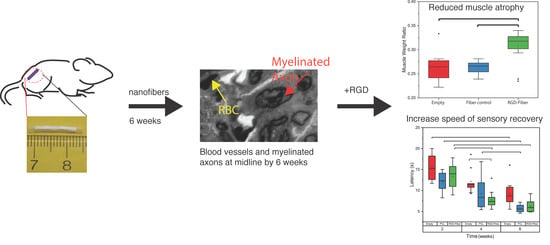
RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury
Abstract
1. Introduction
2. Results
2.1. Nanofibrous Nerve Constructs
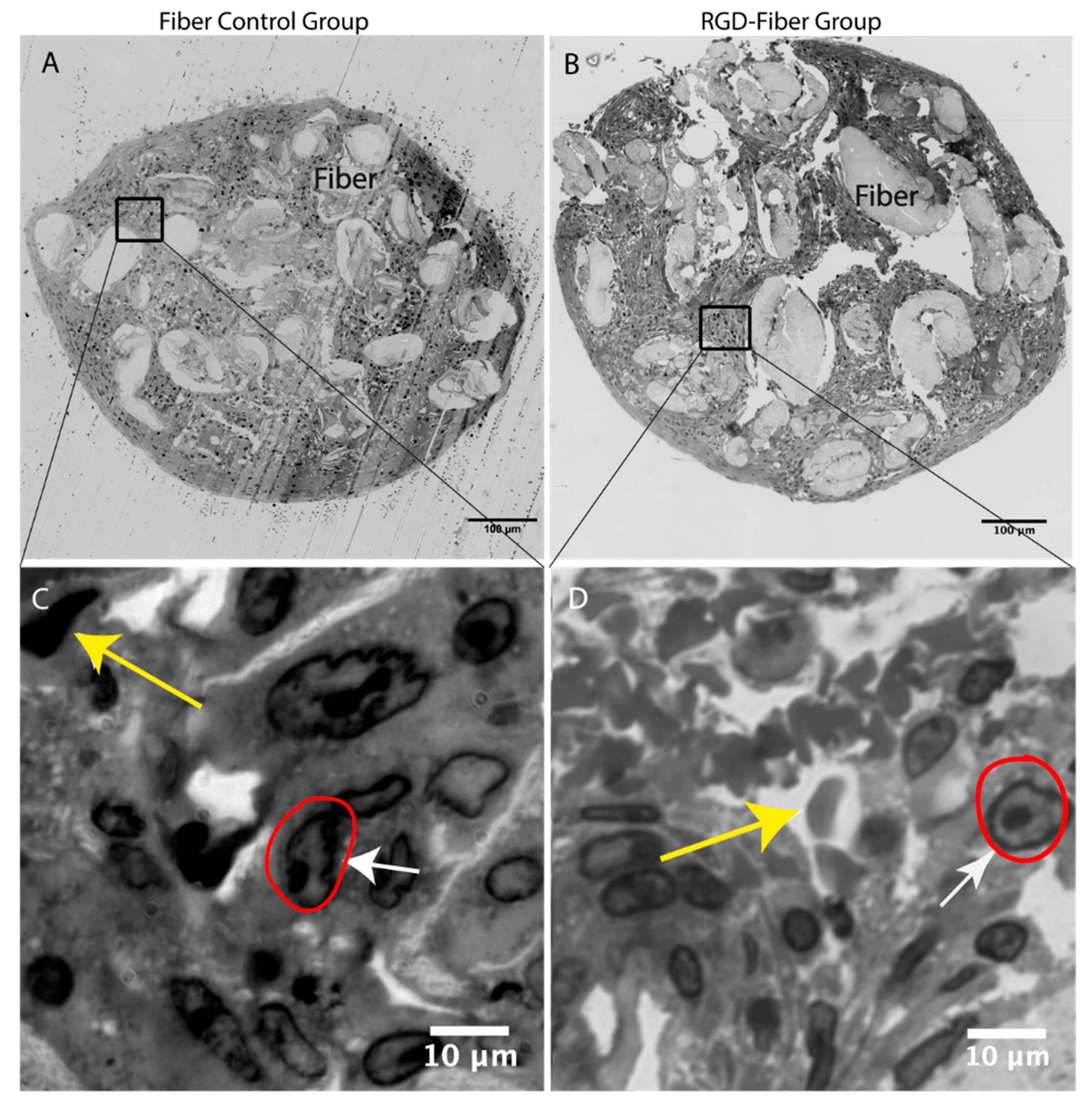
2.2. Tissue Analysis
2.3. Functional Assessment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nerve Guidance Conduits Fabrication Methods
4.3. Experimental Design and Animals
4.3.1. Surgical Procedures
4.3.2. Tissue Analysis
4.3.3. Functional Assessment
Motor Recovery
Sensory Recovery
4.3.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farahpour, M.R.; Ghayour, S.J. Effect of in situ delivery of acetyl-l-carnitine on peripheral nerve regeneration and functional recovery in transected sciatic nerve in rat. Int. J. Surg. 2014, 12, 1409–1415. [Google Scholar]
- Muheremu, A.; Ao, Q. Past, Present, and Future of Nerve Conduits in the Treatment of Peripheral Nerve Injury. Biomed. Res. Int. 2015, 2015, 237507. [Google Scholar] [CrossRef] [PubMed]
- Braga Silva, J.; Marchese, G.M.; Cauduro, C.G.; Debiasi, M. Nerve conduits for treating peripheral nerve injuries: A systematic literature review. Hand Surg. Rehabil. 2017, 36, 71–85. [Google Scholar] [CrossRef]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Hoben, G.; Yan, Y.; Iyer, N.; Newton, P.; Hunter, D.A.; Moore, A.M.; Sakiyama-Elbert, S.E.; Wood, M.D.; Mackinnon, S.E. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand 2015, 10, 396–402. [Google Scholar] [CrossRef]
- Patel, N.P.; Lyon, K.A.; Huang, J.H. An update-tissue engineered nerve grafts for the repair of peripheral nerve injuries. Neural Regen. Res. 2018, 13, 764–774. [Google Scholar]
- Saheb-Al-Zamani, M.; Yan, Y.; Farber, S.J.; Hunter, D.A.; Newton, P.; Wood, M.D.; Stewart, S.A.; Johnson, P.J.; Mackinnon, S.E. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp. Neurol. 2013, 247, 165–177. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- De Ruiter, G.C.; Malessy, M.J.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical Engineering Strategies for Peripheral Nerve Repair: Surgical Applications, State of the Art, and Future Challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Mendonça, A.C.; Barbieri, C.H.; Mazzer, N. Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J. Neurosci. Methods 2003, 129, 183–190. [Google Scholar] [CrossRef]
- Zhou, W.; Stukel, J.M.; Cebull, H.L.; Willits, R.K. Tuning the Mechanical Properties of Poly(Ethylene Glycol) Microgel-Based Scaffolds to Increase 3D Schwann Cell Proliferation. Macromol. Biosci. 2016, 16, 535–544. [Google Scholar] [CrossRef]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2012, 100, 406–423. [Google Scholar] [CrossRef]
