Bio-Based Electrospun Fibers for Wound Healing
Abstract
:1. Introduction
2. Wound Healing
Wound Dressing
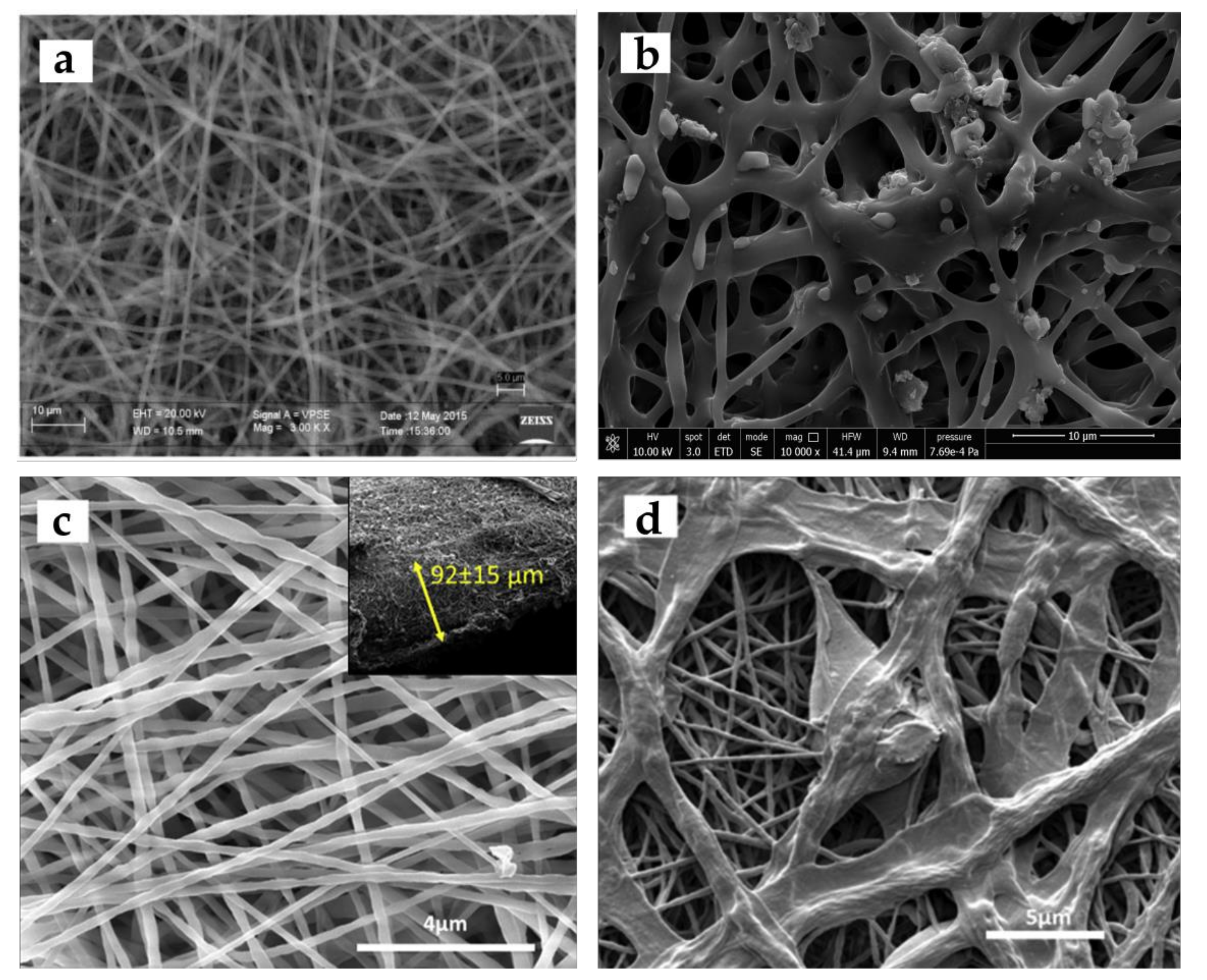
3. Electrospinning Process and Its Advantages for Wound Healing Applications
Effect of Electrospinning Parameters on Biological Applications
4. Multifunctional Wound Dressings
4.1. Drug Loaded Electrospun Wound Dressings
4.2. Electrospun Wound Dressings with Antibacterial Activity
4.3. Electrospun Wound Dressings Loaded with Bioactive Molecules
5. Application of Bio-Based Electrospun Fibers in Wound Healing
5.1. Application of Cellulose-Electrospun Nanofibers in Wound Healing (Including Its Composite)
5.2. Application of Chitosan Electrospun Fibers in Wound Healing
5.3. Application of PHA Electrospun Fibers in Wound Healing
5.4. Application of PLA-Based Electrospun Fibers in Wound Healing
6. Translational Approaches
6.1. Drug Delivery Electrospun Fibers
6.2. Clinical Trials of Electrospun Wound Dressings
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio. Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, Y.; Xue, Y.; Cheng, X.; Zhao, W.; Wang, J.; He, R.; Wan, Q.; Pei, X. Tazarotene Released from Aligned Electrospun Membrane Facilitates Cutaneous Wound Healing by Promoting Angiogenesis. ACS Appl. Mater. Interfaces 2019, 11, 36141–36153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110083. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA—Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Khan, N.; Misra, M.; Koch, T.; Mohanty, A. Applications of electrospun nanofibers in the biomedical field. SURG J. 2012, 5, 63–73. [Google Scholar]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonca, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Sun, Y.; Kang, J.; Liu, J.; Wang, Y.; Li, Y.; Zhang, L.; Ning, G. Electrospun fibrous mat based on silver (I) metal-organic frameworks-polylactic acid for bacterial killing and antibiotic-free wound dressing. Chem. Eng. J. 2020, 390, 124523. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Ilomuanya, M.O.; Adebona, A.C.; Wang, W.; Sowemimo, A.; Eziegbo, C.L.; Silva, B.O.; Adeosun, S.O.; Joubert, E.; De Beer, D. Development and characterization of collagen-based electrospun scaffolds containing silver sulphadiazine and Aspalathus linearis extract for potential wound healing applications. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A Macroporous Hydrogel Dressing with Enhanced Antibacterial and Anti-Inflammatory Capabilities for Accelerated Wound Healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Koivuniemi, R.; Hakkarainen, T.; Kiiskinen, J.; Kosonen, M.; Vuola, J.; Valtonen, J.; Luukko, K.; Kavola, H.; Yliperttula, M. Clinical study of nanofibrillar cellulose hydrogel dressing for skin graft donor site treatment. Adv. Wound Care 2020, 9, 199–210. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Q.; You, J.; Cheng, Y.; Hong, C.; Chen, Z.; Jiang, T.; Hao, T. Adhesion loss mechanism based on carboxymethyl cellulose-filled hydrocolloid dressings in physiological wounds environment. Carbohydr. Polym. 2020, 235, 115953. [Google Scholar] [CrossRef]
- Chin, C.-Y.; Ng, P.-Y.; Ng, S.-F. Moringa oleifera standardised aqueous leaf extract-loaded hydrocolloid film dressing: In vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model. Drug Deliv. Transl. Res. 2019, 9, 453–468. [Google Scholar] [CrossRef]
