Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Chi-DHA Conjugate
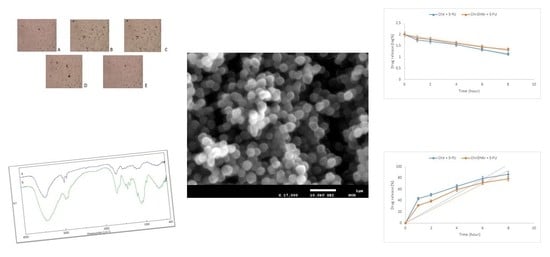
2.2. Morphological Characterization of Chi-DHAr Nanoparticles
2.3. Drug Loading and Release
2.4. Cytotoxicity Studies
2.5. Cellular Uptake Studies of 5-FU
3. Materials and Methods
3.1. Chemicals
3.2. Cell Cultures
3.3. Instrumentation
3.4. Preparation of Chi-DHA Conjugate
3.5. Synthesis of Chi-DHAr Nanoparticles
3.6. Drug Loading and Release
3.7. Cell Viability
3.8. Cellular Uptake Studies of 5-FU
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muzzarelli, R.A.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current Views on Fungal Chitin/Chitosan, Human Chitinases, Food Preservation, Glucans, Pectins and Inulin: A Tribute to Henri Braconnot, Precursor of the Carbohydrate Polymers Science, on the Chitin Bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, X.; Liu, C.; Xing, K.; Zhang, J.; Sun, G.; Li, X.; Chen, X. Preparation, Characterization, and Antibacterial Activity of Oleic Acid-Grafted Chitosan Oligosaccharide Nanoparticles. Front. Biol. China 2009, 4, 321–327. [Google Scholar] [CrossRef]
- Larsson, M.; Huang, W.-C.; Hsiao, M.-H.; Wang, Y.-J.; Nydén, M.; Chiou, S.-H.; Liu, D.-M. Biomedical Applications and Colloidal Properties of Amphiphilically Modified Chitosan Hybrids. Prog. Polym. Sci. 2013, 38, 1307–1328. [Google Scholar] [CrossRef]
- Méndez, P.A.; Vásquez, G.M.; Gartner, C.; López, B.L. Chitosan/OA Nanoparticle as Delivery System for Celecoxib: Parameters Affecting the Particle Size, Encapsulation, and Release. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Chen, X.; Liu, C.; Meng, X.; Park, H.J. Self-Assembled Nanoparticles Based on Linoleic-Acid Modified Carboxymethyl-Chitosan as Carrier of Adriamycin (ADR). Curr. Appl. Phys. 2007, 7, e125–e129. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. The Influence of Multivalent Phosphate Structure on the Properties of Ionically Cross-Linked Chitosan Films for Controlled Drug Release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA- and DHA-Rich Oils: An Overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Cirillo, G.; Picci, N.; Matricardi, P.; Alhaique, F. Molecularly Imprinted Polymers for 5-Fluorouracil Release in Biological Fluids. Molecules 2007, 12, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Pandey, J.P.; Sen, G. Sesbania Gum Based Hydrogel as Platform for Sustained Drug Delivery: An ‘in Vitro’ Study of 5-Fu Release. Int. J. Biol. Macromol. 2018, 113, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Zeng, F.; Chen, L.; Peng, Z.; Kong, L.X. Synthesis and Characterization of Folate Conjugated Chitosan and Cellular Uptake of Its Nanoparticles in HT-29 Cells. Carbohydr. Res. 2011, 346, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial Properties of Chitosan Nanoparticles: Mode of Action and Factors Affecting Activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Manikandan, A.; Sathiyabama, M. Preparation of Chitosan Nanoparticles and Its Effect on Detached Rice Leaves Infected with Pyricularia Grisea. Int. J. Biol. Macromol. 2016, 84, 58–61. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.F.; Apolinário, A.C.; Rangel-Yagui, C.O.; Stephano, M.A.; Tavares, L.C. Chitosan Nanoparticles for the Delivery of a New Compound Active against Multidrug-Resistant Staphylococcus Aureus. J. Drug Deliv. Sci. Technol. 2020, 55, 101363. [Google Scholar] [CrossRef]
- Abd-Elsalam, W.H.; ElKasabgy, N.A. Mucoadhesive Olaminosomes: A Novel Prolonged Release Nanocarrier of Agomelatine for the Treatment of Ocular Hypertension. Int. J. Pharm. 2019, 560, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? | Molecular Pharmaceutics. Available online: https://pubs.acs.org/doi/abs/10.1021/mp500817h (accessed on 2 March 2020).
