Age-Related Trajectories of General Fluid Cognition and Functional Decline in the Health and Retirement Study: A Bivariate Latent Growth Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Cognitive Assessments
2.2.2. Functional Limitation
2.3. Analyses
2.3.1. Longitudinal Factor Analysis
2.3.2. Latent Trajectory Models
3. Results
3.1. Longitudinal Factor Analysis (LFA)
3.2. Univariate Trajectory Models
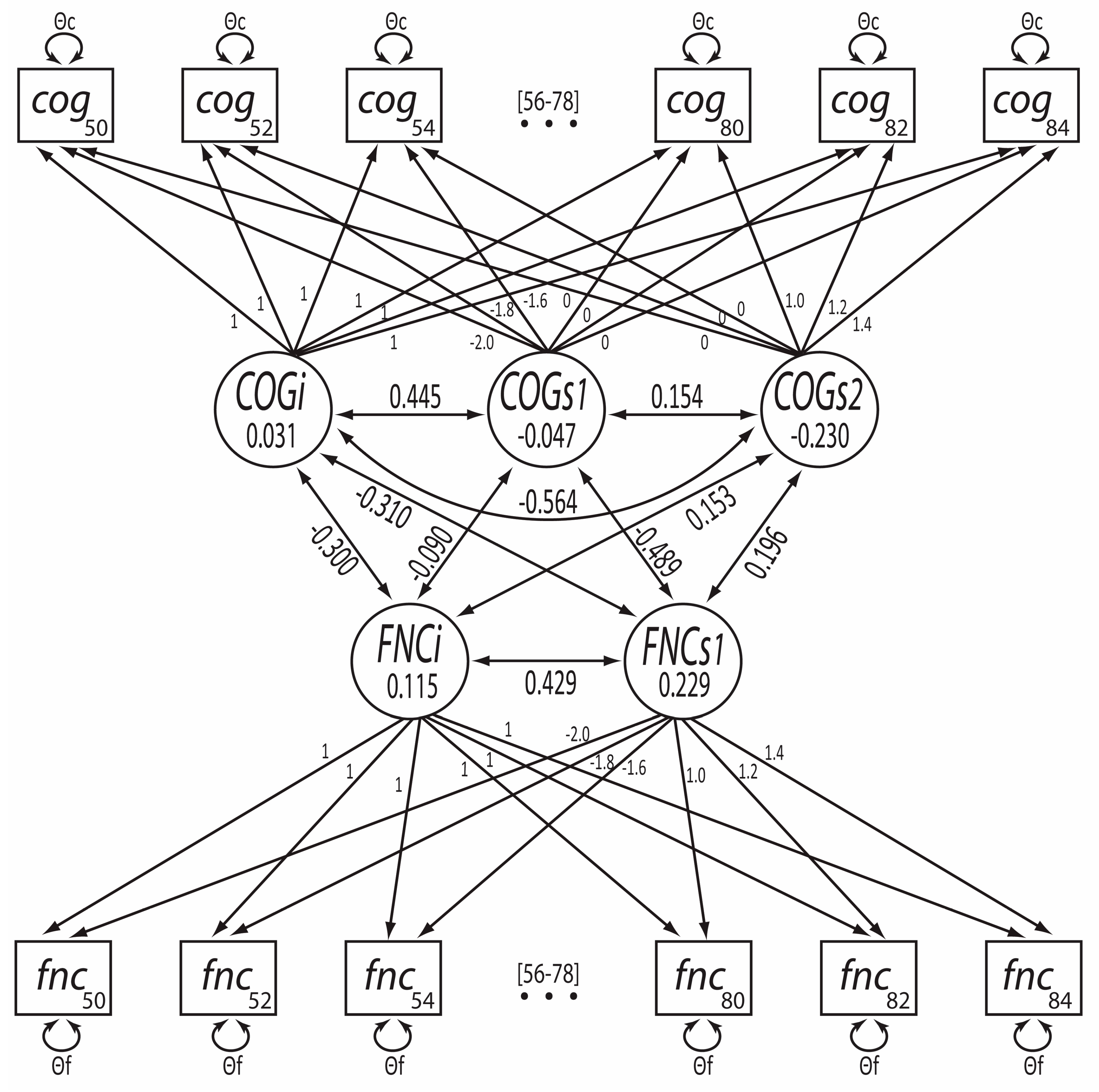
3.3. Bivariate Trajectory Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aichberger, M. C., M. A. Busch, F. M. Reischies, A. Ströhle, A. Heinz, and M. A. Rapp. 2010. Effect of physical inactivity on cognitive performance after 2.5 years of follow-up. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry 23: 7–15. [Google Scholar] [CrossRef]
- Aichele, Stephen, Paolo Ghisletta, Janie Corley, Alison Pattie, Adele M. Taylor, John M. Starr, and Ian J. Deary. 2018. Fluid Intelligence Predicts Change in Depressive Symptoms in Later Life: The Lothian Birth Cohort 1936. Psychological Science 29: 1984–95. [Google Scholar] [CrossRef] [PubMed]
- Aichele, Stephen, Patrick Rabbitt, and Paolo Ghisletta. 2015. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychology and Aging 30: 598–612. [Google Scholar] [CrossRef] [PubMed]
- Aichele, Stephen, Patrick Rabbitt, and Paolo Ghisletta. 2019. Illness and intelligence are comparatively strong predictors of individual differences in depressive symptoms following middle age. Aging & Mental Health 23: 122–31. [Google Scholar] [CrossRef]
- Baltes, Paul B., Ulman Lindenberger, and Ursula M. Staudinger. 2006. Lifespan theory in developmental psychology. In Handbook of Child Psychology. Hoboken: Wiley, vol. 6, pp. 569–664. [Google Scholar]
- Bravell, Marie Ernsth, Steven H. Zarit, and Boo Johansson. 2011. Self-reported activities of daily living and performance-based functional ability: A study of congruence among the oldest old. European Journal of Ageing 8: 199–209. [Google Scholar] [CrossRef]
- Brown, Timothy A. 2015. Confirmatory Factor Analysis for Applied Research, 2nd ed. New York: Guilford Press. [Google Scholar]
