Structure-Based Discovery of Potential HPV E6 and EBNA1 Inhibitors: Implications for Cervical Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Protein Structures
2.2. Protein and Ligand Preparation
2.3. Molecular Docking Studies (E6 Proteins of HPV16 and HPV18)
2.4. Predictive Evaluation of ADMET Properties
2.5. Re-Docking Top Compounds against EBNA1
2.6. Further Re-Docking of Top Compounds against All 3 Oncoproteins Using DockThor-VS
2.7. Visualizing Protein–Ligand Interaction Profiles
2.8. Prediction of Biological Activity of Lead Compounds
2.9. Molecular Dynamics Simulation
2.10. Molecular Mechanics Poisson–Boltzmann Surface Area Calculations
2.11. Structural Similarity Search of Top Compounds
3. Results and Discussion
3.1. Retrieving Protein Structures
3.2. Molecular Docking Analysis
3.2.1. Docking against HPV16 E6
3.2.2. Docking against HPV18 E6
3.2.3. Average Docking Scores for Both HPV16 and HPV18 E6 Proteins
3.3. Predictive Evaluation of Drug-Like Properties: ADMET Profiling
3.4. Re-Docking Top Compounds against EBNA1
3.5. Further Re-Docking of Top Compounds against All Three Oncoproteins Using DockThor
3.6. Exploring Molecular Interactions
3.7. Biological Activity Prediction of Selected Lead Compounds
3.8. MD Simulation Analysis
3.8.1. Test for Convergence
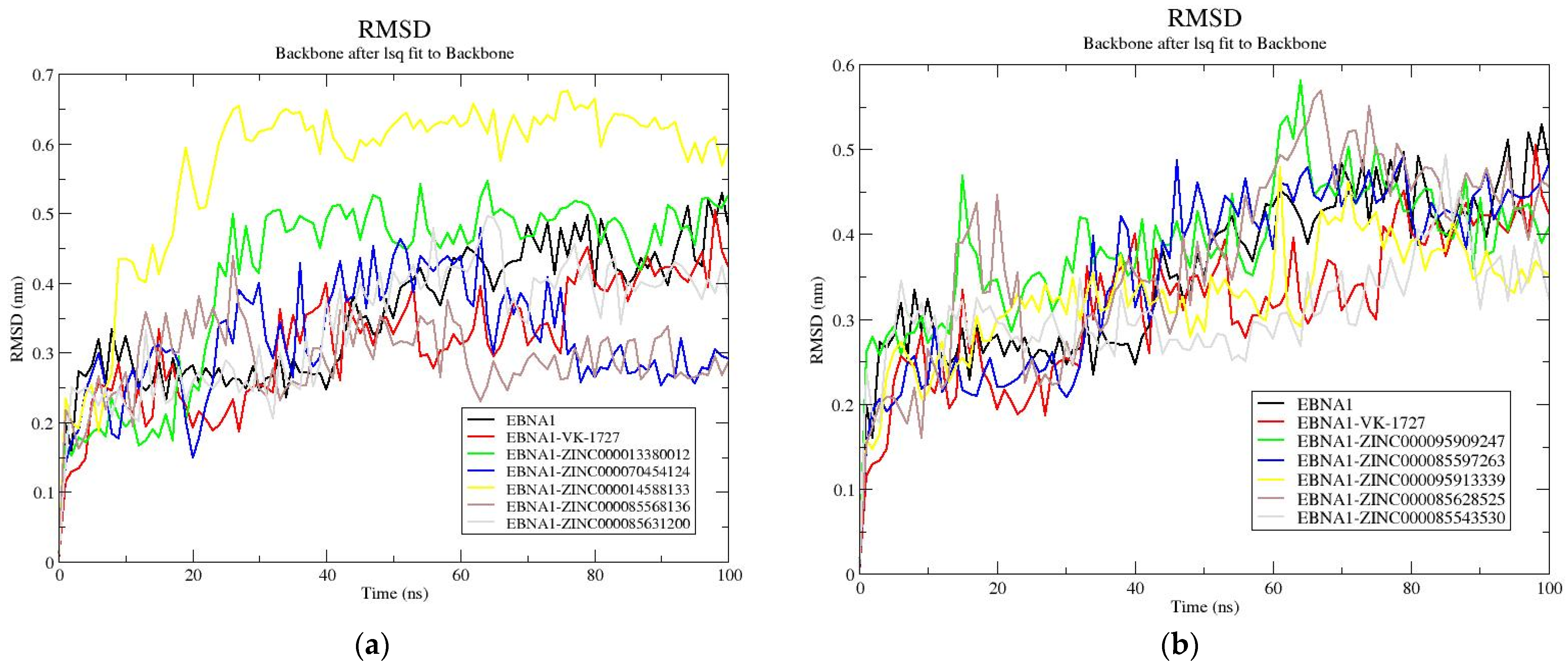
3.8.2. RMSD Analysis
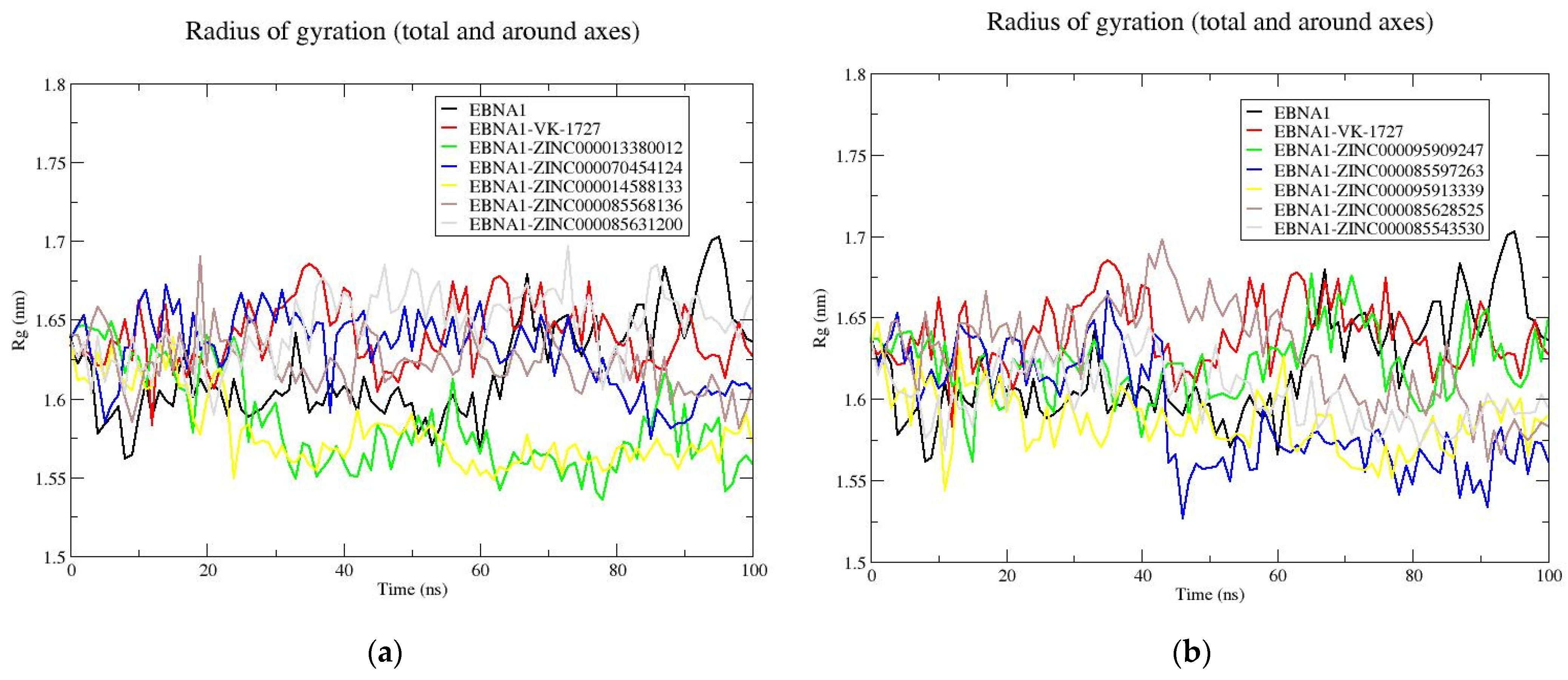
3.8.3. Rg Analysis
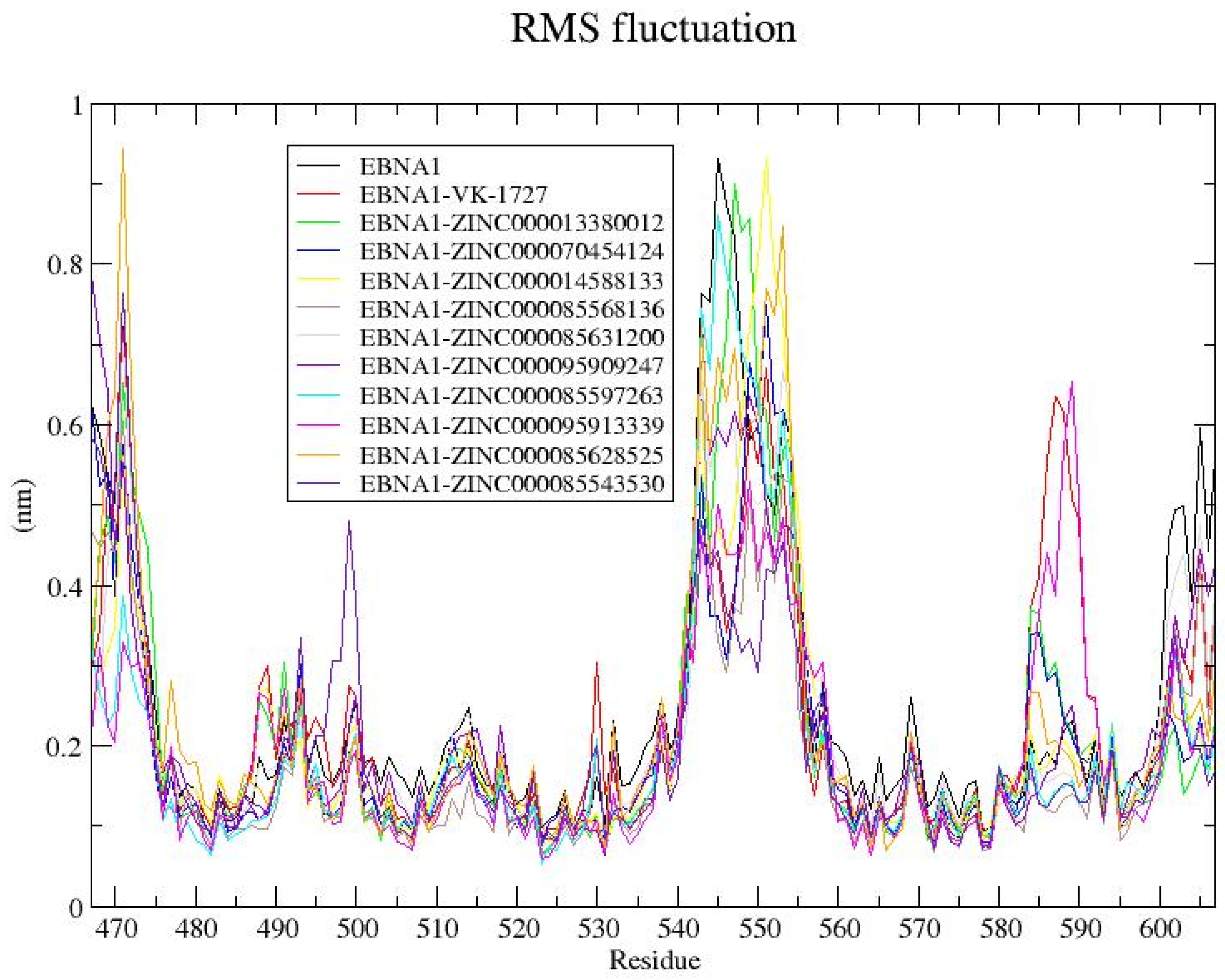
3.8.4. RMSF Analysis
3.9. Post-MD MM/PBSA Analysis
3.9.1. Enthalpy-Based Estimates of Binding Energy of the Protein–Ligand Complexes
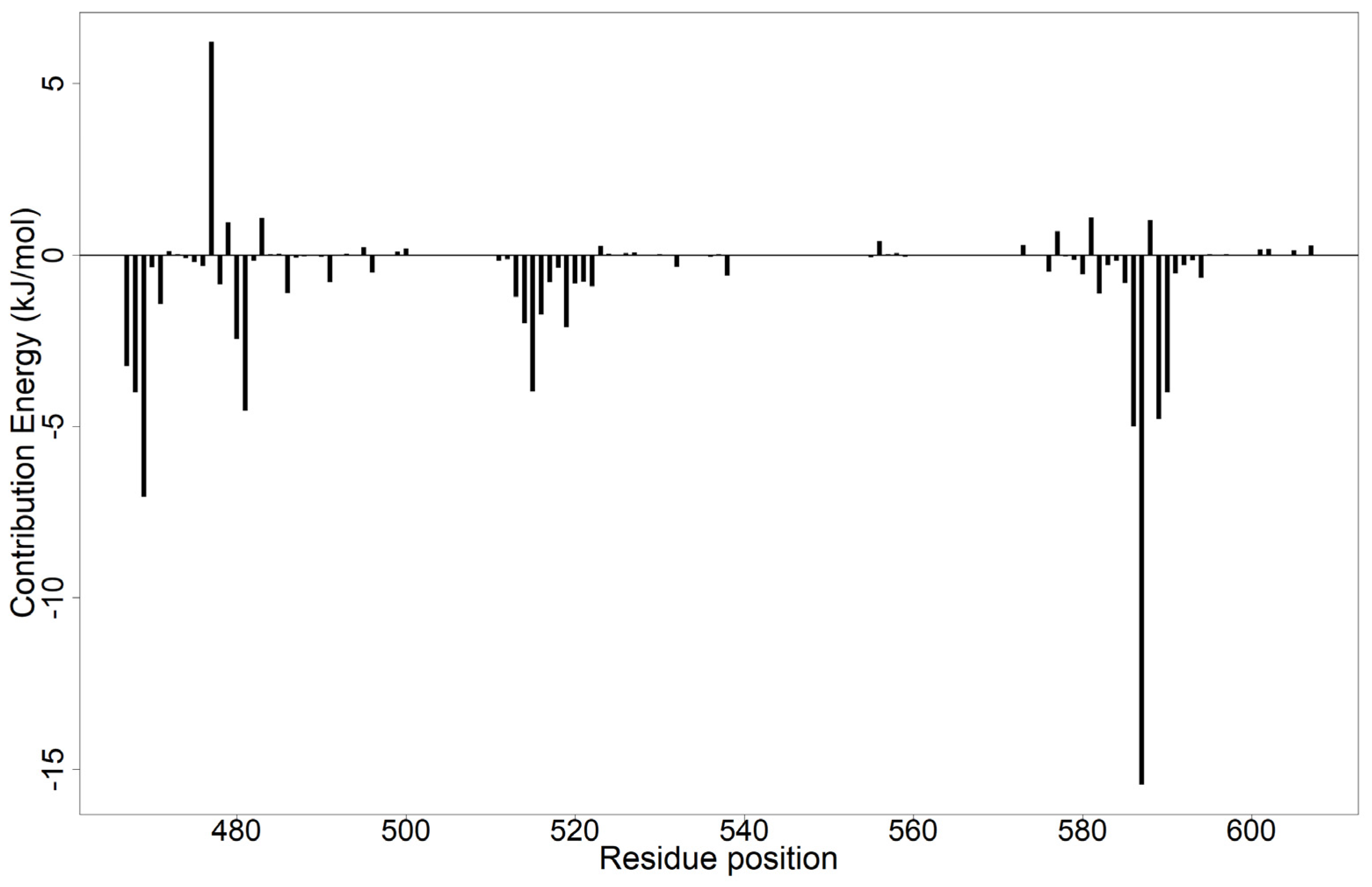
3.9.2. Per-Residue Energy Contributions
3.10. Structural Similarity Search of the Potential Candidates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiller, J.T.; Lowy, D.R. An Introduction to Virus Infections and Human Cancer. In Recent Results in Cancer Research. Fortschritte der Krebsforschung. Progres dans les Recherches sur le Cancer; Springer: Berlin/Heidelberg, Germany, 2021; Volume 217, pp. 1–11. [Google Scholar]
- Shih, W.-L.; Fang, C.-T.; Chen, P.-J. Anti-Viral Treatment and Cancer Control. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 269–290. ISBN 0080-0015. [Google Scholar]
- Shi, Y.; Peng, S.-L.; Yang, L.-F.; Chen, X.; Tao, Y.-G.; Cao, Y. Co-Infection of Epstein-Barr Virus and Human Papillomavirus in Human Tumorigenesis. Chin. J. Cancer 2016, 35, 16. [Google Scholar] [CrossRef] [PubMed]
- Trama, J.P.; Trikannad, C.; Yang, J.J.; Adelson, M.E.; Mordechai, E. High-Risk HPV Genotype Distribution According to Cervical Cytology and Age. Open Forum Infect. Dis. 2022, 9, ofac595. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, A.G.; Boyes, A.W.; Asibey, S.O.; Oldmeadow, C.; Mackenzie, L.J. Prevalence and Correlates of Modifiable Risk Factors for Cervical Cancer and HPV Infection among Senior High School Students in Ghana: A Latent Class Analysis. BMC Public Health 2023, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S. Human Papillomavirus and Cervical Cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, C.; Mohamed, Y.; Engel, D.; Sidibe, A.; Holloway, M.; Bloem, P.; Kumar, S.; Brotherton, J.; Reis, V.; Morgan, C. Integrating HPV Vaccination Programs with Enhanced Cervical Cancer Screening and Treatment, a Systematic Review. Vaccine 2022, 40, A116–A123. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.K.; Abe, S.K.; Thilagaratnam, S.; Haruyama, R.; Pathak, R.; Jayasekara, H.; Togawa, K.; Bhandari, A.K.C.; Shankar, A.; Nessa, A.; et al. Towards Elimination of Cervical Cancer—Human Papillomavirus (HPV) Vaccination and Cervical Cancer Screening in Asian National Cancer Centers Alliance (ANCCA) Member Countries. Lancet Reg. Health–West. Pac. 2023, 39, 100860. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.-A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2021, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Echelman, D.; Feldman, S. Management of Cervical Precancers: A Global Perspective. Hematol. Oncol. Clin. N. Am. 2012, 26, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.L.; Thierry, F. Human Papillomavirus Proteins as Prospective Therapeutic Targets. Microb. Pathog. 2013, 58, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.; Yadav, R.; Pankaj, S.; Shahi, S.K. Immunology of HPV-Mediated Cervical Cancer: Current Understanding. Int. Rev. Immunol. 2021, 40, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V.; Faizo, A.A.A. Control of Human Papillomavirus Gene Expression by Alternative Splicing. Virus Res. 2017, 231, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.K.; Timchenko, V.N.; Shakmaeva, M.A.; Kaplina, T.A.; Subbotina, M.D.; Bannova, S.L.; Fedorova, A.V.; Sukhovetskaya, V.F.; Pavlova, E.B.; Pavlova, N.V. EBV Mononucleosis in Children in Modern Conditions. Child. Infect. 2020, 19, 23–28. [Google Scholar] [CrossRef]
- Hjalgrim, H.; Askling, J.; Rostgaard, K.; Hamilton-Dutoit, S.; Frisch, M.; Zhang, J.-S.; Madsen, M.; Rosdahl, N.; Konradsen, H.B.; Storm, H.H.; et al. Characteristics of Hodgkin’s Lymphoma after Infectious Mononucleosis. N. Engl. J. Med. 2003, 349, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Balfour, H.H.; Dunmire, S.K.; Hogquist, K.A. Infectious Mononucleosis. Clin. Transl. Immunol. 2015, 4, e33. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Tang, K.; Qian, J.; Deng, H.; Zeng, M.; Zheng, S.; Ding, K.; Du, Y.; Sun, R. Immunotherapy for EBV-Associated Nasopharyngeal Carcinoma. Crit. Rev. Oncog. 2018, 23, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Duan, X.; Chen, X.; Zhan, M.; Peng, H.; Meng, Y.; Li, X.; Li, X.-Y.; Pang, G.; Dou, X. Immunotherapeutic Approaches in EBV-Associated Nasopharyngeal Carcinoma. Front. Immunol. 2023, 13, 1079515. [Google Scholar] [CrossRef]
- Orem, J.; Sandin, S.; Mbidde, E.; Mangen, F.W.; Middeldorp, J.; Weiderpass, E. Epstein-Barr Virus Viral Load and Serology in Childhood Non-Hodgkin’s Lymphoma and Chronic Inflammatory Conditions in Uganda: Implications for Disease Risk and Characteristics. J. Med. Virol. 2014, 86, 1796–1803. [Google Scholar] [CrossRef]
- Kanda, T.; Yajima, M.; Ikuta, K. Epstein-Barr Virus Strain Variation and Cancer. Cancer Sci. 2019, 110, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Pagano, J.; Whitehurst, C.; Andrei, G. Antiviral Drugs for EBV. Cancers 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Krumm, A.; Schubach, W.H. Promoter-Specific Targeting of Human SWI-SNF Complex by Epstein-Barr Virus Nuclear Protein 2. J. Virol. 2000, 74, 8893–8903. [Google Scholar] [CrossRef] [PubMed]