- Zheng, J.; Kontoveros, D.; Lin, F.; Hua, G.; Reneker, D.H.; Becker, M.L.; Willits, R.K. Enhanced Schwann cell attachment and alignment using one-pot “dual click” GRGDS and YIGSR derivatized nanofibers. Biomacromolecules 2015, 16, 357–363. [Google Scholar] [CrossRef]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef]
- Moskow, J.; Ferrigno, B.; Mistry, N.; Jaiswal, D.; Bulsara, K.; Rudraiah, S.; Kumbar, S.G. Review: Bioengineering approach for the repair and regeneration of peripheral nerve. Bioact. Mater. 2019, 4, 107–113. [Google Scholar] [CrossRef]
- Koh, H.S.; Yong, T.; Teo, W.E.; Chan, C.K.; Puhaindran, M.E.; Tan, T.C.; Lim, A.; Lim, B.H.; Ramakrishna, S. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J. Neural Eng. 2010, 7, 046003. [Google Scholar] [CrossRef]
- Itoh, S.; Matsuda, A.; Kobayashi, H.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Effects of a laminin peptide (YIGSR) immobilized on crab-tendon chitosan tubes on nerve regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 375–382. [Google Scholar] [CrossRef]
- Mammadov, B.; Sever, M.; Gecer, M.; Zor, F.; Ozturk, S.; Akgun, H.; Tekinay, A.B. Sciatic nerve regeneration induced by glycosaminoglycan and laminin mimetic peptide nanofiber gels. R. Soc. Chem. 2016, 6, 110535–110547. [Google Scholar] [CrossRef]
- Silantyeva, E.A.; Nasir, W.; Carpenter, J.; Manahan, O.; Becker, M.L.; Willits, R.K. Accelerated Neural Differentiation of Mouse Embryonic Stem Cells on Aligned GYIGSR-functionalized Nanofibers. Acta Biomater. 2018, 75, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.A.S.; Xie, S.; Barker, I.A.; Zheng, J.; Reneker, D.H.; Dove, A.P.; Becker, M.L. Directed differentiation and neurite extension of mouse embryonic stem cell on aligned poly (lactide) nanofibers functionalized with YIGSR peptide. Biomaterials 2013, 34, 9089–9095. [Google Scholar] [CrossRef]
- Philip, D.L.; Silantyeva, E.A.; Becker, M.L.; Willits, R.K. RGD-Functionalized Nanofibers Increase Early GFAP Expression during Neural Differentiation of Mouse Embryonic Stem Cells. Biomacromolecules 2019, 20, 1443–1454. [Google Scholar] [CrossRef]