- Clouston, Sean A., Paul Brewster, Diana Kuh, Marcus Richards, Rachel Cooper, Rebecca Hardy, Marcie S. Rubin, and Scott M. Hofer. 2013. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiologic Reviews 35: 33–50. [Google Scholar] [CrossRef]
- Collyer, Taya A., Anne M. Murray, Robyn L. Woods, Elsdon Storey, Trevor T. -J. Chong, Joanne Ryan, Suzanne G. Orchard, Amy Brodtmann, Velandai K. Srikanth, Raj C. Shah, and et al. 2022. Association of Dual Decline in Cognition and Gait Speed With Risk of Dementia in Older Adults. JAMA Network Open 5: e2214647. [Google Scholar] [CrossRef]
- Deary, Ian J., Wendy Johnson, Alan J. Gow, Alison Pattie, Caroline E. Brett, Timothy C. Bates, and John M. Starr. 2011. Losing one’s grip: A bivariate growth curve model of grip strength and nonverbal reasoning from age 79 to 87 years in the Lothian Birth Cohort 1921. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 66: 699–707. [Google Scholar] [CrossRef]
- Demnitz, Naiara, Patrick Esser, Helen Dawes, Vyara Valkanova, Heidi Johansen-Berg, Klaus P. Ebmeier, and Claire Sexton. 2016. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait & Posture 50: 164–74. [Google Scholar] [CrossRef]
- Devlieger, Ines, Axel Mayer, and Yves Rosseel. 2016. Hypothesis Testing Using Factor Score Regression: A Comparison of Four Methods. Educational and Psychological Measurement 76: 741–70. [Google Scholar] [CrossRef]
- Edwards, Mary Margaret. 1990. The Reliability and Validity of Self-Report Activities of Daily Living Scales. Canadian Journal of Occupational Therapy 57: 273–78. [Google Scholar] [CrossRef]
- Fiocco, Alexandra J., and Kristine Yaffe. 2010. Defining successful aging: The importance of including cognitive function over time. Archives of Neurology 67: 876–80. [Google Scholar] [CrossRef] [PubMed]
- Fisher, Gwenith G., John J. McArdle, Ryan J. McCammon, Amanda Sonnega, and David R. Weir. 2014. New Measures of Fluid Intelligence in the HRS. Available online: https://hrs.isr.umich.edu/publications/biblio/5959 (accessed on 3 September 2022).
