Reflections and New Perspectives on Face Cognition as a Specific Socio-Cognitive Ability
Abstract
1. Socio-Cognitive Abilities
Face Cognition as a Basic Socio-Cognitive Ability
2. How Special Are Faces?
Individual Differences in Face Cognition
3. On Accuracy- and Speed-Related Face Cognition Abilities
3.1. Theoretical Accounts of Face Specificity Versus Generality in Accuracy and Speed
4. Conditions of Face Specificity
4.1. No Specificity in Speed: Studies Reporting a Unitary Ability Structure in Easy Tasks
4.2. Specificity in Speed: Studies Reporting a Differentiated Ability Structure in Easy Tasks
4.3. What Differentiates the Studies?
5. Proposal for an Explanatory Model of Face Cognition Specificity
6. Neurocognitive Mechanisms Underlying Specificity in Accuracy and Speed
7. Future Directions
Proposed Differential Functions of Accuracy and Speed in FC
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | A nomological net is a representation of a construct, along with its measurements in which the relationships between the measured variables and their corresponding construct, as well as the relationships between different, more or less similar constructs is being summarized. |
| 2 | We refer to mechanisms as the interplay of psychological (or other, for example neural or genetic) processes, reflecting the laws underlying the pattern of interrelations between observed variables. In our use of the term, mechanisms are not necessarily identical with the cause of a phenomenon, but represent a common set of rules underlying the phenomenon. |
References
- Adolphs, Ralph. 2009. The social brain: Neural basis of social knowledge. Annual Review of Psychology 60: 693–716. [Google Scholar] [CrossRef] [PubMed]
- Bargh, John A. 1982. Attention and automaticity in the processing of self-relevant information. Journal of Personality and Social Psychology 43: 425–36. [Google Scholar] [CrossRef]
- Barton, Jason J. S. 2008. Structure and function in acquired prosopagnosia: Lessons from a series of 10 patients with brain damage. Journal of Neuropsychology 2, Pt 1: 197–225. [Google Scholar] [CrossRef] [PubMed]
- Becker, Nicolas, Florian Schmitz, Anja Göritz, and Frank Spinath. 2016. Sometimes more is better, and sometimes less is better: Task complexity moderates the response time accuracy correlation. Journal of Intelligence 4: 11. [Google Scholar] [CrossRef]
- Bentin, Shlomo, Truett Allison, Aina Puce, Erik Perez, and Gregory McCarthy. 1996. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience 8: 551–65. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, Michal, and Galit Yovel. 2015. Two neural pathways of face processing: A critical evaluation of current models. Neuroscience and Biobehavioral Reviews 55: 536–46. [Google Scholar] [CrossRef]
- Bodamer, Joachim. 1947. Die Prosop-Agnosie. Archiv Fuer Psychiatrie Und Nervenkrankheiten 179: 6–53. [Google Scholar] [CrossRef]
- Bruce, Vicky, and Andrew Young. 1986. Understanding face recognition. Journal of Clinical Ultrasound 3: 304–6. [Google Scholar] [CrossRef]
- Bukach, Cindy M., Isabel Gauthier, and Michael J. Tarr. 2006. Beyond faces and modularity: The power of an expertise framework. Trends in Cognitive Sciences 10: 159–66. [Google Scholar] [CrossRef]
- Burns, Edwin J., Taylor Arnold, and Cindy M. Bukach. 2019. P-curving the fusiform face area: Meta-analyses support the expertise hypothesis. Neuroscience & Biobehavioral Reviews 104: 209–21. [Google Scholar] [CrossRef]
- Calder, Andrew J., and Andrew W. Young. 2005. Understanding the recognition of facial identity and facial expression. Nature Reviews Neuroscience 6: 641–51. [Google Scholar] [CrossRef] [PubMed]
- Calder, Andrew J., Jill Keane, Andrew W. Young, and Michael Dean. 2000. Configural information in facial expression perception. Journal of Experimental Psychology: Human Perception and Performance 26: 527–51. [Google Scholar] [CrossRef]
- Carroll, John B. 1978. How shall we study individual differences in cognitive abilities?—Methodological and theoretical perspectives. Intelligence 2: 87–115. [Google Scholar] [CrossRef]
- Carroll, John B. 1993. Human Cognitive Abilities: A Survey of Factor—Analytic Studies. Cambridge: Cambridge University Press. [Google Scholar]