- Chamorro, A.M.; Thomas, M.C.V.; Mieras, A.S.; Leiva, A.; Martínez, M.P.; Yeste, M.M.S.H.; Grupo, U. Multicenter randomized controlled trial comparing the effectiveness and safety of hydrocellular and hydrocolloid dressings for treatment of category II pressure ulcers in patients at primary and long-term care institutions. Int. J. Nurs. Stud. 2019, 94, 179–185. [Google Scholar] [CrossRef]
- Zhang, K.; Bai, X.; Yuan, Z.; Cao, X.; Jiao, X.; Li, Y.; Qin, Y.; Wen, Y.; Zhang, X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 2019, 204, 70–79. [Google Scholar] [CrossRef]
- Khalid, A.; Naeem, N.; Khan, T.; Wahid, F. Polysaccharide Composites as a Wound-Healing Sponge. Adv. Appl. Polysacch. Their Compos. 2020, 73, 1. [Google Scholar]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P. Bio-multifunctional alginate/chitosan/Fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Lazzeri, L.; Cascone, M.G.; Danti, S.; Serino, L.P.; Moscato, S.; Bernardini, N. Gelatine/PLLA sponge-like scaffolds: Morphological and biological characterization. J. Mater. Sci. Mater. Med. 2007, 18, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Wang, X.W.; Zhang, T.; Shi, B.H.; Zheng, B.; Zhang, D.D.; Hu, Y.Y.; Ma, L. Chitosan-calcium alginate dressing promotes wound healing: A preliminary study. Wound Repair Regen. 2020, 28, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ma, Y.; Tan, H.; Yuan, G.; Li, S.; Li, J.; Jia, Y.; Zhou, T.; Niu, X.; Hu, X. Alginate membrane dressing toughened by chitosan floccule to load antibacterial drugs for wound healing. Polym. Test. 2019, 79, 106039. [Google Scholar] [CrossRef]
- Zare-Gachi, M.; Daemi, H.; Mohammadi, J.; Baei, P.; Bazgir, F.; Hosseini-Salekdeh, S.; Baharvand, H. Improving anti-hemolytic, antibacterial and wound healing properties of alginate fibrous wound dressings by exchanging counter-cation for infected full-thickness skin wounds. Mater. Sci. Eng. C 2020, 107, 110321. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In Vitro and In Vivo Comparison Study of Electrospun PLA and PLA/PVA/SA Fiber Membranes for Wound Healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef] [Green Version]
- Alberti, T.B.; Coelho, D.S.; de Prá, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 1–16. [Google Scholar] [CrossRef]
- Dhivya, S.; Vijaya Padma, V.; Santhini, E. Wound dressings–a review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Ren, G.; Lv, L. Wound healing and inflammation characteristics of the submicrometric mats prepared from electrospinning. J. Bioact. Compat. Polym. 2018, 34, 83–96. [Google Scholar] [CrossRef]
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol. 2019, 127, 27–38. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nanofibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Liu, X.; Nielsen, L.H.; Qu, H.; Christensen, L.P.; Rantanen, J.; Yang, M. Stability of lysozyme incorporated into electrospun fibrous mats for wound healing. Eur. J. Pharm. Biopharm. 2019, 136, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. ACS Appl. Mater. Interfaces 2020, 12, 3371–3381. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, E.; Esmaeili, A.; Eslahi, N.; Shokrgozar, M.A.; Simchi, A. Fabrication and Characterization of Core-Shell Electrospun Fibrous Mats Containing Medicinal Herbs for Wound Healing and Skin Tissue Engineering. Mar. Drugs 2019, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. NPG Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Ullah, S.; Khan, M.Q.; Hashmi, M.; Nam, P.D.; Kato, Y.; Tamada, Y.; Kim, I.S. Manuka honey incorporated cellulose acetate nanofibrous mats: Fabrication and in vitro evaluation as a potential wound dressing. Int. J. Biol. Macromol. 2020, 155, 479–489. [Google Scholar] [CrossRef]