- Liu, L.; Lv, Q.; Zhang, Q.; Zhu, H.; Liu, W.; Deng, G.; Wu, Y.; Shi, C.; Li, H.; Li, L. Preparation of Carboxymethyl Chitosan Microspheres and Their Application in Hemostasis. Disaster Med. Public Health Prep. 2017, 11, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.I.; Ruffo, M.; Malivindi, R.; Vattimo, A.F.; Pezzi, V.; Puoci, F. Molecularly Imprinted Polymers (MIPs) as Theranostic Systems for Sunitinib Controlled Release and Self-Monitoring in Cancer Therapy. Pharmaceutics 2020, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanoencapsulation of Zerumbone in Oleic Acid-Modified Chitosan Nanoparticles. In Proceedings of the 2017 International Conference on Computational Biology and Bioinformatics, Newark, NJ, USA, 18–20 October 2017. Available online: https://dl.acm.org/doi/abs/10.1145/3155077.3155097 (accessed on 30 December 2019).
- Lakkakula, J.R.; Kurapati, R.; Tynga, I.; Abrahamse, H.; Raichur, A.M.; Krause, R.W.M. Cyclodextrin Grafted Calcium Carbonate Vaterite Particles: Efficient System for Tailored Release of Hydrophobic Anticancer or Hormone Drugs. RSC Adv. 2016, 6, 104537–104548. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Candamano, S.; Ruffo, M.; Vattimo, A.F.; Spanedda, M.V.; Puoci, F. Molecularly Imprinted Microrods via Mesophase Polymerization. Molecules 2018, 23, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anticancer Activity of a Quercetin-based Polymer Towards HeLa Cancer Cells. Available online: http://ar.iiarjournals.org/content/32/7/2843.short (accessed on 5 December 2019).
- Varshosaz, J.; Hassanzadeh, F.; Sadeghi, H.; Khadem, M. Galactosylated Nanostructured Lipid Carriers for Delivery of 5-FU to Hepatocellular Carcinoma. J. Liposome Res. 2012, 22, 224–236. [Google Scholar] [CrossRef] [PubMed]







| Sample | Mean Diameter | Polydispersity Index |
|---|---|---|
| Chi-DHAr nanoparticles | 421.8 ± 0.1 nm | 0.063 |
| Chir nanoparticles | 402 ± 0.1 nm | 0.081 |
| Sample | DLE (%) | DLC (%) |
|---|---|---|
| Chir + 5-FU | 27.73 ± 0.14 | 5.62 ± 0.23 |
| Chi-DHAr + 5-FU | 33.74 ± 0.19 | 7.9 ± 0.26 |
| Sample | Mean Diameter | Polydispersity Index |
|---|---|---|
| Chir + 5-FU | 421 ± 0.3 | 0.08 |
| Chi-DHAr + 5-FU | 442 ± 0.2 | 0.07 |
| Sample | Zero Order (r2) | First Order (r2) |
|---|---|---|
| Chir + 5-FU | 0.9876 | 0.9917 |
| Chi-DHAr + 5-FU | 0.9663 | 0.9954 |
| Sample | Amount of 5-FU in MCF-7 Cells (μg) | Amount of 5-FU in HeLa Cells (μg) |
|---|---|---|
| Chi-DHAr + 5-FU nanoparticles | 5.4 ± 0.36 | 6.2 ± 0.2 |
| Chir + 5-FU nanoparticles | 3.5 ± 0.36 | 3.9 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffo, M.; Parisi, O.I.; Patitucci, F.; Dattilo, M.; Malivindi, R.; Amone, F.; Morelli, C.; Nigro, A.; Sisci, D.; Puoci, F. Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect. J. Funct. Biomater. 2020, 11, 48. https://doi.org/10.3390/jfb11030048
Ruffo M, Parisi OI, Patitucci F, Dattilo M, Malivindi R, Amone F, Morelli C, Nigro A, Sisci D, Puoci F. Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect. Journal of Functional Biomaterials. 2020; 11(3):48. https://doi.org/10.3390/jfb11030048
Chicago/Turabian StyleRuffo, Mariarosa, Ortensia Ilaria Parisi, Francesco Patitucci, Marco Dattilo, Rocco Malivindi, Fabio Amone, Catia Morelli, Alessandra Nigro, Diego Sisci, and Francesco Puoci. 2020. "Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect" Journal of Functional Biomaterials 11, no. 3: 48. https://doi.org/10.3390/jfb11030048
APA StyleRuffo, M., Parisi, O. I., Patitucci, F., Dattilo, M., Malivindi, R., Amone, F., Morelli, C., Nigro, A., Sisci, D., & Puoci, F. (2020). Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect. Journal of Functional Biomaterials, 11(3), 48. https://doi.org/10.3390/jfb11030048