- Klinke, O.; Feederle, R.; Delecluse, H.-J. Genetics of Epstein–Barr Virus MicroRNAs. Semin. Cancer Biol. 2014, 26, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Trompet, E.; Snoeck, R. Novel Therapeutics for Epstein–Barr Virus. Molecules 2019, 24, 997. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.A.P.; Neto, P.J.N.; Lima, L.P.M.; Gonçalves Júnior, J.; Teixeira Junior, A.G.; Teodoro, I.P.P.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr Virus (EBV) and Cervical Carcinoma: A Meta-Analysis. Gynecol. Oncol. 2018, 148, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Gupta, I.; Vranic, S.; Al Moustafa, A.-E. Human Papillomaviruses and Epstein–Barr Virus Interactions in Colorectal Cancer: A Brief Review. Pathogens 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-M.; Cheng, Y.-W.; Wang, Y.-C.; Wu, T.-C.; Chen, C.-Y.; Lee, H. Up-Regulation of FOXM1 by E6 Oncoprotein through the MZF1/NKX2-1 Axis Is Required for Human Papillomavirus–Associated Tumorigenesis. Neoplasia 2014, 16, 961–971. [Google Scholar] [CrossRef]
- Diab, A.; Gem, H.; Swanger, J.; Kim, H.Y.; Smith, K.; Zou, G.; Raju, S.; Kao, M.; Fitzgibbon, M.; Loeb, K.R.; et al. FOXM1 Drives HPV+ HNSCC Sensitivity to WEE1 Inhibition. Proc. Natl. Acad. Sci. USA 2020, 117, 28287–28296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, X.; Wei, Z.; Xie, M.; Li, W.; Liu, G.; Guo, H.; Yang, J.; Wei, W.; Zhang, S. Ubiquitination of the HPV Oncoprotein E6 Is Critical for E6/E6AP-Mediated P53 Degradation. Front. Microbiol. 2019, 10, 2483. [Google Scholar] [CrossRef]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/P53 Complex Required for HPV-Mediated Degradation of P53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Soto, A.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin Induces G2 Phase Arrest and Apoptosis with the Activation of P53 in an E6 Expression-independent Manner in HPV-positive Human Cervical Cancer-derived Cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Soumia, M.; Hajji, H.; El Mzibri, M.; Younes, F.Z.; Mohammed, B.; Mohamed, B.; Benaissa, M. In-Silico Molecular Modeling Studies to Identify Novel Potential Inhibitors of HPV E6 Protein. Vaccines 2022, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Hoseini Tabatabaie, F.; Hosseini, S.Y.; Hashemi, S.M.A.; Safaie, A.; Sarvari, J. A Preliminary Sequence Analysis of the Epstein-Barr Virus Nuclear Antigen 1 (EBNA1) Carboxy-Terminal Region in Cervical and Ovarian Cancers. Iran. J. Pathol. 2023, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hung, S.C.; Kieff, E. Epstein-Barr Virus Nuclear Antigen 1 Activates Transcription from Episomal but Not Integrated DNA and Does Not Alter Lymphocyte Growth. Proc. Natl. Acad. Sci. USA 2001, 98, 15233–15238. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Du, S.; Liu, L.; Xie, Y.; Zuo, L.; Yang, J.; Hu, J.; Yue, W.; Zhang, J.; Cao, P.; et al. Epstein-Barr Virus Nuclear Antigen 1 Recruits Cyclophilin A to Facilitate the Replication of Viral DNA Genome. Front. Microbiol. 2019, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Alipour, A.H.; Hashemi, S.M.A.; Moattari, A.; Farhadi, A.; Sarvari, J. Epstein-Barr Virus Nuclear Antigen 1 Increases the Expression of Viral Oncogenes and Cellular Genes in the HeLa Cell Line. Int. J. Mol. Cell. Med. 2022, 11, 346–356. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, S. Past, Present, and Future of Targeting Ras for Cancer Therapies. Mini-Rev. Med. Chem. 2016, 16, 345–357. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, Z. Editorial (Thematic Issue: The Elephant in the Room: Targeting Ras for Therapeutic Development). Mini-Rev. Med. Chem. 2016, 16, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chaudhai, R.; Zhang, S. Polypharmacology in Drug Development: A Minireview of Current Technologies. ChemMedChem 2016, 11, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Kefale, B.; Engidaw, M.T.; Tesfa, D.; Molla, M.; Tegegne, G.T. Medication-Related Problems among Patients with Cervical Cancers at Oncology Centers of University of Gondar Comprehensive Specialized Hospital: A Hospital-Based Retrospective Study. J. Oncol. Pharm. Pract. 2024, 30, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ryszkiewicz, P.; Malinowska, B.; Schlicker, E. Polypharmacology: Promises and New Drugs in 2022. Pharmacol. Rep. 2023, 75, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Jamir, E.; Sarma, H.; Priyadarsinee, L.; Kiewhuo, K.; Nagamani, S.; Sastry, G.N. Polypharmacology Guided Drug Repositioning Approach for SARS-CoV2. PLoS ONE 2023, 18, e0289890. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, D.; Lucidi, A.; Rotili, D.; Mai, A. Epigenetic Polypharmacology: A New Frontier for Epi-drug Discovery. Med. Res. Rev. 2020, 40, 190–244. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lou, H.-X. Strategies to Diversify Natural Products for Drug Discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Chen, A.; Li, Y.; Xia, M.; Guo, P.; Yao, S.; Chen, S. Fucosterol Exhibits Selective Antitumor Anticancer Activity against HeLa Human Cervical Cell Line by Inducing Mitochondrial Mediated Apoptosis, Cell Cycle Migration Inhibition and Downregulation of m-TOR/PI3K/Akt Signalling Pathway. Oncol. Lett. 2018, 15, 3458–3463. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Suryavanshi, S.A.; Kaul-Ghanekar, R. The Aqueous Extract of Ficus Religiosa Induces Cell Cycle Arrest in Human Cervical Cancer Cell Lines SiHa (HPV-16 Positive) and Apoptosis in HeLa (HPV-18 Positive). PLoS ONE 2013, 8, e70127. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, C.; Arul Murugan, N.; Priyakumar, U.D. Structure-Based Drug Repurposing: Traditional and Advanced AI/ML-Aided Methods. Drug Discov. Today 2022, 27, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; Joshi, H.V.; Shah, U.A.; Patel, J.K. A Review on Computational Software Tools for Drug Design and Discovery. Indo Glob. J. Pharm. Sci. 2022, 12, 53–81. [Google Scholar] [CrossRef]
- Chen, C.Y.-C.C. TCM Database@Taiwan: The World’s Largest Traditional Chinese Medicine Database for Drug Screening in Silico. PLoS ONE 2011, 6, e15939. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful New Tools for Exploring 3D Structures of Biological Macromolecules for Basic and Applied Research and Education in Fundamental Biology, Biomedicine, Biotechnology, Bioengineering and Energy Sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Prlić, A.; Altunkaya, A.; Bi, C.; Bradley, A.R.; Christie, C.H.; Di Costanzo, L.; Duarte, J.M.; Dutta, S.; Feng, Z.; et al. The RCSB Protein Data Bank: Integrative View of Protein, Gene and 3D Structural Information. Nucleic Acids Res. 2017, 45, D271–D281. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.M.; Fischer, N.; Paknia, E.; Chari, A.; Stark, H. Atomic-Resolution Protein Structure Determination by Cryo-EM. Nature 2020, 587, 157–161. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A Public Information System for Analyzing Bioactivities of Small Molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 Update: Improved Access to Chemical Data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Malecka, K.A.; Fera, D.; Schultz, D.C.; Hodawadekar, S.; Reichman, M.; Donover, P.S.; Murphy, M.E.; Marmorstein, R. Identification and Characterization of Small Molecule Human Papillomavirus E6 Inhibitors. ACS Chem. Biol. 2014, 9, 1603–1612. [Google Scholar] [CrossRef]