- Ćepulić, Dominik-Borna, Florian Schmitz, and Andrea Hildebrandt. 2020. Do time-on-task effects reveal face specificity in object cognition? Journal of Cognitive Psychology 32: 423–41. [Google Scholar] [CrossRef]
- Ćepulić, Dominik-Borna, Oliver Wilhelm, Werner Sommer, and Andrea Hildebrandt. 2018. All categories are equal, but some categories are more equal than others: The psychometric structure of object and face cognition. Journal of Experimental Psychology: Learning Memory and Cognition 44: 1254–68. [Google Scholar] [CrossRef]
- Chernorizov, Alexander M., Zhong-qing Jiang, Anastasia V. Petrakova, and Yuri P. Zinchenko. 2016. Face cognition in humans: Psychophysiological, developmental, and cross-cultural aspects. Psychology in Russia: State of the Art 9: 37–50. [Google Scholar] [CrossRef]
- Cheung, Olivia S., and Isabel Gauthier. 2010. Selective Interference on the Holistic Processing of Faces in Working Memory. Journal of Experimental Psychology: Human Perception and Performance 36: 448–61. [Google Scholar] [CrossRef]
- Cosmides, Leda, and John Tooby. 1994. Beyond intuition and instinct blindness: Toward an evolutionarily rigorous cognitive science. Cognition 50: 41–77. [Google Scholar] [CrossRef]
- Cross, John F., Jane Cross, and James Daly. 1971. Sex, race, age, and beauty as factors in recognition of faces. Perception & Psychophysics 10: 393–96. [Google Scholar] [CrossRef]
- Danthiir, Vanessa, Richard D. Roberts, Ralf Schulze, and Oliver Wilhelm. 2005. Mental speed: On frameworks, paradigms, and a platform for the future. Handbook of Understanding and Measuring Intelligence, 27–46. [Google Scholar] [CrossRef]
- Diamond, Rhea, and Susan Carey. 1986. Why Faces Are and Are Not Special. An Effect of Expertise. Journal of Experimental Psychology: General 115: 107–17. [Google Scholar] [CrossRef]
- Donders, Frans C. 1969. On the speed of mental processes: Attention and Performance II. Acta Psychologica 30: 412–30. First published 1868. [Google Scholar] [CrossRef]
- Dowdle, Logan T., Geoffrey Ghose, Kamil Ugurbil, Essa Yacoub, and Luca Vizioli. 2021. Clarifying the role of higher-level cortices in resolving perceptual ambiguity using ultra high field fMRI. NeuroImage 227: 117654. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Julien, and Habib Benali. 2005. Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology 15: 161–67. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, Brad, and Galit Yovel. 2015. A Revised Neural Framework for Face Processing. Annual Review of Vision Science 1: 393–416. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, Brad, and Ken Nakayama. 2006. The Cambridge Face Memory Test: Results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia 44: 576–85. [Google Scholar] [CrossRef]
- Dunbar, Robin I. M. 1998. The social brain hypothesis. Evolutionary Anthropology: Issues, News, and Reviews 6: 178–90. [Google Scholar] [CrossRef]
- Dunbar, Robin I. M. 2003. The social brain: Mind, language, and society in evolutionary perspective. Annual Review of Anthropology 32: 163–81. [Google Scholar] [CrossRef]
- Dunbar, Robin I. M., and Susanne Shultz. 2007. Evolution in the social brain. Science 317: 1344–47. [Google Scholar] [CrossRef] [PubMed]
- Dunst, Beate, Mathias Benedek, Emanuel Jauk, Sabine Bergner, Karl Koschutnig, Markus Sommer, Anja Ischebeck, Birgit Spinath, Martin Arendasy, Markus Bühner, and et al. 2014. Neural efficiency as a function of task demands. Intelligence 42: 22–30. [Google Scholar] [CrossRef]
- Eimer, Martin. 2000. The face-specific N170 component reflects late stages in the structural encoding of faces. NeuroReport 11: 2319–24. [Google Scholar] [CrossRef]
- Evans, Jonathan S. B. T., and Keith E. Stanovich. 2013. Dual-process theories of higher cognition. Perspectives on Psychological Science 8: 223–41. [Google Scholar] [CrossRef]
- Farah, Martha J., James W. Tanaka, and H. Maxwell Drain. 1995. What causes the face inversion effect? Journal of Experimental Psychology: Human Perception and Performance 21: 628–34. [Google Scholar] [CrossRef]
- Farah, Martha J., Kevin D. Wilson, Maxwell Drain, and James. N. Tanaka. 1998. What is “Special” about face perception? Psychological Review 105: 482–98. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, Dawn P., and Kevin S. McGrew. 1998. Interpreting intelligence tests from contemporary Gf-Gc Theory: Joint confirmatory factor Aaalysis of the WJ-R and KAIT in a non-white sample. Journal of School Psychology 36: 151–82. [Google Scholar] [CrossRef]