- Folstein, Marshal F., Susan E. Folstein, and Paul R. McHugh. 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–98. [Google Scholar] [CrossRef]
- Fonda, Stephanie, and A. Regula Herzog. 2004. Documentation of Physical Functioning Measured in the Health and Retirement Study and Asset and Health Dynamics among the Oldest Old Study. Available online: https://hrs.isr.umich.edu/publications/biblio/5607 (accessed on 3 September 2022).
- Gerstorf, Denis, Nilam Ram, Ulman Lindenberger, and Jacqui Smith. 2013. Age and time-to-death trajectories of change in indicators of cognitive, sensory, physical, health, social, and self-related functions. Developmental Psychology 49: 1805–21. [Google Scholar] [CrossRef]
- Gonzales, Mitzi M., Chen-Pin Wang, Myla Quiben, Daniel MacCarthy, Sudha Seshadri, Mini Jacob, and Helen Hazuda. 2020. Joint trajectories of cognition and gait speed in Mexican American and European American older adults: The San Antonio longitudinal study of aging. International Journal of Geriatric Psychiatry 35: 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, Rebecca F., Andrea LC Schneider, Yun Zhou, Josef Coresh, Edward Green, Naresh Gupta, David S. Knopman, Akiva Mintz, Arman Rahmim, A. Richey Sharrett, and et al. 2017. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA 317: 1443–50. [Google Scholar] [CrossRef]
- Grimm, Kevin J., Nilam Ram, and Ryne Estabrook. 2017. Growth Modeling: Structural Equation and Multilevel Modeling Approaches. New York: Guilford Press. [Google Scholar]
- Hall, Katherine S., Harvey J. Cohen, Carl F. Pieper, Gerda G. Fillenbaum, William E. Kraus, Kim M. Huffman, Melissa A. Cornish, Andrew Shiloh, Christy Flynn, Richard Sloane, and et al. 2017. Physical Performance Across the Adult Life Span: Correlates With Age and Physical Activity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 72: 572–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, Li-Tze, and Peter M. Bentler. 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal 6: 1–55. [Google Scholar] [CrossRef]
- Huber, Peter J. 1967. The Behavior of Maximum Likelihood Estimates under Nonstandard Conditions. In Fifth Berkeley Symposium on Mathematical Statistics and Probability: Weather Modification, January 7. Berkeley: Statistical Laboratory of the University of California. [Google Scholar]
- Jayakody, Oshadi, Monique Breslin, Emmeline Ayers, Joe Verghese, Nir Barzilai, Sofiya Milman, Erica Weiss, and Helena M. Blumen. 2021. Relative Trajectories of Gait and Cognitive Decline in Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 77: 1230–38. [Google Scholar] [CrossRef]
- Johnson, Julene K., Li-Yung Lui, and Kristine Yaffe. 2007. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 62: 1134–41. [Google Scholar] [CrossRef]
- Kuh, Diana, Rachel Cooper, Rebecca Hardy, Jack Guralnik, Marcus Richards, and Musculoskeletal Study Team. 2009. Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosomatic Medicine 71: 38–48. [Google Scholar] [CrossRef] [PubMed]
- Liao, Wen-Ling, and Yu-Hung Chang. 2020. Age trajectories of disability in instrumental activities of daily living and disability-free life expectancy among middle-aged and older adults in Taiwan: An 11-year longitudinal study. BMC Geriatrics 20: 530. [Google Scholar] [CrossRef] [PubMed]
- McArdle, John J., Emilio Ferrer-Caja, Fumiaki Hamagami, and Richard W. Woodcock. 2002. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology 38: 115–42. [Google Scholar] [CrossRef]
- McArdle, John J., Gwenith G. Fisher, and Kelly M. Kadlec. 2007. Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992–2004. Psychology and Aging 22: 525. [Google Scholar] [CrossRef] [PubMed]
- McCammon, Ryan J., Gwenith G. Fisher, Halimah Hassan, Jessica D. Faul, Willard L. Rodgers, and David R. Weir. 2022. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992–2018 (Version 7.0). Available online: https://hrs.isr.umich.edu/sites/default/files/biblio/COGIMP9218_dd.pdf (accessed on 18 October 2022).