- Messick, T.E.; Smith, G.R.; Soldan, S.S.; McDonnell, M.E.; Deakyne, J.S.; Malecka, K.A.; Tolvinski, L.; van den Heuvel, A.P.J.; Gu, B.-W.; Cassel, J.A.; et al. Structure-Based Design of Small-Molecule Inhibitors of EBNA1 DNA Binding Blocks Epstein-Barr Virus Latent Infection and Tumor Growth. Sci. Transl. Med. 2019, 11, aau5612. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15—Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Shoichet, B.K. ZINC—A Free Database of Commercially Available Compounds for Virtual Screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kwofie, S.; Broni, E.; Yunus, F.; Nsoh, J.; Adoboe, D.; Miller, W.; Wilson, M. Molecular Docking Simulation Studies Identifies Potential Natural Product Derived-Antiwolbachial Compounds as Filaricides against Onchocerciasis. Biomedicines 2021, 9, 1682. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Artemova, S.; Jaillet, L.; Redon, S. Automatic Molecular Structure Perception for the Universal Force Field. J. Comput. Chem. 2016, 37, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Kondapuram, S.K.; Sarvagalla, S.; Coumar, M.S. Docking-Based Virtual Screening Using PyRx Tool: Autophagy Target Vps34 as a Case Study. In Molecular Docking for Computer-Aided Drug Design Fundamentals, Techniques, Resources and Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 463–477. [Google Scholar] [CrossRef]
- Dallakyan, S.; Arthur, J. Olson Small Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, H.; Yu, K.; Jin, Z. Molecular Docking-Based Computational Platform for High-Throughput Virtual Screening. CCF Trans. High Perform. Comput. 2022, 4, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, EfficientOptimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Barret, R. Lipinski’s Rule of Five. In Therapeutical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Plinski, E.F.; Plinska, S. Veber’s Rules in Terahertz Light. Res. Sq. 2020, preprint. [Google Scholar] [CrossRef]
- Lee, E.K.; Kim, S.Y.; Noh, K.-W.; Joo, E.H.; Zhao, B.; Kieff, E.; Kang, M.-S. Small Molecule Inhibition of Epstein–Barr Virus Nuclear Antigen-1 DNA Binding Activity Interferes with Replication and Persistence of the Viral Genome. Antivir. Res. 2014, 104, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Thompson, S.; Schultz, D.C.; Zhu, W.; Jiang, H.; Luo, C.; Lieberman, P.M. Discovery of Selective Inhibitors against Ebna1 via High Throughput in Silico Virtual Screening. PLoS ONE 2010, 5, e10126. [Google Scholar] [CrossRef] [PubMed]
- Messick, T.E.; Tolvinski, L.; Zartler, E.R.; Moberg, A.; Frostell, Å.; Smith, G.R.; Reitz, A.B.; Lieberman, P.M. Biophysical Screens Identify Fragments That Bind to the Viral DNA-Binding Proteins EBNA1 and LANA. Molecules 2020, 25, 1760. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, S.; Jonniya, N.A.; Sk, M.F.; Rani, A.; Kar, P.; Jha, H.C. Identification of Potential Inhibitors against Epstein–Barr Virus Nuclear Antigen 1 (EBNA1): An Insight from Docking and Molecular Dynamic Simulations. ACS Chem. Neurosci. 2021, 12, 3060–3072. [Google Scholar] [CrossRef] [PubMed]
- Guedes, I.A.; Mü Ller Pereira Da Silva, M.; Galheigo, M.; Krempser, E.; Silva De Magalhã Es, C.; Correa Barbosa, H.J.; Dardenne, L.E. DockThor-VS: A Free Platform for Receptor-Ligand Virtual Screening. J. Mol. Biol. 2024, in press. [Google Scholar] [CrossRef]
- Guedes, I.A.; Costa, L.S.C.; dos Santos, K.B.; Karl, A.L.M.; Rocha, G.K.; Teixeira, I.M.; Galheigo, M.M.; Medeiros, V.; Krempser, E.; Custódio, F.L.; et al. Drug Design and Repurposing with DockThor-VS Web Server Focusing on SARS-CoV-2 Therapeutic Targets and Their Non-Synonym Variants. Sci. Rep. 2021, 11, 5543. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Parasuraman, S. Prediction of Activity Spectra for Substances. J. Pharmacol. Pharmacother. 2011, 2, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of Activity Spectra for Biologically Active Substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen Web Server: An Automatic OPLS-AA Parameter Generator for Organic Ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; Vilseck, J.Z.; Tirado-Rives, J.; Jorgensen, W.L. 1.14*CM1A-LBCC: Localized Bond-Charge Corrected CM1A Charges for Condensed-Phase Simulations. J. Phys. Chem. B 2017, 121, 3864–3870. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMOL: An Open-Source Molecular Graphics Tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Lynn, A. G_mmpbsa—A GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A Knowledgebase for Drugs, Drug Actions and Drug Targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef] [PubMed]
- Caballero Alfonso, A.Y.; Chayawan, C.; Gadaleta, D.; Roncaglioni, A.; Benfenati, E. A KNIME Workflow to Assist the Analogue Identification for Read-Across, Applied to Aromatase Activity. Molecules 2023, 28, 1832. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Senese, S.; Li, C.-M.; Hu, Q.; Huang, Y.; Damoiseaux, R.; Torres, J.Z. Large-Scale Chemical Similarity Networks for Target Profiling of Compounds Identified in Cell-Based Chemical Screens. PLoS Comput. Biol. 2015, 11, e1004153. [Google Scholar] [CrossRef] [PubMed]
- Nabati, F.; Moradi, M.; Mohabatkar, H. In Silico Analyzing the Molecular Interactions of Plant-Derived Inhibitors against E6AP, P53, and c-Myc Binding Sites of HPV Type 16 E6 Oncoprotein. Mol. Biol. Res. Commun. 2020, 9, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zanier, K.; Stutz, C.; Kintscher, S.; Reinz, E.; Sehr, P.; Bulkescher, J.; Hoppe-Seyler, K.; Travé, G.; Hoppe-Seyler, F. The E6AP Binding Pocket of the HPV16 E6 Oncoprotein Provides a Docking Site for a Small Inhibitory Peptide Unrelated to E6AP, Indicating Druggability of E6. PLoS ONE 2014, 9, e112514. [Google Scholar] [CrossRef] [PubMed]
- Dhamodharan, P.; Ponnusamy, N.; Odumpatta, R.; Lulu, S.; Arumugam, M. Computational Investigation of Marine Bioactive Compounds against E6 Oncoprotein of Human Papilloma Virus-HPV16. J. Appl. Pharm. Sci. 2018, 8, 023–032. [Google Scholar] [CrossRef]
- Kumar, S.; Jena, L.; Sahoo, M.; Kakde, M.; Daf, S.; Varma, A.K. In Silico Docking to Explicate Interface between Plant-Originated Inhibitors and E6 Oncogenic Protein of Highly Threatening Human Papillomavirus 18. Genom. Inform. 2015, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Sarvazad, H.; Rahnejat, N.; Rostampour, R.; Rad, M.G.; Eskandari-Roozbahani, N. Investigation of Potential Antiviral Natural Products with an Effect on HPV18 E6 Protein by Molecular Docking Method. Funct. Foods Health Dis. 2021, 11, 586. [Google Scholar] [CrossRef]
- Atabaki, V.; Pourahmad, J.; Hosseinabadi, T. Phytochemical Compounds from Jurinea Macrocephala Subsp.Elbursensis and Their Cytotoxicity Evaluation. S. Afr. J. Bot. 2021, 137, 399–405. [Google Scholar] [CrossRef]