- Miao, J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wounddressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Ghorbani, S.; Eyni, H.; Tiraihi, T.; Asl, L.S.; Soleimani, M.; Atashi, A.; Beiranvand, S.P.; Warkiani, M.E. Combined effects of 3D bone marrow stem cell-seeded wet-electrospun poly lactic acid scaffolds on full-thickness skin wound healing. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 905–912. [Google Scholar] [CrossRef]
- Malgarim Cordenonsi, L.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Scherman Schapoval, E.E.; Del Fante, C.; Vigani, B.; Miele, D.; et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109745. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, G.; Cai, R.; Wang, Y.; Liu, L.; Zuo, H.; Zhao, P.; Umar, A.; Mao, C.; Xia, Q.; He, H. Bioinspired design of AgNPs embedded silk sericin-based sponges for efficiently combating bacteria and promoting wound healing. Mater. Des. 2019, 180, 107940. [Google Scholar] [CrossRef]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Barani, H. Morphological and mechanical properties of drawn poly(l-lactide) electrospun twisted yarns. Polym. Eng. Sci. 2018, 58, 1091–1096. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2019, 9, 1901287. [Google Scholar] [CrossRef]
- Günday, C.; Anand, S.; Gencer, H.B.; Munafò, S.; Moroni, L.; Fusco, A.; Donnarumma, G.; Ricci, C.; Hatir, P.C.; Türeli, N.G.; et al. Ciprofloxacin-loaded polymeric nanoparticles incorporated electrospun fibers for drug delivery in tissue engineering applications. Drug Deliv. Transl. Res. 2020, 10, 706–720. [Google Scholar] [CrossRef]
- Dart, A.; Bhave, M.; Kingshott, P. Antimicrobial Peptide-Based Electrospun Fibers for Wound Healing Applications. Macromol. Biosci. 2019, 19, e1800488. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun Core–Shell Fibrous 2D Scaffold with Biocompatible Poly (Glycerol Sebacate) and Poly-L-Lactic Acid for Wound Healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- .Wang, J.; Windbergs, M. Functional electrospun fibers for the treatment of human skin wounds. Eur. J. Pharm. Biopharm. 2017, 119, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zou, T.; Li, S.; Jing, J.; Xia, X.; Liu, X. Drug-Loaded Zein Nanofibers Prepared Using a Modified Coaxial Electrospinning Process. AAPS PharmSciTech 2013, 14, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.-C.; Zhang, C.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Designer fibers from 2D to 3D–Simultaneous and controlled engineering of morphology, shape and size. Chem. Eng. J. 2018, 334, 89–98. [Google Scholar] [CrossRef]
- Ura, D.P.; Karbowniczek, J.E.; Szewczyk, P.K.; Metwally, S.; Kopyscianski, M.; Stachewicz, U. Cell Integration with Electrospun PMMA Nanofibers, Microfibers, Ribbons, and Films: A Microscopy Study. Bioengineering 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of Electrospun Polymer Nanofibers with Diverse Morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef] [Green Version]