- McDonough, Ian M., Gérard N. Bischof, Kristen M. Kennedy, Karen M. Rodrigue, Michelle E. Farrell, and Denise C. Park. 2016. Discrepancies between fluid and crystallized ability in healthy adults: A behavioral marker of preclinical Alzheimer’s disease. Neurobiology of Aging 46: 68–75. [Google Scholar] [CrossRef]
- Montero-Odasso, Manuel, Mark Speechley, Susan W. Muir-Hunter, Frederico Pieruccini-Faria, Yanina Sarquis-Adamson, Vladimir Hachinski, Louis Bherer, Michael Borrie, Jennie Wells, Amit X. Garg, and et al. 2020. Dual decline in gait speed and cognition is associated with future dementia: Evidence for a phenotype. Age and Ageing 49: 995–1002. [Google Scholar] [CrossRef]
- Montero-Odasso, Manuel, Mark Speechley, Susan W. Muir-Hunter, Yanina Sarquis-Adamson, Luciano A. Sposato, Vladimir Hachinski, Michael Borrie, Jennie Wells, Alanna Black, Ervin Sejdić, and et al. 2018. Motor and Cognitive Trajectories Before Dementia: Results from Gait and Brain Study. Journal of the American Geriatrics Society 66: 1676–83. [Google Scholar] [CrossRef]
- Montero-Odasso, Manuel, Quincy J. Almeida, Louis Bherer, Amer M. Burhan, Richard Camicioli, Julien Doyon, Sarah Fraser, Susan Muir-Hunter, Karen Z. H. Li, Teresa Liu-Ambrose, and et al. 2019. Consensus on Shared Measures of Mobility and Cognition: From the Canadian Consortium on Neurodegeneration in Aging (CCNA). The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 74: 897–909. [Google Scholar] [CrossRef]
- National Institute on Aging. n.d. Gateway to Global Aging Data, Produced by the Program on Global Aging, Health & Policy, University of Southern California with Funding from the National Institute on Aging (R01 AG030153). Available online: https://g2aging.org/ (accessed on 15 October 2022).
- Peel, Nancye May, Linson John Alapatt, Lee Vanessa Jones, and Ruth Eleanor Hubbard. 2019. The Association Between Gait Speed and Cognitive Status in Community-Dwelling Older People: A Systematic Review and Meta-analysis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 74: 943–48. [Google Scholar] [CrossRef]
- R Core Team. 2021. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 12 March 2021).
- Richards, Marcus, David Strachan, Rebecca Hardy, Diana Kuh, and Michael Wadsworth. 2005. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine 67: 602–8. [Google Scholar] [CrossRef]
- Ritchie, Stuart J., Elliot M. Tucker-Drob, John M. Starr, and Ian J. Deary. 2016. Do Cognitive and Physical Functions Age in Concert from Age 70 to 76? Evidence from the Lothian Birth Cohort 1936. The Spanish Journal of Psychology 19: E90. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, Annie, Andrea M. Piccinin, Scott M. Hofer, Boo Johansson, and Graciela Muniz Terrera. 2018. An examination of the heterogeneity in the pattern and association between rates of change in grip strength and global cognition in late life. A multivariate growth mixture modelling approach. Age and Ageing 47: 692–97. [Google Scholar] [CrossRef] [PubMed]