- Flanagan, Dawn P., and Shauna G. Dixon. 2014. The Cattell-Horn-Carroll theory of cognitive abilities. In Encyclopedia of Special Education. Edited by Cecil R. Reynolds, Kimberly J. Vannest and Elaine Fletcher-Janzen. New York: Wiley Online Library. [Google Scholar] [CrossRef]
- Garrido, Lúcia, Frank Eisner, Carolyn McGettigan, Lauren Stewart, Disa Sauter, John R. Hanley, Stefan R. Schweinberger, Jason. D. Warren, and Brad Duchaine. 2009. Developmental phonagnosia: A selective deficit of vocal identity recognition. Neuropsychologia 47: 123–31. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, Isabel. 2018. Domain-specific and domain-general individual differences in visual object recognition. Current Directions in Psychological Science 27: 97–102. [Google Scholar] [CrossRef]
- Gauthier, Isabel, and Cindy Bukach. 2007. Should we reject the expertise hypothesis? Cognition 103: 322–30. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, Isabel, Kim M. Curby, Pawel Skudlarski, and Russell A. Epstein. 2005. Individual differences in FFA activity suggest independent processing at different spatial scales. Cognitive Affective and Behavioral Neuroscience 5: 222–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gauthier, Isabel, R. W. McGugin, J. J. Richler, Grit Herzmann, Magen Speegle, and Ana E. Van Gulick. 2014. Experience moderates overlap between object and face recognition, suggesting a common ability. Journal of Vision 14: 7. [Google Scholar] [CrossRef]
- Goldhammer, Frank, Johannes Naumann, Annette Stelter, Krisztina Tóth, Heiko Rölke, and Eckhard Klieme. 2014. The time on task effect in reading and problem solving is moderated by task difficulty and skill: Insights from a computer-based large-scale assessment. Journal of Educational Psychology 106: 608–26. [Google Scholar] [CrossRef]
- Gunnery, Sarah D., Judith A. Hall, and Mollie A. Ruben. 2013. The deliberate duchenne smile: Individual differences in expressive control. Journal of Nonverbal Behavior 37: 29–41. [Google Scholar] [CrossRef]
- Guttman, Louis. 1954. A new approach to factor analysis: The Radex. In Mathematical Thinking in the Social Sciences. Edited by Paul F. Lazarsfeld. New York: Free Press, pp. 258–348. [Google Scholar]
- Hammar, Åsa. 2012. Automatic information processing. In Encyclopedia of the Sciences of Learning. Edited by Norbert M. Seel. Berlin/Heidelberg: Springer. [Google Scholar] [CrossRef]
- Happé, Francesca, Jennifer L. Cook, and Geoffrey Bird. 2017. The structure of social cognition: In (ter) dependence of sociocognitive processes. Annual Review of Psychology 68: 243–67. [Google Scholar] [CrossRef]
- Haxby, James V., Elizabeth A. Hoffman, and Ida Gobbini. 2000. The distributed human neural system for face perception. Trends in Cognitive Sciences 4: 223–33. [Google Scholar] [CrossRef]
- Herzmann, Grit, Olga Kunina, Werner Sommer, and Oliver Wilhelm. 2010. Individual differences in face cognition: Brain-behavior relationships. Journal of Cognitive Neuroscience 22: 571–89. [Google Scholar] [CrossRef]
- Herzmann, Grit, Vanessa Danthiir, Annekathrin Schacht, Werner Sommer, and Oliver Wilhelm. 2008. Toward a comprehensive test battery for face cognition: Assessment of the tasks. Behavior Research Methods 40: 840–57. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, Andrea, Annekathrin Schacht, Werner Sommer, and Oliver Wilhelm. 2012. Measuring the speed of recognising facially expressed emotions. Cognition & Emotion 26: 650–66. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Astrid Kiy, Martin Reuter, Werner Sommer, and Oliver Wilhelm. 2016. Face and emotion expression processing and the serotonin transporter polymorphism 5-HTTLPR/rs22531. Genes Brain and Behavior 15: 453–64. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Oliver Wilhelm, Florian Schmiedek, Grit Herzmann, and Werner Sommer. 2011. On the specificity of face cognition compared with general cognitive functioning across adult age. Psychology and Aging 26: 701–15. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Oliver Wilhelm, Grit Herzmann, and Werner Sommer. 2013. Face and object cognition across adult age. Psychology and Aging 28: 243–48. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Sally Olderbak, and Oliver Wilhelm. 2015a. Facial emotion expression, Individual differences in. International Encyclopedia of the Social & Behavioral Sciences, 667–75. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Werner Sommer, Annekathrin Schacht, and Oliver Wilhelm. 2015b. Perceiving and remembering emotional facial expressions—A basic facet of emotional intelligence. Intelligence 50: 52–67. [Google Scholar] [CrossRef]