- Rafiuddin Ahmed, M.; Jayakumar, R. Peripheral nerve regeneration in RGD peptide incorporated collagen tubes. Brain Res. 2003, 993, 208–216. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, K.; Ma, T.; Huang, L.; Xia, B.; Zhu, S.; Yang, Y.; Liu, Z.; Quan, X.; Luo, K.; et al. Noncovalent Bonding of RGD and YIGSR to an Electrospun Poly(epsilon-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv. Healthc. Mater. 2017, 6, 1600860. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Koka, R.; Hadlock, T.A. Quantification of Functional Recovery Following Rat Sciatic Nerve Transection. Exp. Neurol. 2001, 168, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Browne, T. Overcoming short gaps in peripheral nerve repair: Conduits and human acellular nerve allograft. Hand 2014, 9, 131–137. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.; Mi, R.; Hoke, A.; Leong, K. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Mukhatyar, V.; Karumbaiah, L.; Yeh, J.; Bellamkonda, R. Tissue Engineering Strategies Designed to Realize the Endogenous Regenerative Potential of Peripheral Nerves. Adv. Mater. 2009, 21, 4670–4679. [Google Scholar] [CrossRef]
- Wood, M.D.; Moore, A.M.; Hunter, D.A.; Tuffaha, S.; Borschel, G.H.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009, 5, 959–968. [Google Scholar] [CrossRef]
- Lee, A.C.; Yu, V.M.; Lowe, J.B.; Brenner, M.J.; Hunter, D.A.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp. Neurol. 2003, 184, 295–303. [Google Scholar] [CrossRef]
- Marquardt, L.M.; Sakiyama-Elbert, S.E. Engineering peripheral nerve repair. Curr. Opin. Biotechnol. 2013, 24, 887–892. [Google Scholar] [CrossRef]
- LeBlanc, A.C.P.; Poduslo, J.F. Axonal modulation of myelin gene expression in the peripheral nerve. J. Neurosci. Res. 1990, 26, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Clements, I.P.; Kim, Y.T.; English, A.W.; Lu, X.; Chung, A.; Bellamkonda, R.V. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials 2009, 30, 3834–3846. [Google Scholar] [CrossRef] [PubMed]
- Triolo, D.; Dina, G.; Taveggia, C.; Vaccari, I.; Porrello, E.; Rivellini, C.; Domi, T.; La Marca, R.; Cerri, F.; Bolino, A.; et al. Vimentin regulates peripheral nerve myelination. Development 2012, 139, 1359–1367. [Google Scholar] [CrossRef]
- De Luca, A.C.; Lacour, S.P.; Raffoul, W.; di Summa, P.G. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration? Neural Regen. Res. 2014, 9, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Menko, A.S.; Bleaken, B.M.; Libowitz, A.A.; Zhang, L.; Stepp, M.A.; Walker, J.L. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol. Biol. Cell 2014, 25, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Monte-Raso, V.V.; Barbieri, C.H.; Mazzer, N.; Yamasita, A.C.; Barbieri, G. Is the Sciatic Functional Index always reliable and reproducible? J. Neurosci. Methods 2008, 170, 255–261. [Google Scholar] [CrossRef]