- Rosseel, Yves. 2012. Lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software 48: 1–36. [Google Scholar] [CrossRef]
- Salthouse, Timothy A. 2009. When does age-related cognitive decline begin? Neurobiology of Aging 30: 507–14. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, Nikolaos, Steven Albert, Jennifer J. Manly, and Yaakov Stern. 2006. Education and rates of cognitive decline in incident Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry 77: 308–16. [Google Scholar] [CrossRef]
- Schaie, K. Warner. 2005. Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study. Oxford: Oxford University Press. [Google Scholar]
- Steiger, James H. 1990. Structural Model Evaluation and Modification: An Interval Estimation Approach. Multivariate Behavioral Research 25: 173–80. [Google Scholar] [CrossRef]
- Tabbarah, Melissa, Eileen M. Crimmins, and Teresa E. Seeman. 2002. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 57: M228–35. [Google Scholar] [CrossRef]
- Tian, Qu, Susan M. Resnick, Michelle M. Mielke, Kristine Yaffe, Lenore J. Launer, Palmi V. Jonsson, Giulia Grande, Anna-Karin Welmer, Erika J. Laukka, Stefania Bandinelli, and et al. 2020. Association of Dual Decline in Memory and Gait Speed With Risk for Dementia Among Adults Older Than 60 Years: A Multicohort Individual-Level Meta-analysis. JAMA Network Open 3: e1921636. [Google Scholar] [CrossRef]
- van der Willik, Kimberly D., Silvan Licher, Elisabeth J. Vinke, Maria J. Knol, Sirwan K. L. Darweesh, Jos N. van der Geest, Sanne B. Schagen, M. Kamran Ikram, Annemarie I. Luik, and M. Arfan Ikram. 2021. Trajectories of Cognitive and Motor Function between Ages 45 and 90 Years: A Population-Based Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 76: 297–306. [Google Scholar] [CrossRef]
- Widaman, Keith F., Emilio Ferrer, and Rand D. Conger. 2010. Factorial Invariance within Longitudinal Structural Equation Models: Measuring the Same Construct across Time. Child Development Perspectives 4: 10–18. [Google Scholar] [CrossRef]
- Yeung, Michael K., Sophia L. Sze, Jean Woo, Timothy Kwok, David H. K. Shum, Ruby Yu, and Agnes S. Chan. 2016. Altered Frontal Lateralization Underlies the Category Fluency Deficits in Older Adults with Mild Cognitive Impairment: A Near-Infrared Spectroscopy Study. Frontiers in Aging Neuroscience 8: 59. [Google Scholar] [CrossRef] [PubMed]
- Zammit, Andrea R., Annie Robitaille, Andrea M. Piccinin, Graciela Muniz-Terrera, and Scott M. Hofer. 2019. Associations Between Aging-Related Changes in Grip Strength and Cognitive Function in Older Adults: A Systematic Review. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 74: 519–27. [Google Scholar] [CrossRef] [PubMed]