- Hildebrandt, Andrea, Werner Sommer, Grit Herzmann, and Oliver Wilhelm. 2010. Structural Invariance and Age-Related Performance Differences in Face Cognition. Psychology and Aging 25: 794–810. [Google Scholar] [CrossRef] [PubMed]
- Horn, John L., and Scott M. Hofer. 1992. Major abilities and development in the adult period. In Intellectual Development. Edited by Robert J. Sternberg and Cynthia A. Berg. Cambridge: Cambridge University Press, pp. 44–99. [Google Scholar]
- Kaltwasser, Laura, Andrea Hildebrandt, Guillermo Recio, Oliver Wilhelm, and Werner Sommer. 2014. Neurocognitive mechanisms of individual differences in face cognition: A replication and extension. Cognitive Affective and Behavioral Neuroscience 14: 861–78. [Google Scholar] [CrossRef]
- Kanwisher, Nancy. 2000. Domain specificity in face perception. Nature Neuroscience 3: 759–63. [Google Scholar] [CrossRef]
- Kanwisher, Nancy. 2010. Functional specificity in the human brain: A window into the functional architecture of the mind. Proceedings of the National Academy of Sciences of the United States of America 107: 11163–70. [Google Scholar] [CrossRef]
- Kanwisher, Nancy, and Galit Yovel. 2006. The fusiform face area: A cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B: Biological Sciences 361: 2109–28. [Google Scholar] [CrossRef]
- Kanwisher, Nancy, Josh McDermott, and Marvin Chun. 1997. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience 17: 4302–11. [Google Scholar] [CrossRef]
- Karimi-Rouzbahani, Hamid, Farzad Ramezani, Alexandra Woolgar, Aninaq Rich, and Masoud Ghodrati. 2021. Perceptual difficulty modulates the direction of information flow in familiar face recognition. NeuroImage 233: 117896. [Google Scholar] [CrossRef]
- Karmiloff-Smith, Annette. 1992. Nature, nurture and PDP: Preposterous developmental postulates? Connection Science 4: 253–269. [Google Scholar] [CrossRef]
- Karmiloff-Smith, Annette, Edward Klima, Ursula Bellugi, Julia Grant, and Simon Baron-Cohen. 1995. Is there a social module? Language, face processing, and theory of mind in individuals with Williams syndrome. Journal of Cognitive Neuroscience 7: 196–208. [Google Scholar] [CrossRef]
- Kaufmann, Jürgen M., Stefan R. Schweinberger, and A. Mike Burton. 2009. N250 ERP correlates of the acquisition of face representations across different images. Journal of Cognitive Neuroscience 21: 625–41. [Google Scholar] [CrossRef]
- Keith, Timothy Z., and Matthew R. Reynolds. 2010. Cattell–Horn–Carroll abilities and cognitive tests: What we’ve learned from 20 years of research. Psychology in the Schools 47: 635–50. [Google Scholar]
- Kiy, Astrid, Oliver Wilhelm, Andrea Hildebrandt, Martin Reuter, and Werner Sommer. 2013. On the genetic basis of face cognition and its relation to fluid cognitive abilities. Genes Brain and Behavior 12: 438–45. [Google Scholar] [CrossRef]
- LaBerge, David, and S. Jay Samuels. 1974. Toward a theory of automatic information processing in reading. Cognitive Psychology 6: 293–323. [Google Scholar] [CrossRef]
- Liu, Xinyang, Andrea Hildebrandt, Guillermo Recio, Werner Sommer, Xinxia Cai, and Oliver Wilhelm. 2017. Individual differences in the speed of facial emotion recognition show little specificity but are strongly related with general mental speed: Psychometric, neural and genetic evidence. Frontiers in Behavioral Neuroscience 11. [Google Scholar] [CrossRef]
- Liu, Xinyang, Andrea Hildebrandt, Kristina Meyer, Werner Sommer, and Changsong Zhou. 2020. Patterns of individual differences in fiber tract integrity of the face processing brain network support neurofunctional models. NeuroImage 204: 116–229. [Google Scholar] [CrossRef]
- McGrew, Kevin S. 2009. CHC theory and the human cognitive abilities project: Standing on the shoulders of the giants of psychometric intelligence research. Intelligence 37: 1–10. [Google Scholar] [CrossRef]