- Munro, C.A.; Szalai, J.P.; Mackinnon, S.E.; Midha, R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve 1998, 21, 1095–1097. [Google Scholar] [CrossRef]
- Calabrese, J.; Cushing, M.A.; Ittel, S.D. Sterically Hindered Magnesium Aryloxides. Inorg. Chem. 1988, 27, 867–870. [Google Scholar] [CrossRef]
- Wilson, J.A.H.; Hopkins, S.A.; Wright, P.M. ‘Immortal’ ring-opening polymerization of ω-pentadecalactone by Mg(BHT)2(THF)2. Polym. Chem. 2014, 5, 2691–2694. [Google Scholar] [CrossRef]
- Ning, X.; Guo, J.; Wolfert, M.A.; Boons, G.J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem. Int. Ed. 2008, 47, 2253–2255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xie, S.; Lin, F.; Hua, G.; Yu, T.; Reneker, D.H.; Becker, M.L. 4-Dibenzocyclooctynol (DIBO) as an initiator for poly(ε-caprolactone): Copper-free clickable polymer and nanofiber-based scaffolds. Polym. Chem. 2013, 4, 2215–2218. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Groos, S.; Reale, E.; Luciano, L. Re-evaluation of epoxy resin sections for light and electron microscopic immunostaining. J. Histochem. Cytochem. 2001, 49, 397–406. [Google Scholar] [CrossRef] [PubMed]
- De Medinaceli, L.; Freed, W.J.; Wyatt, R.J. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp. Neurol. 1982, 77, 634–643. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavanaugh, M.; Silantyeva, E.; Pylypiv Koh, G.; Malekzadeh, E.; Lanzinger, W.D.; Willits, R.K.; Becker, M.L. RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury. J. Funct. Biomater. 2019, 10, 24. https://doi.org/10.3390/jfb10020024
Cavanaugh M, Silantyeva E, Pylypiv Koh G, Malekzadeh E, Lanzinger WD, Willits RK, Becker ML. RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury. Journal of Functional Biomaterials. 2019; 10(2):24. https://doi.org/10.3390/jfb10020024
Chicago/Turabian StyleCavanaugh, McKay, Elena Silantyeva, Galina Pylypiv Koh, Elham Malekzadeh, William D. Lanzinger, Rebecca Kuntz Willits, and Matthew L. Becker. 2019. "RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury" Journal of Functional Biomaterials 10, no. 2: 24. https://doi.org/10.3390/jfb10020024
APA StyleCavanaugh, M., Silantyeva, E., Pylypiv Koh, G., Malekzadeh, E., Lanzinger, W. D., Willits, R. K., & Becker, M. L. (2019). RGD-Modified Nanofibers Enhance Outcomes in Rats after Sciatic Nerve Injury. Journal of Functional Biomaterials, 10(2), 24. https://doi.org/10.3390/jfb10020024