- Chang, M.W.; Lindstrom, W.; Olson, A.J.; Belew, R.K. Analysis of HIV Wild-Type and Mutant Structures via in Silico Docking against Diverse Ligand Libraries. J. Chem. Inf. Model. 2007, 47, 1258–1262. [Google Scholar] [CrossRef]
- Indari, O.; Kumar Singh, A.; Tiwari, D.; Chandra Jha, H.; Nath Jha, A. Deciphering Antiviral Efficacy of Malaria Box Compounds against Malaria Exacerbating Viral Pathogens- Epstein Barr Virus and SARS-CoV-2, an in Silico Study. Med. Drug Discov. 2022, 16, 100146. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, S.; Momoh, R.; Lin, L.; Mallareddy, J.R.; Krstenansky, J.L. Identification of Potential Binding Pocket on Viral Oncoprotein HPV16 E6: A Promising Anti-Cancer Target for Small Molecule Drug Discovery. BMC Mol. Cell Biol. 2019, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Pathan, S.K.; Wadher, S.J. In Silico PASS Analysis and Determination of Antimycobacterial, Antifungal, and Antioxidant Efficacies of Maslinic Acid in an Extract Rich in Pentacyclic Triterpenoids. Int. J. Mycobacteriol. 2016, 5, 417–425. [Google Scholar] [CrossRef]
- Stiasny, A.; Freier, C.P.; Kuhn, C.; Schulze, S.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Dannecker, C.; Jeschke, U.; et al. The Involvement of E6, P53, P16, MDM2 and Gal-3 Inthe Clinical Outcome of Patients with Cervical Cancer. Oncol. Lett. 2017, 14, 4467–4476. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Pujals, A.; Favre, L.; Debernardi, J.; Wiels, J. The BCL-2 Family Protein Inhibitor ABT-737 as an Additional Tool for the Treatment of EBV-Associated Post-Transplant Lymphoproliferative Disorders. Mol. Oncol. 2020, 14, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Chabay, P. Advances in the Pathogenesis of EBV-Associated Diffuse Large B Cell Lymphoma. Cancers 2021, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Hongyu, L.; Hongling, S.; Qian, X.; Dongrui, D.; Shixuan, W.; Yunping, L.; Ding, M. Expression of Pin1 and Ki67 in Cervical Cancer and Their Significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Zhu, T.; Zhou, J.; Xu, Q.; Lu, Y.; Ma, D. Pin1 Contributes to Cervical Tumorigenesis by Regulating Cyclin D1 Expression. Oncol. Rep. 2006, 16, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Z.; Shi, F.; Wang, J. Pin1 Modulates Chemo-Resistance by up-Regulating FoxM1 and the Involvements of Wnt/β-Catenin Signaling Pathway in Cervical Cancer. Mol. Cell. Biochem. 2016, 413, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, J.; Zhang, T.; Liu, N. LPCAT1 Functions as an Oncogene in Cervical Cancer through Mediating JAK2/STAT3 Signaling. Exp. Cell Res. 2022, 421, 113360. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.L.; Macdonald, A. JAK2 Inhibition Impairs Proliferation and Sensitises Cervical Cancer Cells to Cisplatin-Induced Cell Death. Cancers 2019, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Arellano, A.R.; Lopez-Pulido, E.I.; Martínez-Neri, P.A.; Chávez, C.E.; Lucano, R.G.; Fafutis-Morris, M.; Aguilar-Lemarroy, A.; Muñoz-Valle, J.F.; Pereira-Suárez, A.L. STAT3 Activation Is Required for the Antiapoptotic Effects of Prolactin in Cervical Cancer Cells. Cancer Cell Int. 2015, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Chen, L.; Shao, J.; Jiang, C.; Zhao, Y.; Li, Y.; Ke, H.; Zhang, R.; Zhu, J.; Yu, M. Lanatoside C Inhibits Human Cervical Cancer Cell Proliferation and Induces Cell Apoptosis by a Reduction of the JAK2/STAT6/SOCS2 Signaling Pathway. Oncol. Lett. 2021, 22, 740. [Google Scholar] [CrossRef] [PubMed]
- Senba, M.; Buziba, N.; Mori, N.; Fujita, S.; Morimoto, K.; Wada, A.; Toriyama, K. Human Papillomavirus Infection Induces NF-ΚB Activation in Cervical Cancer: A Comparison with Penile Cancer. Oncol. Lett. 2011, 2, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Akbarpour Arsanjani, A.; Abuei, H.; Behzad-Behbahani, A.; Bagheri, Z.; Arabsolghar, R.; Farhadi, A. Activating Transcription Factor 3 Inhibits NF-κB P65 Signaling Pathway and Mediates Apoptosis and Cell Cycle Arrest in Cervical Cancer Cells. Infect. Agent Cancer 2022, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Nees, M.; Geoghegan, J.M.; Hyman, T.; Frank, S.; Miller, L.; Woodworth, C.D. Papillomavirus Type 16 Oncogenes Downregulate Expression of Interferon-Responsive Genes and Upregulate Proliferation-Associated and NF-ΚB-Responsive Genes in Cervical Keratinocytes. J. Virol. 2001, 75, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Sui, L.; Wang, J.; Li, F. Phenyllactic Acid Promotes Cell Migration and Invasion in Cervical Cancer via IKK/NF-ΚB-Mediated MMP-9 Activation. Cancer Cell Int. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- James, M.A.; Lee, J.H.; Klingelhutz, A.J. Human Papillomavirus Type 16 E6 Activates NF-ΚB, Induces CIAP-2 Expression, and Protects against Apoptosis in a PDZ Binding Motif-Dependent Manner. J. Virol. 2006, 80, 5301–5307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, L.; Gong, J.; Shi, C.; Wang, Z.; Ye, B.; Xuan, A.; He, X.; Long, D.; Zhu, X.; et al. NF-ΚB Pathway Link with ER Stress-Induced Autophagy and Apoptosis in Cervical Tumor Cells. Cell Death Discov. 2017, 3, 17059. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, L.; Li, E.; Dong, G. Application of Molecular Dynamics Simulation in Biomedicine. Chem. Biol. Drug Des. 2022, 99, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Allu, A.S.; Tiriveedhi, V. Cancer Salt Nostalgia. Cells 2021, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanic, A.; Zagrovic, B. Determination of Ensemble-Average Pairwise Root Mean-Square Deviation from Experimental B-Factors. Biophys. J. 2010, 98, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Abagyan, R. Methods of Protein Structure Comparison. In Methods in Molecular Biology (Clifton, N.J.); NIH Public Access: Bethesda, MD, USA, 2011; Volume 857, pp. 231–257. [Google Scholar]
- Koliński, M.; Dec, R.; Dzwolak, W. Multiscale Modeling of Amyloid Fibrils Formed by Aggregating Peptides Derived from the Amyloidogenic Fragment of the A-Chain of Insulin. Int. J. Mol. Sci. 2021, 22, 12325. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, M.Y.; Bogatyreva, N.S.; Galzitskaya, O.V. Radius of Gyration as an Indicator of Protein Structure Compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Shityakov, S.; Skorb, E.V.; Nosonovsky, M. Topological Bio-Scaling Analysis as a Universal Measure of Protein Folding. R. Soc. Open Sci. 2022, 9, 220160. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, D.; Zhou, S.; Liu, H.; Liu, H.; Yao, X. Molecular Dynamics Simulations and Novel Drug Discovery. Expert Opin. Drug Discov. 2018, 13, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kwofie, S.; Dankwa, B.; Enninful, K.; Adobor, C.; Broni, E.; Ntiamoah, A.; Wilson, M. Molecular Docking and Dynamics Simulation Studies Predict Munc18b as a Target of Mycolactone: A Plausible Mechanism for Granule Exocytosis Impairment in Buruli Ulcer Pathogenesis. Toxins 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Parvaiz, N.; Ahmad, F.; Yu, W.; MacKerell, A.D.; Azam, S.S. Discovery of Beta-Lactamase CMY-10 Inhibitors for Combination Therapy against Multi-Drug Resistant Enterobacteriaceae. PLoS ONE 2021, 16, e0244967. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Yusof, U.K.; Salim, N. Deep Learning Based Methods for Molecular Similarity Searching: A Systematic Review. Processes 2023, 11, 1340. [Google Scholar] [CrossRef]