- McGugin, Rankin W., Ana E. Van Gulick, and Isabel Gauthier. 2016. Cortical thickness in fusiform face area predicts face and object recognition performance. Journal of Cognitive Neuroscience 28: 282–94. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Kristina, Benjamín Garzón, Martin Lövdén, and Andrea Hildebrandt. 2019a. Are global and specific interindividual differences in cortical thickness associated with facets of cognitive abilities, including face cognition? Royal Society Open Science 6. [Google Scholar] [CrossRef]
- Meyer, Kristina, Florian Schmitz, Oliver Wilhelm, and Andrea Hildebrandt. 2019b. Perceiving faces: Too much, too fast?—Face specificity in response caution. Journal of Experimental Psychology: Human Perception and Performance 45: 16–38. [Google Scholar] [CrossRef]
- Meyer, Kristina, Hadiseh Nowparast Rostami, Guang Ouyang, Stefan Debener, Werner Sommer, and Andrea Hildebrandt. 2021. Mechanisms of face specificity–differentiating speed and accuracy in face cognition by event-related potentials of central processing. Cortex 134: 114–33. [Google Scholar] [CrossRef] [PubMed]
- Morton, John, and Mark H. Johnson. 1991. CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review 98: 164–81. [Google Scholar] [CrossRef] [PubMed]
- Motes, Michael A., Rafael Malach, and Maria Kozhevnikov. 2008. Object-processing neural efficiency differentiates object from spatial visualizers. NeuroReport 19: 1727–31. [Google Scholar] [CrossRef]
- Neta, Maital, and Paul J. Whalen. 2011. Individual differences in neural activity during a facial expression vs. identity working memory task. NeuroImage 56: 1685–92. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, Aljoscha C., and Andreas Fink. 2009. Intelligence and neural efficiency. Neuroscience and Biobehavioral Reviews 33: 1004–23. [Google Scholar] [CrossRef] [PubMed]
- Neumann, Markus F., and Stefan R. Schweinberger. 2008. N250r and N400 ERP correlates of immediate famous face repetition are independent of perceptual load. Brain Research 1239: 181–90. [Google Scholar] [CrossRef] [PubMed]
- Neuner, Frank, and Stefan R. Schweinberger. 2000. Neuropsychological impairments in the recognition of faces, voices, and personal names. Brain and Cognition 44: 342–66. [Google Scholar] [CrossRef]
- Nowparast Rostami, Hadiseh, Werner Sommer, C. Zhou, Oliver Wilhelm, and Andrea Hildebrandt. 2017. Structural encoding processes contribute to individual differences in face and object cognition: Inferences from psychometric test performance and event-related brain potentials. Cortex 95: 192–210. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, Maureen, and Joy P. Guilford. 1975. Six factors of behavioral cognition: Understanding other people. Journal of Educational Measurement 12: 255–71. [Google Scholar] [CrossRef]
- Oberauer, Klaus, Heinz-Martin Süß, Oliver Wilhelm, and Werner W. Wittman. 2003. The multiple faces of working memory: Storage, processing, supervision, and coordination. Intelligence 31: 167–93. [Google Scholar] [CrossRef]
- Oberauer, Klaus, Heinz-Martin Süß, Ralf Schulze, Oliver Wilhelm, and Werner W. Wittmann. 2000. Working memory capacity—Facets of a cognitive ability construct. Personality and Individual Differences 29: 1017–45. [Google Scholar] [CrossRef]
- Oberauer, Klaus, Oliver Wilhelm, and Florian Schmiedek. 2005. Experimental strategies in multivariate research. In Multivariate Research Strategies: Festschrift in Honor of Werner W. Wittmann. Edited by André Beauducel, Bernhard Biehl, Michael Bosnjak, Wolgang Conrad, Gisela Schönberger and Dietrich Wagener. Düren: Shaker Verlag. [Google Scholar]
- Olderbak, Sally, Andrea Hildebrandt, and Oliver Wilhelm. 2015. Examining age-related shared variance between face cognition, vision, and self-reported physical health: A test of the common cause hypothesis for social cognition. Frontiers in Psychology 6: 1–17. [Google Scholar] [CrossRef]
- Olderbak, Sally, Oliver Wilhelm, Andrea Hildebrandt, and Jordi Quoidbach. 2019. Sex differences in facial emotion perception ability across the lifespan. Cognition and Emotion 33: 579–88. [Google Scholar] [CrossRef]
- Ouyang, Guang, Andrea Hildebrandt, Florian Schmitz, and Christoph S. Herrmann. 2020. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. NeuroImage 205: 116304. [Google Scholar] [CrossRef]
