high average Nap (LMW PAHs) concentrations observed in muscles (6.69 µg/kg) and livers (41.71 µg/kg) could be explained by the high solubility of Nap in water and its high bioavailability rendering it strongly absorbed in the water environment [
1,
79]. Indeed, due to their high solubility, LMW PAHs have an accumulation rate three times higher than HMW PAHs one in aquatic organisms [
80]. These results agree with those obtained in other previous studies [
1,
73,
79]. However, bioaccumulation is controlled by several factors, such as the duration of exposure, the quality and mode of feeding, the species, the nature of the molecule, the physicochemical quality of the medium (temperature, salinity, pH, etc.), as well as the absorption and removal rates of contaminants [
79]. The abundance of Nap and Phe could be linked to the release of used fuel and motor oils into the wild from the phosphate processing plant and quarry machinery. In addition, automobile exhaust gases can be noted as a result of atmospheric depositions. Indeed, it is known that PAHs with two and three aromatic rings, such as Nap and Phe, are characteristic of PAHs of petrogenic origin [
71,
81]. This abundance of Nap and Phe has also been observed in other studies [
1,
71,
73].
The double pyrogenic origin of PAHs observed in this study is in agreement with other studies. This may be due to the complexity of the parameters that influence the PAHs distribution in the environment [
39,
40]. However, attention should be paid to the petrogenic origin of PAHs in fish. Indeed, the high concentrations of LMW PAHs observed could be linked to their high solubility in water. This renders them more bioavailable, associated to the fact that HMW PAHs can be easily removed from fishes [
73,
82]. In addition, it is known that in tropical environments, Nap and Phe can also have a biological source [
83]. The main entry routes for PAHs into the lagoon complex can be marine intrusion, especially during low water and high tides, runoff, and rivers after leaching of mining and urban soils, as well as atmospheric depositions.
The average concentrations of tPAHs and HMW PAHs in the muscles are generally lower than those obtained in the internal organs (gill, liver). These high concentrations of HMW PAHs in the gills are thought to be due to the fact that they are better bound to particulate matter naturally retained by the gills [
73]. The strong accumulation of PAHs in livers could be explained by the important physiological role that play livers in fish’s metabolism [
84,
85,
86]. This strong accumulation by internal organs is consistent with results obtained by other studies [
73,
76,
87]. In addition, these differences in PAHs contents in organs can be controlled by the physicochemical parameters of PAHs, lipid contents, and the metabolic capacity of each organ [
76]. The overall decrease in organic pollutant content with increasing biological parameters has also been observed in other studies [
1,
88].
Decreases in concentrations with increasing fish sizes observed for some PAH molecules could be due to the dilution of concentrations during fish growth and low accumulation rate in old individuals. In fact, Douben [
89] indicated that the accumulation of contaminants could reach a stable state after a certain age in fish. So, any further growth would dilute the existing concentrations. In addition, the oldest individuals have a more developed enzymatic system and elimination pathways (excretion or reproduction) than the youngest [
45,
90]. It can also be mentioned that the changes in fish diets with increasing fish sizes [
29] may contribute to these decreases of concentrations since accumulation of contaminants is also linked to the composition of diet. Thus, adults that feed on less contaminated preys may be less exposed to contaminant accumulation.
The HQ and CR indicate that consumption of the fish studied does not present a risk related to PAHs on the health of consumers according to the exposure scenarios of the present study. However, increasing ingested quantities may increase risks related to PAH. These results are consistent with those obtained in some fishery products from the Persian Gulf [
1]. However, the values of HQ and CR in children are always higher than in adults. This observation may be due to their low body weight, their physiological predisposition, the fragility of their organism, and their less developed enzymatic system [
91,
92].