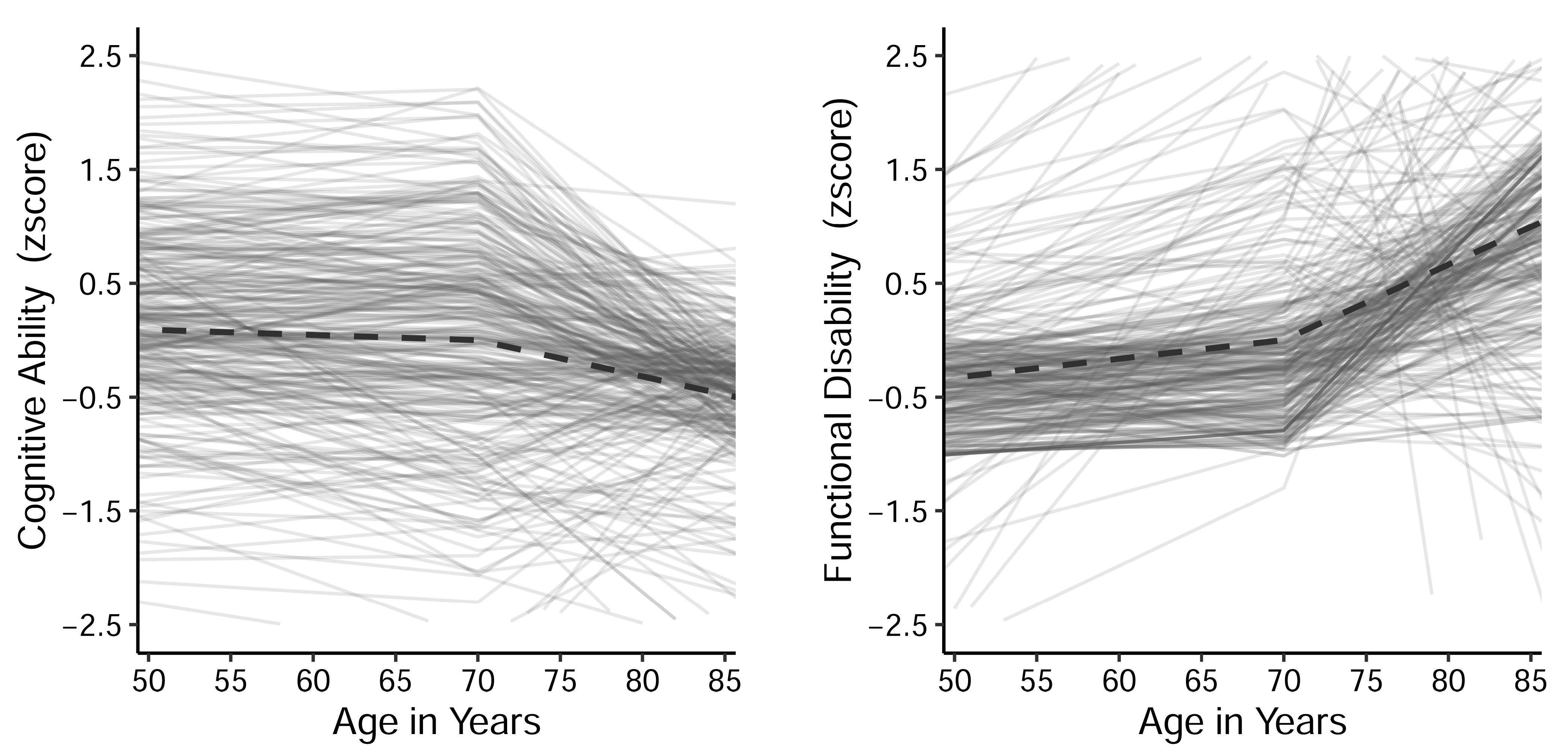
| Variable | Summary Statistics | |||||
|---|---|---|---|---|---|---|
| All Comers (N = 14,489) | Completers (N = 11,033) | |||||
| Age in years as of 2010 | M = 64.9, SD = 9.6 | M = 64.3, SD = 9.2 | ||||
| Women | n = 8126 (56.1%) | n = 6372 (57.8%) | ||||
| Years of education | M = 13.1, SD = 2.9 | M = 13.2, SD = 2.9 | ||||
| Mean (SD) by Wave | ||||||
| 2010 | 2012 | 2016 | 2010 | 2012 | 2016 | |
| Cognitive Ability | n = 14,489 | n = 13,277 | n = 10,900 | |||
| Fluid Reasoning | 497.3 (43.4) | 522.8 (31.4) | 521.8 (31.3) | 499.2 (142.8) | 523.9 (31.0) | 521.9 (31.3) |
| Executive Function | 3.1 (1.9) | 3.1 (1.9) | 3.1 (1.9) | 3.1 (1.9) | 3.1 (1.9) | 3.1 (1.9) |
| Category fluency | 17.4 (7.1) | 17.9 (7.3) | 16.7 (6.6) | 17.8 (7.1) | 18.2 (7.4) | 16.7 (6.6) |
| Recall Memory | 10.1 (3.3) | 10.0 (3.4) | 9.7 (3.5) | 10.3 (3.2) | 10.1 (3.3) | 9.8 (3.5) |
| Factor Score | −0.1 (0.9) | 0.1 (0.8) | 0.1 (0.7) | −0.1 (0.8) | 0.1 (0.8) | 0.1 (0.8) |
| Functional Limitation | n = 14,489 | n = 13,430 | n = 11,205 | |||
| ADL | 0.3 (0.9) | 0.4 (1.0) | 0.5 (1.2) | 0.3 (0.8) | 0.3 (0.8) | 0.5 (1.2) |
| IADL | 0.1 (0.4) | 0.1 (0.5) | 0.2 (0.7) | 0.1 (0.4) | 0.1 (0.5) | 0.2 (0.7) |
| Mobility | 2.6 (2.7) | 2.7 (2.7) | 3.0 (2.9) | 2.5 (2.6) | 2.6 (2.6) | 3.0 (2.9) |
| Factor Score | −0.1 (0.9) | −0.1 (1.0) | 0.1 (1.1) | −0.2 (0.8) | −0.2 (0.9) | 0.1 (1.2) |
| Model | Χ2(df) | TLI | RMSEA [95%CI] | AIC |
|---|---|---|---|---|
| Cognitive Ability | ||||
| Intercept-only | 1124 (186) | .948 | .019 [.018, .020] | 80,554 |
| Linear Slope | 906 (183) | .959 | .017 [.016, .018] | 80,343 |
| Spline, KP = 70/71y | 670 (179) | .971 | .014 [.014, .015] | 80,114 |
| Functional limitation | ||||
| Intercept-only | 3731 (186) | .781 | .036 [.035, .037] | 96,756 |
| Linear Slope | 2120 (183) | .880 | .027 [.025, .028] | 95,151 |
| Spline, KP = 70/71y | 1346 (179) | .927 | .021 [.019, .022] | 94,386 |
| Bivariate | ||||
| Cognitive Ability (linear slope) + Functional limitation (linear slope) | 3265 (685) | .915 | .017 [.016–.017] | 200,094 |
| Cognitive Ability (spline, KP = 70/71) + Functional limitation (linear slope) | 3005 (679) | .922 | .016 [.015–.016] | 199,846 |
| Parameter | Estimate | S.E. | Z | p |
|---|---|---|---|---|
| Means | ||||
| Intercept of Cognitive Ability | .031 | .012 | 2.673 | .008 |
| Slope (pre-70y) of Cognitive Ability | −.047 | .011 | −4.442 | <.001 |
| Slope (post-70y) of Cognitive Ability | −.230 | .016 | −14.376 | <.001 |
| Intercept of Functional limitation | .115 | .009 | 12.293 | <.001 |
| Slope of Functional limitation | .229 | .008 | 27.195 | <.001 |