- Quinones Sanchez, Juan F., Xinyang Liu, Changsong Zhou, and Andrea Hildebrandt. 2021. Nature and Nurture Shape Structural Connectivity in the Face Processing Brain Network. NeuroImage 229: 117736. [Google Scholar] [CrossRef]
- Ratcliff, Roger. 1978. A theory of memory retrieval. Psychological Review 85: 59–108. [Google Scholar] [CrossRef]
- Reingold, Eyal M., Neil Charness, Richard S. Schultetus, and Dave M. Stampe. 2001. Perceptual automaticity in expert chess players: Parallel encoding of chess relations. Psychonomic Bulletin & Review 8: 504–10. [Google Scholar] [CrossRef]
- Richler, Jennifer J., and Isabel Gauthier. 2014. A Meta-Analysis and Review of Holistic Face Processing. Psychological Bulletin 140: 1281–302. [Google Scholar] [CrossRef] [PubMed]
- Roberts, Richard D., and Lazar Stankov. 1999. Individual differences in speed of mental processing and human cognitive abilities: Toward a taxonomic model. Learning and Individual Differences 11: 1–120. [Google Scholar] [CrossRef]
- Rossion, Bruno, Roberto Caldara, Mohamed Seghier, Anne-Marie Schuller, Francois Lazeyras, and Eugene Mayer. 2003. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain 126: 2381–95. [Google Scholar] [CrossRef] [PubMed]
- Rotshtein, Pia, Joy J. Geng, Jon Driver, and Raymond J. Dolan. 2007. Role of features and second-order spatial relations in face discrimination, face recognition, and individual face skills: Behavioral and functional magnetic resonance imaging data. Journal of Cognitive Neuroscience 19: 1435–52. [Google Scholar] [CrossRef]
- Rypma, Bart, Jeffrey S. Berger, Vivek Prabhakaran, Benjamin Martin Bly, Daniel Y. Kimberg, Bharat B. Biswal, and Mark D’Esposito. 2006. Neural correlates of cognitive efficiency. NeuroImage 33: 969–79. [Google Scholar] [CrossRef]
- Said, Christopher P., Christopher D. Moore, Andrew D. Engell, Alexander Todorov, and James V. Haxby. 2010. Distributed representations of dynamic facial expressions in the superior temporal sulcus. Journal of Vision 10: 11. [Google Scholar] [CrossRef]
- Schneider, W. Joel, and Kevin S. McGrew. 2012. The Cattell-Horn-Carroll model of intelligence. In Contemporary Intellectual Assessment: Theories Tests and Issues. Edited by Dawn P. Flanagan and Patti L. Harrison. New York: The Guilford Press. [Google Scholar]
- Schulze, Ralf. 2005. Modeling structures of intelligence. In Handbook of Understanding and Measuring Intelligence. Edited by Oliver Wilhelm and Randall W. Engle. Thousand Oaks: Sage, pp. 241–63. [Google Scholar]
- Schwartz, Linoy, and Galit Yovel. 2016. The roles of perceptual and conceptual information in face recognition. Journal of Experimental Psychology: General 145: 1493. [Google Scholar] [CrossRef]
- Schwartz, Linoy, and Galit Yovel. 2019. Learning faces as concepts rather than percepts improves face recognition. Journal of Experimental Psychology: Learning Memory and Cognition 45: 1733. [Google Scholar] [CrossRef]
- Schweinberger, Stefan R., and A. Mike Burton. 2011. Person perception 25 years after Bruce and Young (1986): An introduction. British Journal of Psychology 102: 695–703. [Google Scholar] [CrossRef]
- Schweinberger, Stefan R., and Dana Schneider. 2014. Person perception and social cognition. Psychologische Rundschau 65: 212–26. [Google Scholar] [CrossRef]
- Shakeshaft, Nicholas G., and Robert Plomin. 2015. Genetic specificity of face recognition. Proceedings of the National Academy of Sciences 112: 12887–92. [Google Scholar] [CrossRef]
- Shallice, Tim. 1988. From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press. [Google Scholar]
- Skuse, David H., Adriana Lori, Joseph F. Cubells, Irene Lee, Karen N. Conneely, Kaija Puura, Terho Lehtimäki, Elisabeth B. Binder, and Larry J. Young. 2014. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proceedings of the National Academy of Sciences of the United States of America 111: 1987–92. [Google Scholar] [CrossRef] [PubMed]
- Sommer, Werner, Andrea Hildebrandt, Ologa Kunina-Habenicht, Annekathrin Schacht, and Oliver Wilhelm. 2013. Sex differences in face cognition. Acta Psychologica 142: 62–73. [Google Scholar] [CrossRef]
- Sperling, George, and Barbara A. Dosher. 1986. Strategy and optimization in human information processing. In Handbook of Perception and Human Performance. Edited by Kenneth R. Boff, Lloyd Kaufman and James P. Thomas. New York: Wiley, vol. 1, chp. 2. pp. 1–65. [Google Scholar]