- Safizadeh, H.; Simpkins, S.W.; Nelson, J.; Li, S.C.; Piotrowski, J.S.; Yoshimura, M.; Yashiroda, Y.; Hirano, H.; Osada, H.; Yoshida, M.; et al. Improving Measures of Chemical Structural Similarity Using Machine Learning on Chemical–Genetic Interactions. J. Chem. Inf. Model. 2021, 61, 4156–4172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-C.C.; Wang, S.S.; Wu, Y.; Chen, R.-Y.Y.; Yu, D.-Q.Q. Five New Diprenylated Flavonols from the Leaves of Broussonetia Kazinoki. J. Nat. Prod. 2001, 64, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.N.; Ramakrishnaiah, H.; Krishna, V.; Radhika, M. Cytotoxic Activity of Broussonetia Papyrifera(l.) Vent on MCF-7, HeLa and HepG2 Cell Lines. Int. J. Pharm. Pharm. Sci. 2014, 6, 339–342. [Google Scholar]
- Dou, C.-Z.; Liu, Y.-F.; Zhang, L.-L.; Chen, S.-H.; Hu, C.-Y.; Liu, Y.; Zhao, Y.-T. Polyphenols from Broussonetia Papyrifera Induce Apoptosis of HepG2 Cells via Inactivation of ERK and AKT Signaling Pathways. Evid.-Based Complement. Altern. Med. 2021, 2021, 8841706. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, J.; Mirzahosseini-pourranjbar, A.; Hajializadeh, Z.; Kaeidi, A. Anticancer and Cytotoxic Effects of Troxerutin on HeLa Cell Line: An in-Vitro Model of Cervical Cancer. Mol. Biol. Rep. 2020, 47, 6135–6142. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Ding, D.N.; Wang, Y.R.; Liu, S.X.; Peng, C.; Shen, F.; Zhu, X.Y.; Li, C.; Tang, L.P.; Han, F.J. Icariin as a Potential Anticancer Agent: A Review of Its Biological Effects on Various Cancers. Front. Pharmacol. 2023, 14, 1216363. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced Inhibition of SIRT6/NF-κB Triggers Redox Mediated Apoptosis and Enhances Anti-tumor Immunity in Triple-negative Breast Cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Lee, L.-H.; Goh, B.-H. Anti-Cancer Properties of the Naturally Occurring Aphrodisiacs: Icariin and Its Derivatives. Front. Pharmacol. 2016, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, H.; Long, M.; Song, L.; Meng, Z.; Lin, S.; Zhang, Y.; Qin, J. Icariin Attenuates the Tumor Growth by Targeting MiR-1-3p/TNKS2/Wnt/β-Catenin Signaling Axis in Ovarian Cancer. Front. Oncol. 2022, 12, 940926. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, K.; Zhao, F. Icariin Regulates the Proliferation and Apoptosis of Human Ovarian Cancer Cells through MicroRNA-21 by Targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xie, T.; Liu, W. Icariin Inhibits the Growth of Human Cervical Cancer Cells by Inducing Apoptosis and Autophagy by Targeting MTOR/PI3K/AKT Signalling Pathway. JBUON 2019, 24, 990–996. [Google Scholar]
- Li, C.; Yang, S.; Ma, H.; Ruan, M.; Fang, L.; Cheng, J. Influence of Icariin on Inflammation, Apoptosis, Invasion, and Tumor Immunity in Cervical Cancer by Reducing the TLR4/MyD88/NF-ΚB and Wnt/β-Catenin Pathways. Cancer Cell Int. 2021, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Cheng, J.; Mao, R.; Wang, R.; Jin, Y.; Li, C. Icariin-Mediated MiR-875-5p Inhibits Autophagy and Epithelial-Mesenchymal Transition by Regulation of MDM4 in Cervical Cancer. J. Biomed. Nanotechnol. 2022, 18, 2708–2720. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, J.; Iinuma, M. Melicodenines A and B, Novel Diels–Alder Type Adducts Isolated from Melicope Denhamii. Tetrahedron Lett. 2011, 52, 4694–4696. [Google Scholar] [CrossRef]
- Kamperdick, C.; Van, N.H.; Van Sung, T.; Adam, G. Bisquinolinone Alkaloids from Melicope Ptelefolia. Phytochemistry 1999, 50, 177–181. [Google Scholar] [CrossRef]
- Nakashima, K.; Oyama, M.; Ito, T.; Akao, Y.; Witono, J.R.; Darnaedi, D.; Tanaka, T.; Murata, J.; Iinuma, M. Novel Quinolinone Alkaloids Bearing a Lignoid Moiety and Related Constituents in the Leaves of Melicope Denhamii. Tetrahedron 2012, 68, 2421–2428. [Google Scholar] [CrossRef]
- Chelariu-Raicu, A.; Mahner, S.; Moore, K.N.; Lorusso, D.; Coleman, R.L. Integrating Antibody Drug Conjugates in the Management of Gynecologic Cancers. Int. J. Gynecol. Cancer 2023, 33, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Graybill, W.S.; Coleman, R.L. Vintafolide: A Novel Targeted Agent for Epithelial Ovarian Cancer. Futur. Oncol. 2014, 10, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Leamon, C.P. Vintafolide: A Novel Targeted Therapy for the Treatment of Folate Receptor Expressing Tumors. Ther. Adv. Med. Oncol. 2015, 7, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Canevari, S.; Thigpen, T. Targeting the Folate Receptor: Diagnostic and Therapeutic Approaches to Personalize Cancer Treatments. Ann. Oncol. 2015, 26, 2034–2043. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The Folate Receptor as a Rational Therapeutic Target for Personalized Cancer Treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, M.C.; Daniele, G.; Di Maio, M.; Bryce, J.; De Feo, G.; Del Giudice, A.; Perrone, F.; Morabito, A. Vinorelbine for Non-Small Cell Lung Cancer. Expert Opin. Drug Saf. 2010, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kenmotsu, H.; Yamanaka, T.; Nakamura, S.; Tsuboi, M. Randomized Phase III Study of Cisplatin With Pemetrexed and Cisplatin With Vinorelbine for Completely Resected Nonsquamous Non–Small-Cell Lung Cancer: The JIPANG Study Protocol. Clin. Lung Cancer 2018, 19, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Lavacchi, D.; Perrone, G.; Vicini, G.; Tassi, R.; Landini, I.; Grosso, A.; Roviello, G.; Mazzanti, R.; Santomaggio, C.; et al. Vinorelbine in Non-Small Cell Lung Cancer: Real-World Data From a Single-Institution Experience. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2020, 28, 237–248. [Google Scholar]
- Douillard, J.-Y.; Tribodet, H.; Aubert, D.; Shepherd, F.A.; Rosell, R.; Ding, K.; Veillard, A.-S.; Seymour, L.; Le Chevalier, T.; Spiro, S.; et al. Adjuvant Cisplatin and Vinorelbine for Completely Resected Non-Small Cell Lung Cancer: Subgroup Analysis of the Lung Adjuvant Cisplatin Evaluation. J. Thorac. Oncol. 2010, 5, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Coronel, J.A.; Cetina, L.D.C.; Cantú, D.; Cerezo, O.; Hernández, C.S.; Rivera, L.; Chacón, A.P.; Duenas-Gonzalez, A. A Randomized Comparison of Cisplatin and Oral Vinorelbine as Radiosensitizers in Aged or Comorbid Locally Advanced Cervical Cancer Patients. Int. J. Gynecol. Cancer 2013, 23, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Lacava, J.A.; Leone, B.A.; Machiavelli, M.; Romero, A.O.; Perez, J.E.; Elem, Y.L.; Ferreyra, R.; Focaccia, G.; Suttora, G.; Salvadori, M.A.; et al. Vinorelbine as Neoadjuvant Chemotherapy in Advanced Cervical Carcinoma. J. Clin. Oncol. 1997, 15, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Goodbody, A.; Vukovic, J.; Misawa, M. Biotransformation of Anhydrovinblastine to Vinblastine by a Cell-Free Extract of Catharanthus Roseus Cell Suspension Cultures. Phytochemistry 1987, 26, 3233–3234. [Google Scholar] [CrossRef]