| Variances | ||||
| Intercept of Cognitive Ability | .777 | .021 | 36.731 | <.001 |
| Slope (pre-70y) of Cognitive Ability | .142 | .021 | 6.716 | <.001 |
| Slope (post-70y) of Cognitive Ability | .413 | .035 | 11.861 | <.001 |
| Intercept of Functional limitation | .769 | .016 | 49.143 | <.001 |
| Slope of Functional limitation | .150 | .013 | 11.782 | <.001 |
| Residual of Cognitive Ability | .313 | .003 | 94.410 | <.001 |
| Residual of Functional limitation | .475 | .005 | 101.098 | <.001 |
| Std. Estimate | Z | p | ||
| Correlations, Within Processes | ||||
| Intercept of Cognitive Ability ~ Slope (pre-70y) of Cognitive Ability | .445 | 7.158 | <.001 | |
| Slope (post-70y) of Cognitive Ability | −.564 | −12.841 | <.001 | |
| Slope (pre-70y) of Cognitive Ability ~ Slope (post-70y) of Cognitive Ability | .154 | 0.270 | .787 | |
| Intercept of Functional limitation ~ Slope of Functional limitation | .429 | 17.132 | <.001 | |
| Correlations, Across Processes | ||||
| Intercept of Cognitive Ability ~ Intercept of Functional limitation | −.300 | −18.420 | <.001 | |
| Slope of Functional limitation | −.310 | −8.165 | <.001 | |
| Intercept of Functional limitation ~ Slope (pre-70y) of Cognitive Ability | −.090 | −1.759 | .079 | |
| Slope (post-70y) of Cognitive Ability | .153 | 3.535 | <.001 | |
| Slope of Functional limitation ~ Slope (pre-70y) of Cognitive Ability | −.489 | −5.353 | <.001 | |
| Slope (post-70y) of Cognitive Ability | .196 | 2.289 | .022 | |
| Residual of Cognitive Ability ~ Residual of Functional limitation | .007 | 2.238 | .025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handing, E.P.; Jiao, Y.; Aichele, S. Age-Related Trajectories of General Fluid Cognition and Functional Decline in the Health and Retirement Study: A Bivariate Latent Growth Analysis. J. Intell. 2023, 11, 65. https://doi.org/10.3390/jintelligence11040065
Handing EP, Jiao Y, Aichele S. Age-Related Trajectories of General Fluid Cognition and Functional Decline in the Health and Retirement Study: A Bivariate Latent Growth Analysis. Journal of Intelligence. 2023; 11(4):65. https://doi.org/10.3390/jintelligence11040065
Chicago/Turabian StyleHanding, Elizabeth P., Yuqin Jiao, and Stephen Aichele. 2023. "Age-Related Trajectories of General Fluid Cognition and Functional Decline in the Health and Retirement Study: A Bivariate Latent Growth Analysis" Journal of Intelligence 11, no. 4: 65. https://doi.org/10.3390/jintelligence11040065
APA StyleHanding, E. P., Jiao, Y., & Aichele, S. (2023). Age-Related Trajectories of General Fluid Cognition and Functional Decline in the Health and Retirement Study: A Bivariate Latent Growth Analysis. Journal of Intelligence, 11(4), 65. https://doi.org/10.3390/jintelligence11040065