- Stanovich, Keith E., Richard F. West, and Maggie E. Toplak. 2011. The complexity of developmental predictions from dual process models. Developmental Review 31: 103–18. [Google Scholar] [CrossRef]
- Sternberg, Saul. 1969. The discovery of processing stages: Extensions of Donders’ method. Acta Psychologica 30: 276–315. [Google Scholar] [CrossRef]
- Süß, Heinz-Martin, and Andre Beauducel. 2005. Faceted models of intelligence. In Understanding and Measuring Intelligence. Edited by Oliver Wilhelm and Randall W. Engle. Thousand Oaks: Sage, pp. 313–22. [Google Scholar]
- Tanaka, James W., and Diana Simonyi. 2016. The “Parts and Wholes” of face recognition: A review of the literature. Quarterly Journal of Experimental Psychology 69: 1876–89. [Google Scholar] [CrossRef]
- Tanaka, James W., and Iris Gordon. 2011. Features, configuration, and holistic face processing. In The Oxford Handbook of Face Perception. Edited by Gillian Rhodes, Andrew Calder, Mark Johnson and James V. Haxby. Oxford: Oxford University Press, pp. 177–94. [Google Scholar] [CrossRef]
- Tanaka, James W., and Isabel Gauthier. 1997. Expertise in object and face recognition. Psychology of Learning and Motivation, 83–125. [Google Scholar] [CrossRef]
- Tanaka, James W., and Martha J. Farah. 1993. Parts and wholes in face recognition. The Quarterly Journal of Experimental Psychology Section A 46: 225–45. [Google Scholar] [CrossRef]
- Thorndike, Edward L. 1920. Intelligence and its uses. Harper’s Magazine 140: 227–35. [Google Scholar]
- Turano, Maria T., and Maria P. Viggiano. 2017. The relationship between face recognition ability and socioemotional functioning throughout adulthood. Aging Neuropsychology and Cognition 24: 613–30. [Google Scholar] [CrossRef]
- Valentine, Tim. 1988. Upside-down faces: A review of the effect of inversion upon face recognition. British Journal of Psychology 79: 471–91. [Google Scholar] [CrossRef]
- Verhallen, Roeland J., Jenny M. Bosten, Patrick T. Goodbourn, Adam J. Lawrance-Owen, Gary Bargary, and John D. Mollon. 2017. The Oxytocin receptor gene (OXTR) and face recognition. Psychological Science 28: 47–55. [Google Scholar] [CrossRef]
- Vernon, Philip E. 1933. Some characteristics of the good judge of personality. Journal of Social Psychology 4: 42–57. [Google Scholar] [CrossRef]
- Voss, Andreas, Klaus Rothermund, and Jochen Voss. 2004. Interpreting the parameters of the diffusion model: An empirical validation. Memory & Cognition 32: 1206–20. [Google Scholar]
- Wagenmakers, Eric-Jan, Han L. J. Van Der Maas, and Raoul P. P. P. Grasman. 2007. An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin and Review 14: 3–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Yin, Athanasia Metoki, David V. Smith, John D. Medaglia, Yinyin Zang, Susan Benear, Haroon Popal, Ying Lin, and Ingrid R. Olson. 2020. Multimodal mapping of the face connectome. Nature Human Behaviour 4: 397–411. [Google Scholar] [CrossRef]
- Wechsler, D. 1958. The Measurement and Appraisal of Adult Intelligence, 4th ed. Baltimore: Williams & Wilkins Co. [Google Scholar] [CrossRef]
- Wilhelm, Oliver, Andrea Hildebrandt, Karsten Manske, Annekathrin Schacht, and Werner Sommer. 2014. Test battery for measuring the perception and recognition of facial expressions of emotion. Frontiers in Psychology 5: 1–23. [Google Scholar] [CrossRef]
- Wilhelm, Oliver, Grit Herzmann, Olga Kunina, and Werner Sommer. 2007. Face cognition: A set of distinct mental Abilities. Nature Precedings 1. [Google Scholar] [CrossRef]
- Wilhelm, Oliver, Grit Herzmann, Olga Kunina, Vanessa Danthiir, Annekathrin Schacht, and Werner Sommer. 2010. Individual differences in perceiving and recognizing faces-One element of social cognition. Journal of Personality and Social Psychology 99: 530–48. [Google Scholar] [CrossRef]
- Wilmer, Jeremy B. 2017. Individual differences in face recognition: A decade of discovery. Current Directions in Psychological Science 26: 225–30. [Google Scholar] [CrossRef]
- Wilmer, Jeremy B., Laura Germine, Christopher F. Chabris, Garga Chatterjee, Margaret Gerbasi, and Ken Nakayama. 2012. Capturing specific abilities as a window into human individuality: The example of face recognition. Cognitive Neuropsychology 29: 360–92. [Google Scholar] [CrossRef]