- McLauchlan, W.R.; Hasan, M.; Baxter, R.L.; Ian Scott, A. Conversion of Anhydrovinblastine to Vinblastine by Cell-Free Homogenates of Catharanthus Roseus Cell Suspension Cultures. Tetrahedron 1983, 39, 3777–3780. [Google Scholar] [CrossRef]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Velasco, A.; Flores-Tafoya, P.D.J.; Fragoso-Serrano, M.; Leitão, S.G.; Pereda-Miranda, R. Resin Glycosides from Operculina Hamiltonii and Their Synergism with Vinblastine in Cancer Cells. J. Nat. Prod. 2022, 85, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-K.; Shao, Y.; Ding, H.; Hu, L.-H. Synthesis and Structure−Activity Relationship Studies of Cytotoxic Ester and Ether Anhydrovinblastine Derivatives. J. Nat. Prod. 2008, 71, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Kongsema, M.; Wongkhieo, S.; Khongkow, M.; Lam, E.W.F.; Boonnoy, P.; Vongsangnak, W.; Wong-Ekkabut, J. Molecular Mechanism of Forkhead Box M1 Inhibition by Thiostrepton in Breast Cancer Cells. Oncol. Rep. 2019, 42, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.S.; Sanders, D.A.; Rodriguez, R.; Balasubramanian, S. The Transcription Factor FOXM1 Is a Cellular Target of the Natural Product Thiostrepton. Nat. Chem. 2011, 3, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, P.; Chen, L.; Chen, H. Down-Regulation of FoxM1 by Thiostrepton or Small Interfering RNA Inhibits Proliferation, Transformation Ability and Angiogenesis, and Induces Apoptosis of Nasopharyngeal Carcinoma Cells. Int. J. Clin. Exp. Pathol. 2014, 7, 5450–5460. [Google Scholar] [PubMed]
- Jiang, L.; Wu, X.; Wang, P.; Wen, T.; Yu, C.; Wei, L.; Chen, H. Targeting FoxM1 by Thiostrepton Inhibits Growth and Induces Apoptosis of Laryngeal Squamous Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2015, 141, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Karalai, C.; Rat-a-pa, Y.; Ponglimanont, C.; Chantrapromma, K. New Cytotoxic Cardenolide Glycoside from the Seeds of Cerbera manghas. Chem. Pharm. Bull. 2004, 52, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Laphookhieo, S.; Cheenpracha, S.; Karalai, C.; Chantrapromma, S.; Ratapa, Y.; Ponglimanont, C.; Chantrapromma, K. Cytotoxic Cardenolide Glycoside from the Seeds of Cerbera odollam. Phytochemistry 2004, 65, 507–510. [Google Scholar] [CrossRef]
- Karaś, K.; Sałkowska, A.; Dastych, J.; Bachorz, R.A.; Ratajewski, M. Cardiac Glycosides with Target at Direct and Indirect Interactions with Nuclear Receptors. Biomed. Pharmacother. 2020, 127, 110106. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J.D. Novel Ingredients for Weight Loss: New Developments. In Novel Food Ingredients for Weight Control; Elsevier: Amsterdam, The Netherlands, 2007; pp. 218–233. ISBN 9781845690304. [Google Scholar]
- Cai, J.; Zhang, B.-D.; Li, Y.-Q.; Zhu, W.-F.; Akihisa, T.; Kikuchi, T.; Xu, J.; Liu, W.-Y.; Feng, F.; Zhang, J. Cardiac Glycosides from the Roots of Streblus Asper Lour. with Activity against Epstein-Barr Virus Lytic Replication. Bioorg. Chem. 2022, 127, 106004. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Orta, M.L.; Maldonado-Navas, D.; García-Domínguez, I.; López-Lázaro, M. Evaluating the Cancer Therapeutic Potential of Cardiac Glycosides. Biomed Res. Int. 2014, 2014, 794930. [Google Scholar] [CrossRef] [PubMed]
- Ayogu, J.I.; Odoh, A.S. Prospects and Therapeutic Applications of Cardiac Glycosides in Cancer Remediation. ACS Comb. Sci. 2020, 22, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Kumavath, R.; Paul, S.; Pavithran, H.; Paul, M.K.; Ghosh, P.; Barh, D.; Azevedo, V. Emergence of Cardiac Glycosides as Potential Drugs: Current and Future Scope for Cancer Therapeutics. Biomolecules 2021, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac Glycosides Inhibit Cancer through Na/K-ATPase-Dependent Cell Death Induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Qi, M.; Chan, C.; Leung, P.; Ye, G.; Lei, Y.; Liu, A.; Xue, F.; Liu, D.; Ye, W.; et al. Digitoxin Inhibits HeLa Cell Growth through the Induction of G2/M Cell Cycle Arrest and Apoptosis In Vitro and In Vivo. Int. J. Oncol. 2020, 57, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.G.; Rendeiro, M.M.; Cortes, V.F.; Barbosa, L.A.; Quintas, L.E.M. Antagonistic Anticancer Effect of Paclitaxel and Digoxin Combination. J. Cell. Biochem. 2019, 120, 13107–13114. [Google Scholar] [CrossRef] [PubMed]

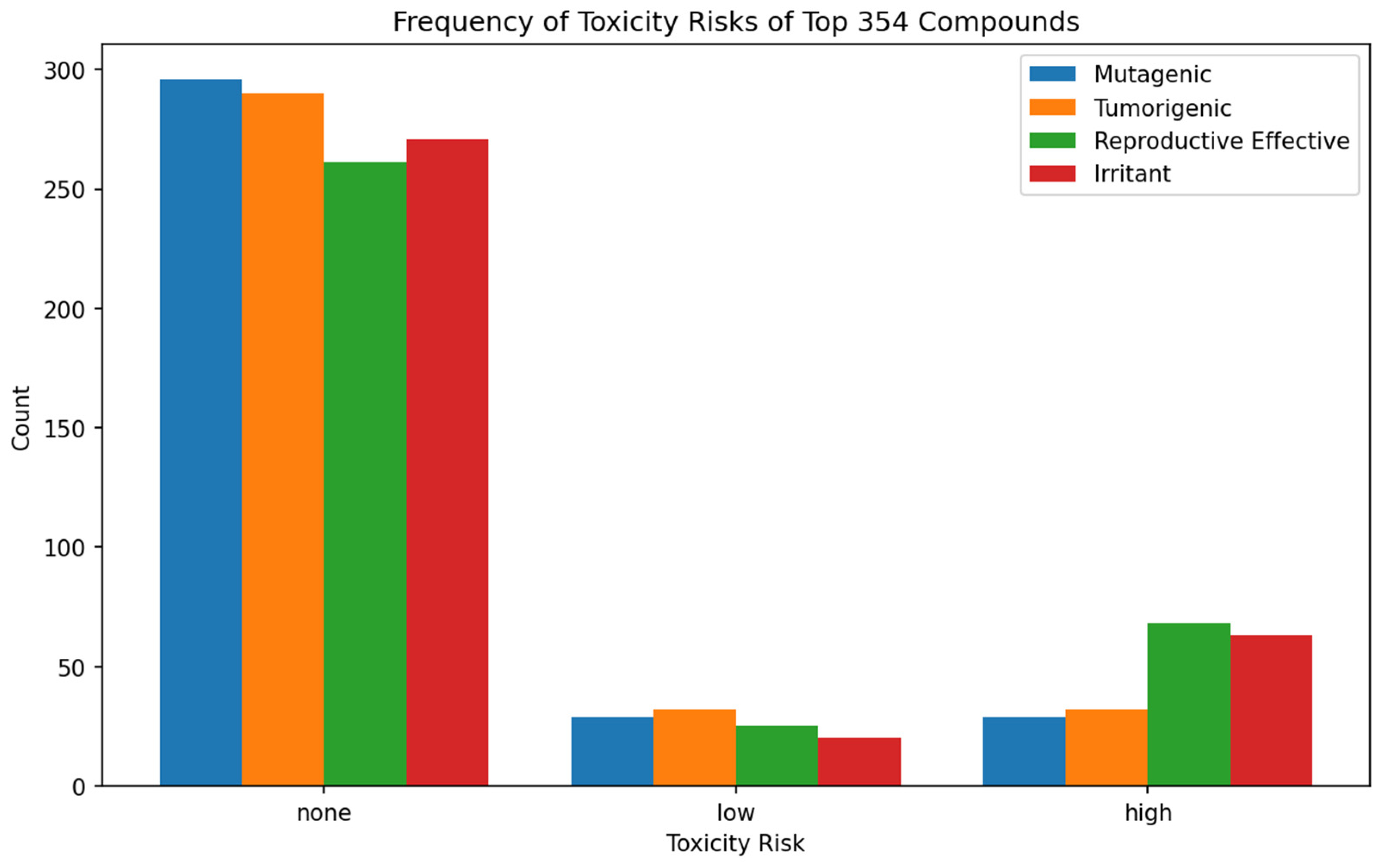
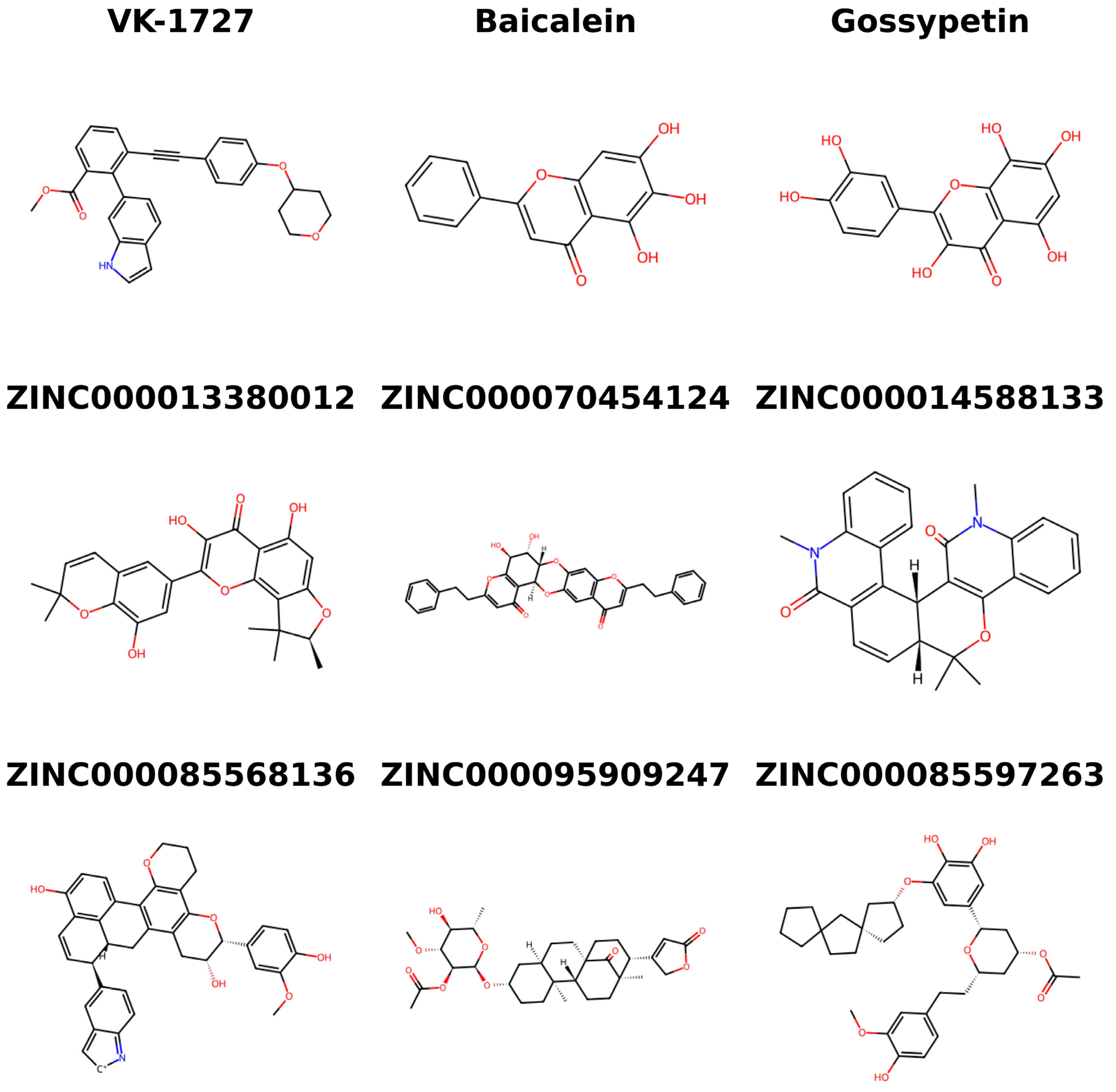
| Compound | Docking Scores (kcal/mol) | |||
|---|---|---|---|---|
| HPV16 E6 | HPV18 E6 | E6 Average | EBNA1 | |
| Baicalein | −7.9 | −6.8 | −7.35 | - |
| Gossypetin | −6.8 | −6.5 | −6.65 | - |
| VK-1727 | - | - | - | −6.6 |
| ZINC000070454124 | −10.8 | −10.7 | −10.75 | −7.3 |
| ZINC000085543530 | −9.8 | −9.8 | −9.8 | −7.3 |
| ZINC000085628525 | −9.8 | −9.4 | −9.6 | −7.6 |
| ZINC000013380012 | −9.5 | −9.7 | −9.6 | −7.0 |
| ZINC000014588133 | −9.3 | −9.6 | −9.45 | −7.1 |
| ZINC000085568150 | −8.7 | −10.1 | −9.4 | −7.5 |
| ZINC000070454501 | −8.6 | −10.1 | −9.35 | −7.6 |
| ZINC000085597263 | −8.6 | −10 | −9.3 | −7.7 |
| ZINC000085568136 | −8.7 | −9.8 | −9.25 | −7.0 |
| ZINC000095909247 | −8.7 | −9.8 | −9.25 | −7.4 |
| ZINC000085631200 | −8.7 | −9.6 | −9.15 | −7.4 |
| ZINC000095913339 | −8.6 | −9.6 | −9.1 | −7.1 |
| ZINC000003588336 | −8.9 | −9.2 | −9.05 | −7.2 |
| ZINC000085543579 | −8.9 | −9.1 | −9 | −7.7 |
| ZINC000095914132 | −8.5 | −9.5 | −9 | −7.6 |
| ZINC000000899994 | −8.9 | −9 | −8.95 | −7.7 |
| ZINC000013378519 | −8.9 | −9 | −8.95 | −7.0 |
| ZINC000014768164 | −8.7 | −9.2 | −8.95 | −7.3 |
| ZINC000070454552 | −8.6 | −9.3 | −8.95 | −7.2 |
| ZINC000013378520 | −8.8 | −9 | −8.9 | −7.3 |
| ZINC000014697269 | −8.7 | −9.1 | −8.9 | −7.3 |
| ZINC000103533241 | −8.5 | −9.1 | −8.8 | −7.0 |
| ZINC000085597267 | −8.6 | −8.9 | −8.75 | −7.3 |
| ZINC000033831887 | −8.8 | −8.5 | −8.65 | −7.8 |
| ZINC000085950180 | −8.8 | −8.5 | −8.65 | −7.6 |
| ZINC000014649947 | −8.6 | −8.7 | −8.65 | −7.3 |
| Compound | Molecular Weight (g/mol) | TPSA (Å2) | Consensus LogP | ESOL Solubility Class | GI Absorption | BBB Permeant | Pgp Substrate |
|---|---|---|---|---|---|---|---|
| ZINC000070454124 | 564.58 | 119.34 | 3.83 | Moderately soluble | High | No | Yes |
| ZINC000031155872 | 484.5 | 124.8 | 2.06 | Soluble | High | No | Yes |
| ZINC000070451048 | 530.65 | 119.49 | 2.97 | Moderately soluble | High | No | No |
| ZINC000085631224 | 561.75 | 95.86 | 4.85 | Poorly soluble | High | No | No |
| ZINC000095911347 | 526.53 | 137.96 | 1.37 | Soluble | High | No | Yes |
| ZINC000103554058 | 526.53 | 137.96 | 5.97 | Poorly soluble | Low | No | No |
| ZINC000085491721 | 596.8 | 15.25 | 4.91 | Poorly soluble | Low | No | No |
| ZINC000085543530 | 497.75 | 32.26 | 7.26 | Poorly soluble | Low | No | No |