- Wilmer, Jeremy B., Laura Germine, Christopher F. Chabris, Garga Chatterjee, Mark Williams, Eric Loken, Ken Nakayama, and Bradley Duchaine. 2010. Human face recognition ability is specific and highly heritable. Proceedings of the National Academy of Sciences of the United States of America 107: 5238–41. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, Jeremy B., Laura T. Germine, and Ken Nakayama. 2014. Face recognition: A model specific ability. Frontiers in Human Neuroscience 8: 1–5. [Google Scholar] [CrossRef]
- Young, Andrew W., Deborah Hellawell, and Dennis C. Hay. 1987. Configurational information in face perception. Perception 16: 747–59. [Google Scholar] [CrossRef]
- Young, Andrew W., Freda Newcombe, Edward H. F. de Haan, Marian Small, and Dennis C. Hay. 1993. Face perception after brain injury. Brain 116: 941–59. [Google Scholar] [CrossRef]
- Yovel, Galit, Jeremy B. Wilmer, and Bradley Duchaine. 2014. What can individual differences reveal about face processing? Frontiers in Human Neuroscience 8: 1–9. [Google Scholar] [CrossRef]
- Zhang, Yang, and Yue Wang. 2007. Neural plasticity in speech acquisition and learning. Bilingualism 10: 147–60. [Google Scholar] [CrossRef]
- Zhu, Qi, Yiying Song, Siyua Hu, Xiaobai Li, Moqian Tian, Zonglei Zhen, Qi Dong, Nany Kanwisher, and Jia Liu. 2010. Heritability of the specific cognitive ability of face perception. Current Biology 20: 137–42. [Google Scholar] [CrossRef]
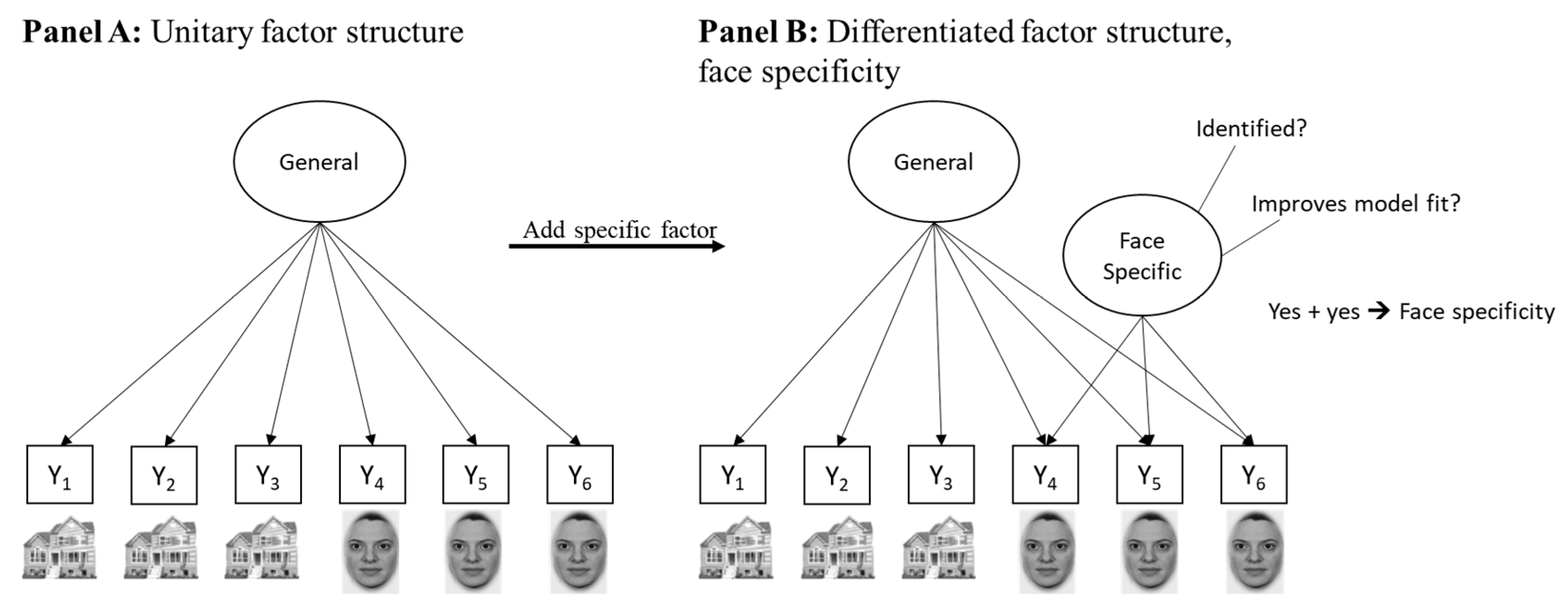


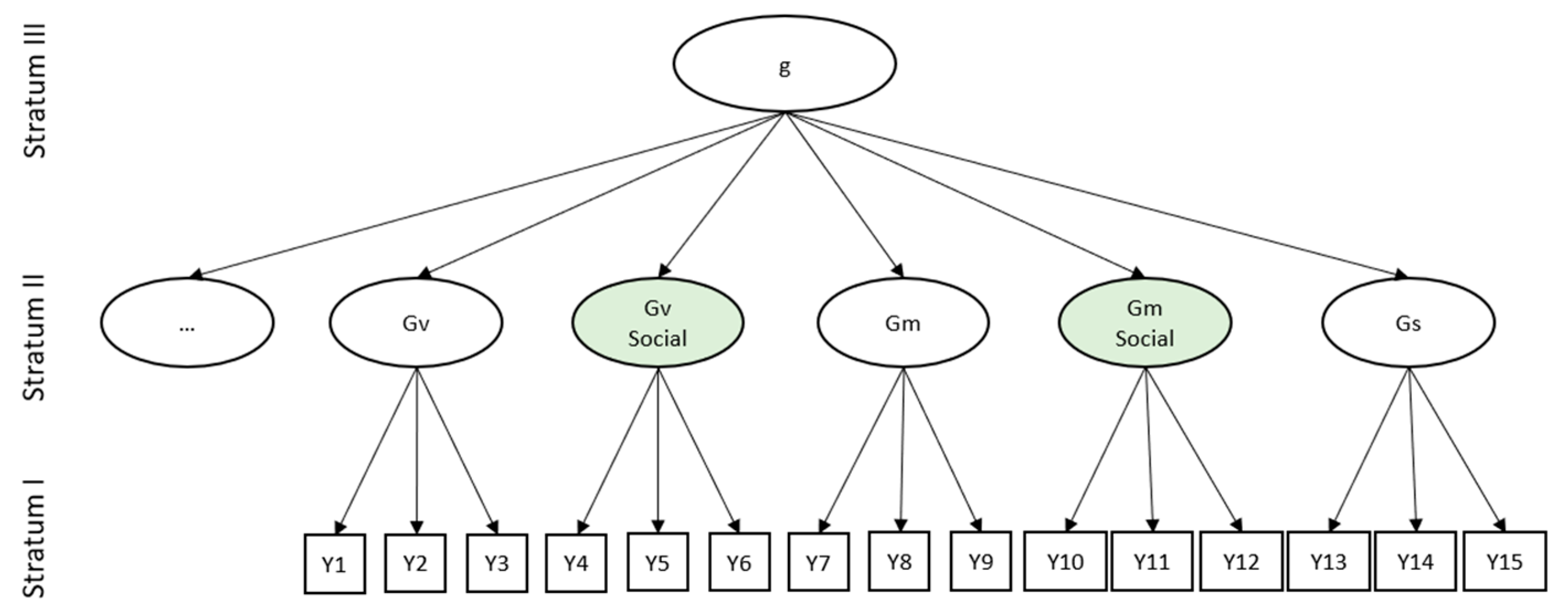
| Task Name | Description | Accuracy (SD) | |
|---|---|---|---|
| Easy Memory | Delayed non-matching to sample | Two faces/houses are presented simultaneously. Participants decide which of the two faces/houses does not match a previously memorized sample stimulus. | 97% (4%) |
| Easy Perception | Simultaneous matching of morphed faces/houses | Two faces/houses are presented simultaneously, each morphed from the same two parent faces. Participants decide whether the stimuli are the same or different. In the same condition, morphs are derived in equal parts from parent stimuli, in different condition at a ratio of 20/80. | 93% (6%) |
| Difficult Memory | Eyewitness testimony | Recognition of distractor faces from a previous task, which had not been required to memorize. In each trial, two faces are presented on the screen, one of which is the target, familiar from a previous task. | 65% (11%) |
| Difficult Perception | Simultaneous matching of spatially manipulated faces | Two faces/houses are presented simultaneously and participants decide whether they are the same or different. In the different condition, the spatial relationship between certain features are different; in 50% of the trials, stimuli are presented upside down. | 69% (11%) |
| Study | Model | Tasks Used in Model | Dependent Variable | Specific Face Factor in Easy Tasks |
|---|---|---|---|---|
| Hildebrandt et al. (2013) | 1 | Hard: PW, SM, FR, AC 1, DR, EY Easy: RS, DNMS, VP, UH, M | Speed, accuracy | no |
| Nowparast Rostami et al. (2017) | 1 | Hard: SM, FR, AC 1, DR, EY Easy: DNMS, M, RS, V | Speed | no |
| Ćepulić et al. (2018) | 1 | Easy: L/R 1 Hard: L/R 1 | Speed | yes |
| Meyer et al. (2021) | 1 | Easy: L/R 1 | Speed | yes |
| 2 | Easy: DNMS | Speed | yes | |
| 3 | Easy: M | Speed | yes | |
| 4 | Easy: RS | Speed | yes | |
| 5 | Easy: V | Speed | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, K.; Sommer, W.; Hildebrandt, A. Reflections and New Perspectives on Face Cognition as a Specific Socio-Cognitive Ability. J. Intell. 2021, 9, 30. https://doi.org/10.3390/jintelligence9020030
Meyer K, Sommer W, Hildebrandt A. Reflections and New Perspectives on Face Cognition as a Specific Socio-Cognitive Ability. Journal of Intelligence. 2021; 9(2):30. https://doi.org/10.3390/jintelligence9020030
Chicago/Turabian StyleMeyer, Kristina, Werner Sommer, and Andrea Hildebrandt. 2021. "Reflections and New Perspectives on Face Cognition as a Specific Socio-Cognitive Ability" Journal of Intelligence 9, no. 2: 30. https://doi.org/10.3390/jintelligence9020030
APA StyleMeyer, K., Sommer, W., & Hildebrandt, A. (2021). Reflections and New Perspectives on Face Cognition as a Specific Socio-Cognitive Ability. Journal of Intelligence, 9(2), 30. https://doi.org/10.3390/jintelligence9020030