| ZINC000085545967 | 580.79 | 118.22 | 4.14 | Poorly soluble | High | No | Yes |
| ZINC000103537846 | 484.5 | 124.8 | 3.47 | Poorly soluble | Low | No | Yes |
| ZINC000085531689 | 568.66 | 105.86 | 2.57 | Moderately soluble | High | No | Yes |
| ZINC000103572309 | 590.66 | 124.8 | 3.44 | Moderately soluble | High | No | Yes |
| ZINC000103580368 | 526.53 | 137.96 | 0.81 | Soluble | High | No | Yes |
| ZINC000085628525 | 516.67 | 97.83 | 4.87 | Poorly soluble | High | No | Yes |
| ZINC000013380012 | 436.45 | 109.36 | 3.98 | Poorly soluble | High | No | No |
| ZINC000095909822 | 446.66 | 74.6 | 4.59 | Moderately soluble | High | No | No |
| ZINC000085530466 | 596.71 | 124.8 | 4.08 | Poorly soluble | Low | No | Yes |
| ZINC000085531511 | 520.7 | 74.76 | 3.27 | Soluble | High | Yes | Yes |
| ZINC000085550046 | 473.73 | 32.26 | 5.16 | Poorly soluble | Low | No | Yes |
| ZINC000103585067 | 510.53 | 125.43 | 1.27 | Soluble | High | No | Yes |
| Compound | Interacting Residues | |
|---|---|---|
| Hydrogen Bonds | Hydrophobic Contacts | |
| EBNA1 | ||
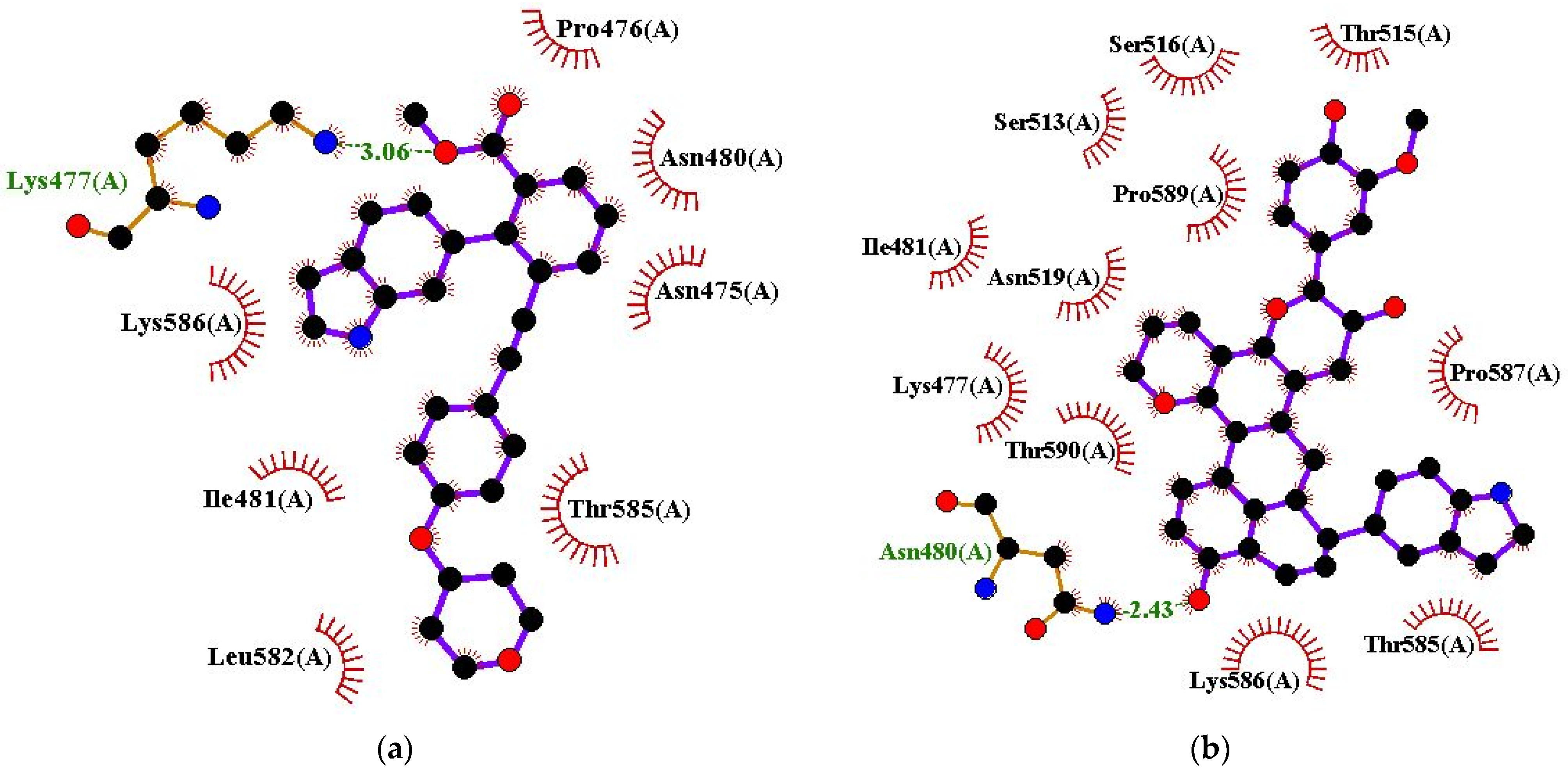
| VK-1727 | Lys477 (3.06 Å) | Asn475, Pro476, Asn480, Ile481, Leu582, Thr585, and Lys586 |
| ZINC000013380012 | Ser516 (3.08 Å), Lys586 (3.25 Å), and Thr590 (2.87 Å) | Lys477, Asn480, Ile481, Thr515, Pro587, and Ala588 |
| ZINC000070454124 | Thr585 (3.05 Å) | Lys477, Asn480, Gly484, Leu488, Arg491, Asp581, and Lys586 |
| ZINC000014588133 | Lys586 (2.66 Å) | Lys477, Ile481, Pro587, and Thr590 |
| ZINC000085568136 | Asn480 (2.43 Å) | Lys477, Ile481, Ser513, Thr515, Ser516, Asn519, Thr585, Lys586, Pro587, Pro589, and Thr590 |
| ZINC000085631200 | Asn480 (2.85 Å) and Thr590 (2.59 and 3.16 Å) | Lys477, Gly484, Leu582, Lys586, and Pro587 |
| ZINC000095909247 | Asn480 (2.77 Å) and Lys586 (2.77 Å) | Lys477, Ile481, Gly484, Leu488, Asp581, Leu582, Thr585, and Pro587 |
| ZINC000085597263 | Lys477 (2.64 Å) and Lys586 (2.54 Å) | Asn480, Ile481, Gly484, Leu488, Asn519, Leu582, Thr585, Pro587, and Thr590 |
| ZINC000095913339 | Lys586 (2.95 Å) | Lys477, Asn480, Ile481, Gly484, Leu582, and Pro587 |
| ZINC000085628525 | - | Lys477, Asn480, Ile481, Ser513, Thr515, Lys586, Pro587, Ala588, Pro589, and Thr590 |
| ZINC000085543530 | - | Asn475, Lys477, Asn480, Ile481, Asn519, Lys586, Pro587, Pro589, and Thr590 |
| HPV16 E6 | ||
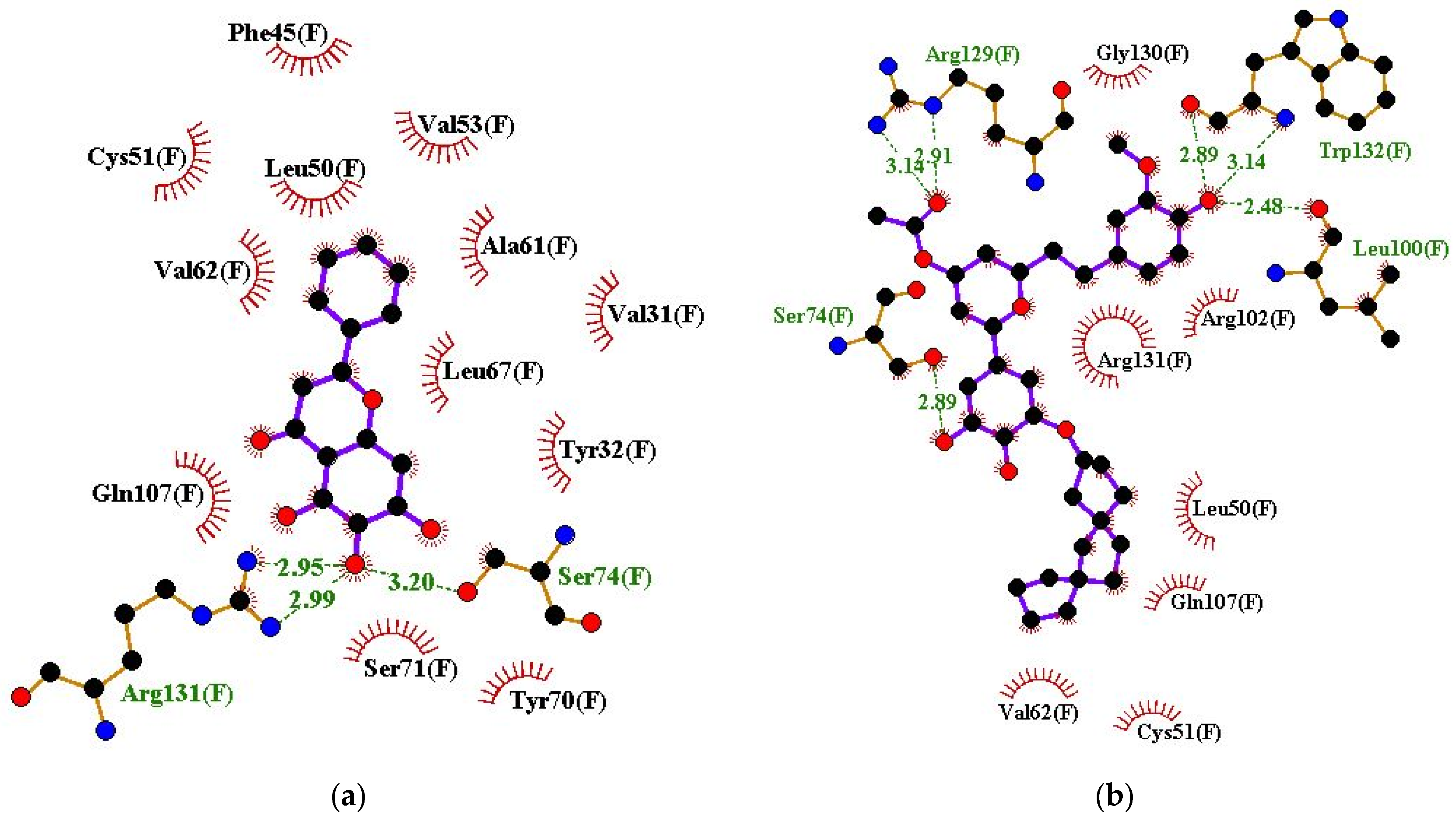
| Baicalein | Ser74 (3.2 Å) and Arg131 (2.95 and 2.99 Å) | Val31, Tyr32, Phe45, Leu50, Cys51, Val53, Ala61, Val62, Leu67, Tyr70, Ser71, and Gln107 |
| Gossypetin | Gln107 (3.01 Å) and Arg131 (3.24 Å) | Leu67, Tyr70, Ser71, Ser74, Arg102, and Thr133 |
| ZINC000013380012 | Ser71 (2.81 and 3.03 Å) and Gln107 (2.82 and 2.88 Å) | Tyr32, Leu50, Cys51, Val53, Val62, Leu67, Tyr70, Arg102, and Arg131 |
| ZINC000070454124 | Arg102 (3.22 Å) and Arg131 (3.01 Å) | Val31, Tyr32, Phe45, Leu50, Cys51, Val53, Val62, Leu67, Ser71, Tyr92, Leu99, Leu100, Gln107, and Trp132 |
| ZINC000014588133 | - | Tyr32, Phe45, Leu50, Cys51, Val53, Val62, Leu100, Arg102, and Arg131 |
| ZINC000085568136 | His78 (3.27 Å) | Tyr32, Leu50, Cys51, Tyr70, Ile73, Ser74, Arg77, and Arg102 |
| ZINC000085631200 | Cys51 (3.12 Å) | Val31, Tyr32, Leu50, Val53, Arg55, Val62, Leu67, Arg102, Gln107, and Arg131 |
| ZINC000095909247 | Arg8 (3.13 and 3.14 Å) | Tyr32, Leu50, Cys51, Val53, Tyr54, Arg55, Val62, Leu67, Tyr70, and Ser71 |
| ZINC000085597263 | Ser74 (2.89 Å), Leu100 (2.48 Å), Arg129 (2.91 and 3.14 Å), and Trp132 (2.89 and 3.14 Å) | Leu50, Cys51, Val62, Arg102, Gln107, Gly130, and Arg131 |
| ZINC000095913339 | - | Tyr32, Leu50, Cys51, Val53, Leu67, Tyr70, Ile73, and Ser74 |
| ZINC000085628525 | Cys51 (2.93 Å), Leu100 (2.79) and Trp132 (2.89 and 3.07 Å) | Tyr32, Phe45, Leu50, Val53, Ala61, Val62, Tyr70, Ser71, Arg102, Gln107, Gly130, and Arg131 |
| ZINC000085543530 | Leu67 (3 Å), Ser71 (2.91 Å), and Ser74 (2.77 Å) | Tyr32, Leu50, Cys51, Val53, Val62, Tyr70, Ile73, Arg77, Arg102, and Gln107 |
| HPV18 E6 | ||
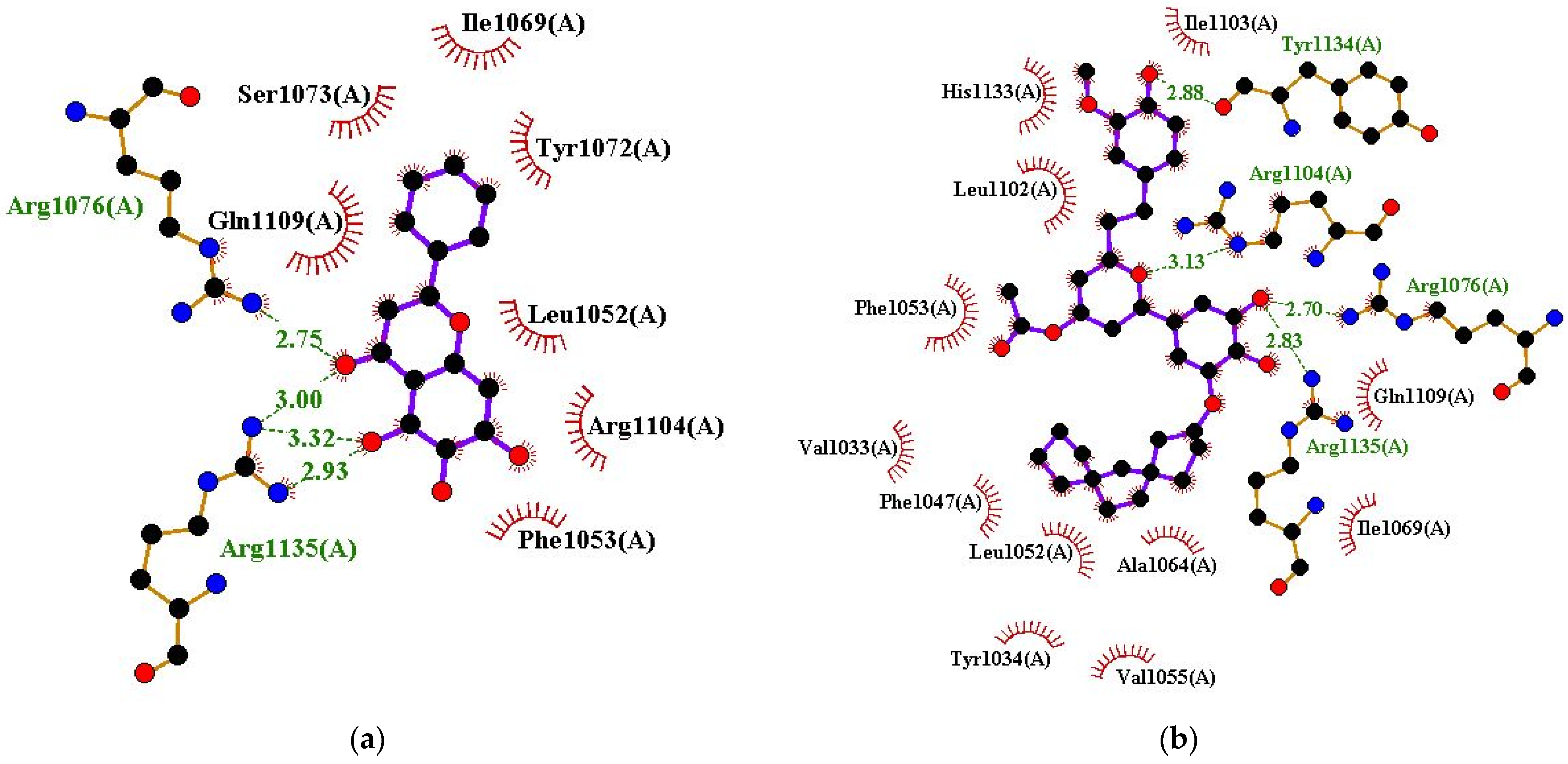
| Baicalein | Arg1076 (2.75 Å) and Arg1135 (2.93, 3.32, and 3 Å) | Leu1052, Phe1053, Ile1069, Tyr1072, Ser1073, Arg1104, and Gln1109 |
| Gossypetin | Ile1069 (2.71 Å), Ser1073 (3.15 Å), and Arg1076 (3.07 Å) | Leu1052, Ala1064, Tyr1072, Arg1104, Gln1109, His1133, and Arg1135 |
| ZINC000013380012 | Arg1135 (2.88 Å) | Tyr1034, Phe1047, Leu1052, Phe1053, Ala1064, Ile1069, Tyr1072, Ser1073, Arg1076, Arg1104, and Gln1109 |
| ZINC000070454124 | Gln1109 (3.12 Å) and Arg1135 (2.94 Å) | Tyr1034, Phe1047, Leu1052, Phe1053, Ala1064, Ile1069, Arg1076, Leu1090, Leu1101, Leu1102, Arg1104, His1133, and Tyr1134 |
| ZINC000014588133 | - | Leu1052, Phe1053, Val1055, Ala1064, Ile1069, Arg1076, Arg1104, and Gln1109 |
| ZINC000085568136 | - | Tyr1012, Leu1052, Phe1053, Val1055, Ile1069, Arg1076, Arg1104, and Gln1109 |
| ZINC000085631200 | Tyr1034 (3.28 Å) | Val1033, Leu1052, Phe1053, Val1055, Ala1064, Ile1069, Tyr1072, and Arg1076 |
| ZINC000095909247 | Ser1073 (2.89 Å), Arg1076 (3.19 Å), Arg1104 (3.1 Å), and Arg1135 (3.01 Å) | Tyr1034, Phe1047, Leu1052, Phe1053, Val1055, Ala1064, Ile1069, Tyr1072, Gln1109, and His1133 |
| ZINC000085597263 | Arg1076 (2.7 Å), Arg1104 (3.13 Å), Tyr1134 (2.88 Å), and Arg1135 (2.83 Å) | Val1033, Tyr1034, Phe1047, Leu1052, Phe1053, Val1055, Ala1064, Ile1069, Leu1102, Ile1103, Gln1109, and His1133 |
| ZINC000095913339 | - | Val1033, Tyr1034, Phe1047, Leu1052, Phe1053, Ala1063, Ala1064, Ile1069, Arg1104, and His1133 |
| ZINC000085628525 | Ile1069 (2.85 Å) and Ser1073 (2.89 Å) | Val1033, Tyr1034, Leu1052, Val1055, Ala1064, Tyr1072, Ile1075, Arg1076, His1080, and Gln1109 |
| ZINC000085543530 | Arg1076 (2.98 Å) | Val1033, Leu1052, Phe1053, Val1055, Ala1063, Ala1064, Ile1069, Arg1104, and Gln1109 |
| Compound | RMSD | Rg | RMSF | Hydrogen Bonds |
|---|---|---|---|---|
| EBNA1 | ||||
| Apo EBNA1 | 0.36 ± 0.09 | 1.62 ± 0.03 | 0.25 ± 0.18 | - |
| VK-1727 | 0.32 ± 0.09 | 1.64 ± 0.02 | 0.23 ± 0.15 | 0.43 ± 0.49 |
| ZINC000013380012 | 0.42 ± 0.13 | 1.58 ± 0.03 | 0.22 ± 0.17 | 0.81 ± 0.88 |
| ZINC000070454124 | 0.32 ± 0.08 | 1.63 ± 0.02 | 0.20 ± 0.14 | 0.40 ± 0.55 |
| ZINC000014588133 | 0.56 ± 0.13 | 1.58 ± 0.02 | 0.21 ± 0.16 | 0.19 ± 0.42 |
| ZINC000085568136 | 0.29 ± 0.05 | 1.62 ± 0.02 | 0.18 ± 0.13 | 1.33 ± 0.71 |
| ZINC000085631200 | 0.34 ± 0.09 | 1.65 ± 0.02 | 0.22 ± 0.15 | 1.30 ± 1.09 |
| ZINC000095909247 | 0.39 ± 0.08 | 1.62 ± 0.02 | 0.22 ± 0.16 | 0.64 ± 0.78 |
| ZINC000085597263 | 0.36 ± 0.11 | 1.59 ± 0.03 | 0.20 ± 0.16 | 2.36 ± 1.13 |
| ZINC000095913339 | 0.33 ± 0.07 | 1.59 ± 0.02 | 0.19 ± 0.13 | 0.41 ± 0.49 |
| ZINC000085628525 | 0.37 ± 0.11 | 1.63 ± 0.03 | 0.23 ± 0.18 | 0.83 ± 0.76 |
| ZINC000085543530 | 0.31 ± 0.06 | 1.60 ± 0.02 | 0.19 ± 0.13 | 0.18 ± 0.43 |
| HPV16 E6 | ||||
| Apo HPV16 E6 | 0.34 ± 0.07 | 1.72 ± 0.02 | 0.17 ± 0.07 | - |
| Baicalein | 0.28 ± 0.06 | 1.75 ± 0.02 | 0.21 ± 0.11 | 0.63 ± 0.98 |
| Gossypetin | 0.35 ± 0.08 | 1.76 ± 0.02 | 0.24 ± 0.13 | 2.24 ± 1.76 |
| ZINC000013380012 | 0.30 ± 0.05 | 1.72 ± 0.02 | 0.17 ± 0.17 | 0.68 ± 0.76 |
| ZINC000070454124 | 0.32 ± 0.08 | 1.77 ± 0.02 | 0.20 ± 0.17 | 1.62 ± 0.89 |
| ZINC000014588133 | 0.48 ± 0.08 | 1.73 ± 0.02 | 0.18 ± 0.10 | 0.39 ±0.56 |
| ZINC000085568136 | 0.41 ±0.07 | 1.74 ± 0.02 | 0.19 ± 0.08 | 0.46 ± 0.55 |
| ZINC000085631200 | 0.33 ± 0.06 | 1.78 ± 0.03 | 0.23 ± 0.17 | 0.35 ± 0.71 |
| ZINC000095909247 | 0.63 ± 0.16 | 1.72 ± 0.04 | 0.27 ± 0.14 | 0.56 ± 0.70 |
| ZINC000085597263 | 0.75 ± 0.18 | 1.85 ± 0.05 | 0.28 ± 0.10 | 1.47 ± 1.00 |
| ZINC000095913339 | 0.26 ± 0.06 | 1.77 ± 0.05 | 0.22 ± 0.08 | 0.54 ± 0.50 |
| ZINC000085628525 | 0.27 ± 0.04 | 1.75 ± 0.02 | 0.16 ± 0.09 | 1.14 ± 1.02 |
| ZINC000085543530 | 0.28 ± 0.06 | 1.76 ± 0.03 | 0.21 ± 0.08 | 0.52 ± 0.57 |
| HPV18 E6 | ||||
| Apo HPV18 E6 | 0.46 ± 0.25 | 1.84 ± 0.10 | 0.40 ± 0.13 | - |
| Baicalein | 0.46 ± 0.18 | 1.70 ± 0.05 | 0.30 ± 0.11 | 1.48 ± 0.65 |
| Gossypetin | 0.18 ± 0.03 | 1.74 ± 0.01 | 0.14 ± 0.05 | 1.61 ± 1.11 |
| ZINC000013380012 | 0.25 ± 0.04 | 1.75 ± 0.02 | 0.18 ± 0.10 | 0.80 ± 0.84 |
| ZINC000070454124 | 0.23 ± 0.04 | 1.73 ± 0.02 | 0.15 ± 0.06 | 1.36 ± 0.91 |
| ZINC000014588133 | 0.27 ± 0.04 | 1.76 ± 0.02 | 0.17 ± 0.08 | 0.21 ± 0.41 |
| ZINC000085568136 | 0.26 ± 0.05 | 1.78 ± 0.02 | 0.16 ± 0.07 | 0.37 ± 0.52 |
| ZINC000085631200 | 0.33 ± 0.10 | 1.76 ± 0.02 | 0.22 ± 0.06 | 0.10 ± 0.33 |
| ZINC000095909247 | 0.20 ± 0.03 | 1.76 ± 0.02 | 0.15 ± 0.07 | 1.57 ± 0.84 |
| ZINC000085597263 | 0.28 ± 0.06 | 1.77 ± 0.02 | 0.18 ± 0.09 | 1.34 ± 1.03 |
| ZINC000095913339 | 0.33 ± 0.13 | 1.82 ± 0.04 | 0.25 ± 0.08 | 0.97 ± 0.97 |
| ZINC000085628525 | 0.35 ± 0.08 | 1.77 ± 0.03 | 0.27 ± 0.08 | 0.25 ± 0.65 |
| ZINC000085543530 | 0.38 ± 0.16 | 1.76 ± 0.03 | 0.27 ± 0.11 | 1.10 ± 0.97 |
| Compound | vdW | Electrostatic | Polar Solvation | SASA | Binding Energy |
|---|---|---|---|---|---|
| EBNA1 | |||||
| VK-1727 | −117.584 ± 2.286 | −17.227 ± 1.564 | 68.307 ± 2.484 | −16.485 ± 0.196 | −82.862 ± 2.367 |
| ZINC000013380012 | −142.616 ± 1.448 | −23.003 ± 1.625 | 65.646 ± 1.737 | −16.998 ± 0.157 | −116.903 ± 1.55 |
| ZINC000070454124 | −151.084 ± 1.979 | −46.31 ± 1.848 | 101.63 ± 2.733 | −20.024 ± 0.227 | −115.733 ± 2.049 |
| ZINC000014588133 | −125.429 ± 1.252 | −50.222 ± 1.114 | 108.897 ± 1.72 | −15.271 ± 0.13 | −82.037 ± 1.222 |
| ZINC000085568136 | −211.009 ± 2.36 | −99.98 ± 2.497 | 186.042 ± 3.267 | −24.743 ± 0.177 | −149.734 ± 2.038 |
| ZINC000085631200 | −155.944 ± 1.213 | 76.406 ± 4.852 | 76.497 ± 5.077 | −19.516 ± 0.164 | −22.304 ± 2.387 |
| ZINC000095909247 | −161.992 ± 2.304 | −34.463 ± 1.79 | 88.3 ± 2.017 | −21.248 ± 0.212 | −129.397 ± 2.297 |
| ZINC000085597263 | −189.418 ± 2.213 | −43.499 ± 1.431 | 132.179 ± 2.343 | −24.333 ± 0.235 | −124.991 ± 2.27 |
| ZINC000095913339 | −29.4 ± 2.416 | −282.814 ± 24.546 | 172.67 ± 14.715 | −5.467 ± 0.419 | −146.078 ± 13.592 |
| ZINC000085628525 | −166.734 ± 2.387 | 189.625 ± 4.134 | 22.396 ± 3.336 | −21.028 ± 0.227 | 24.165 ± 3.502 |
| ZINC000085543530 | −138.653 ± 1.871 | 184.706 ± 2.215 | 17.701 ± 1.827 | −17.204 ± 0.188 | 46.399 ± 2.355 |
| HPV16 E6 | |||||
| Baicalein | −101.895 ± 1.576 | −24.764 ± 2.524 | 53.848 ± 2.289 | −12.766 ± 0.135 | −85.543 ± 1.766 |
| Gossypetin | −115.967 ± 2.867 | −50.543 ± 3.317 | 99.08 ± 3.137 | −14.373 ± 0.231 | −81.853 ± 2.908 |
| ZINC000013380012 | −172.264 ± 1.726 | −36.131 ± 1.176 | 90.437 ± 1.485 | −19.36 ± 0.157 | −137.343 ± 1.744 |
| ZINC000070454124 | −192.666 ± 1.993 | −65.773 ± 1.346 | 126.388 ± 1.44 | −22.811 ± 0.186 | −154.803 ± 2.425 |
| ZINC000014588133 | −85.917 ± 2.305 | −36.3 ± 2.906 | 70.005 ± 4.219 | −13.289 ± 0.381 | −65.693 ± 1.76 |
| ZINC000085568136 | −154.452 ± 2.069 | −32.253 ± 1.478 | 98.567 ± 2.505 | −22.482 ± 0.257 | −110.658 ± 2.193 |
| ZINC000085631200 | −171.491 ± 1.858 | 390.524 ± 4.649 | −38.923 ± 5.732 | −22.436 ± 0.202 | 157.746 ± 2.098 |
| ZINC000095909247 | −124.02 ± 2.802 | −49.058 ± 2.9 | 100.091 ± 2.51 | −18.557 ± 0.255 | −91.618 ± 2.489 |
| ZINC000085597263 | −152.386 ± 2.277 | −36.598 ± 1.329 | 94.31 ± 1.758 | −20.818 ± 0.212 | −115.486 ± 2.182 |
| ZINC000095913339 | −120.438 ± 1.846 | 640.495 ± 5.32 | −15.18 ± 4.005 | −16.878 ± 0.2 | 487.956 ± 2.893 |
| ZINC000085628525 | −211.098 ± 2.097 | 332.533 ± 4.153 | 46.457 ± 3.631 | −25.525 ± 0.149 | 142.202 ± 2.377 |
| ZINC000085543530 | −151.46 ± 2.131 | 338.799 ± 3.318 | 8.698 ± 2.269 | −19.193 ± 0.195 | 176.95 ± 1.801 |
| HPV18 E6 | |||||
| Baicalein | −122.369 ± 1.778 | −34.903 ± 1.711 | 65.906 ± 1.868 | −13.534 ± 0.112 | −104.817 ± 1.385 |
| Gossypetin | −128.577 ± 1.257 | −40.634 ± 1.797 | 75.543 ± 1.458 | −15.07 ± 0.09 | −108.865 ± 1.231 |
| ZINC000013380012 | −183.08 ± 1.882 | −38.721 ± 1.338 | 100.144 ± 2.171 | −22.072 ± 0.188 | −143.562 ± 1.576 |
| ZINC000070454124 | −211.266 ± 1.977 | −45.97 ± 1.739 | 136.442 ± 1.91 | −24.267 ± 0.213 | −145.092 ± 2.12 |
| ZINC000014588133 | −146.336 ± 2.345 | −29.871 ± 1.376 | 86.422 ± 2.204 | −18.83 ± 0.235 | −108.639 ± 1.976 |
| ZINC000085568136 | −188.528 ± 1.056 | −31.536 ± 1.275 | 84.121 ± 2.068 | −21.929 ± 0.122 | −157.826 ± 1.376 |
| ZINC000085631200 | −149.967 ± 2.546 | 227.694 ± 2.755 | 2.349 ± 2.849 | −20.067 ± 0.262 | 60.059 ± 2.292 |
| ZINC000095909247 | −133.976 ± 1.448 | −73.534 ± 2.249 | 114.427 ± 2.09 | −19.629 ± 0.18 | −112.686 ± 2.147 |
| ZINC000085597263 | −209.851 ± 2.767 | −31.126 ± 1.928 | 103.433 ± 3.913 | −26.598 ± 0.319 | −164.172 ± 4.237 |
| ZINC000095913339 | −120.521 ± 2.171 | 291.517 ± 11.731 | 107.367 ± 12.791 | −17.47 ± 0.216 | 260.902 ± 2.655 |
| ZINC000085628525 | −107.125 ± 2.169 | 141.934 ± 7.634 | 31.239 ± 5.66 | −14.648 ± 0.318 | 51.178 ± 3.672 |
| ZINC000085543530 | −183.362 ± 2.412 | 186.255 ± 2.814 | 28.299 ± 2.877 | −23.427 ± 0.174 | 7.617 ± 2.488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broni, E.; Ashley, C.N.; Velazquez, M.; Sakyi, P.O.; Kwofie, S.K.; Miller, W.A., III. Structure-Based Discovery of Potential HPV E6 and EBNA1 Inhibitors: Implications for Cervical Cancer Treatment. Computation 2024, 12, 112. https://doi.org/10.3390/computation12060112
Broni E, Ashley CN, Velazquez M, Sakyi PO, Kwofie SK, Miller WA III. Structure-Based Discovery of Potential HPV E6 and EBNA1 Inhibitors: Implications for Cervical Cancer Treatment. Computation. 2024; 12(6):112. https://doi.org/10.3390/computation12060112
Chicago/Turabian StyleBroni, Emmanuel, Carolyn N. Ashley, Miriam Velazquez, Patrick O. Sakyi, Samuel K. Kwofie, and Whelton A. Miller, III. 2024. "Structure-Based Discovery of Potential HPV E6 and EBNA1 Inhibitors: Implications for Cervical Cancer Treatment" Computation 12, no. 6: 112. https://doi.org/10.3390/computation12060112
APA StyleBroni, E., Ashley, C. N., Velazquez, M., Sakyi, P. O., Kwofie, S. K., & Miller, W. A., III. (2024). Structure-Based Discovery of Potential HPV E6 and EBNA1 Inhibitors: Implications for Cervical Cancer Treatment. Computation, 12(6), 112. https://doi.org/10.3390/computation12060112