- Maleki, H.; Semnani Rahbar, R.; Saadatmand, M.M.; Barani, H. Physical and morphological characterisation of poly(L-lactide) acid-based electrospun fibrous structures: Tunning solution properties. Plast. Rubber Compos. 2018, 47, 438–446. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Toliyat, T.; Dijkstra, P.J. Drug release behavior of electrospun twisted yarns as implantable medical devices. Biofabrication 2016, 8, 35019. [Google Scholar] [CrossRef]
- Barani, H.; Khorashadizadeh, M.; Haseloer, A.; Klein, A. Characterization and Release Behavior of a Thiosemicarbazone from Electrospun Polyvinyl Alcohol Core‒Shell Nanofibers. Polymers 2020, 12, 1488. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Kulkarni, A.A.; Patel, A.; Di Luca, A.; Reis, R.L.; Gomes, M.E.; van Blitterswijk, C.; Moroni, L.; Khademhosseini, A. Amphiphilic beads as depots for sustained drug release integrated into fibrillar scaffolds. J. Control. Release 2014, 187, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Li, T.X.; Ding, X.; Sui, X.; Tian, L.L.; Zhang, Y.; Hu, J.Y.; Yang, X.D. Sustained Release of Protein Particle Encapsulated in Bead-on-String Electrospun Nanofibers. J. Macromol. Sci. Part B 2015, 54, 887–896. [Google Scholar] [CrossRef]
- Li, T.; Ding, X.; Tian, L.; Hu, J.; Yang, X.; Ramakrishna, S. The control of beads diameter of bead-on-string electrospun nanofibers and the corresponding release behaviors of embedded drugs. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Mathur, S.; Klein, A. Antibacterial Ag containing core-shell polyvinyl alcohol-poly (lactic acid) nanofibers for biomedical applications. Polym. Eng. Sci. 2020, 60, 1221–1230. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Gharehaghaji, A.A.; Criscenti, G.; Moroni, L.; Dijkstra, P.J. The influence of process parameters on the properties of electrospun PLLA yarns studied by the response surface methodology. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Moroni, L.; Dijkstra, P.J. Influence of the solvent type on the morphology and mechanical properties of electrospun PLLA yarns. Biofabrication 2013, 5, 035014. [Google Scholar] [CrossRef]
- Danti, S.; D’Alessandro, D.; Mota, C.; Bruschini, L.; Berrettini, S. Applications of bioresorbable polymers in skin and eardrum. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 423–444. [Google Scholar]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Alves, P.E.; Soares, B.G.; Lins, L.C.; Livi, S.; Santos, E.P. Controlled delivery of dexamethasone and betamethasone from PLA electrospun fibers: A comparative study. Eur. Polym. J. 2019, 117, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; He, T.; Huang, M.; Zhang, X.; Yuan, J.; Wei, Y.; Dong, X.; Liu, W.; Ko, F.; et al. Electrospun Sandwich-Structure Composite Membranes for Wound Dressing Scaffolds with High Antioxidant and Antibacterial Activity. Macromol. Mater. Eng. 2017, 303, 1700270. [Google Scholar] [CrossRef]
- Pour Khalili, N.; Moradi, R.; Kavehpour, P.; Islamzada, F. Boron nitride nanotube clusters and their hybrid nanofibers with polycaprolacton: Thermo-pH sensitive drug delivery functional materials. Eur. Polym. J. 2020, 127, 109585. [Google Scholar] [CrossRef]
- Gačanin, J.; Hedrich, J.; Sieste, S.; Glaßer, G.; Lieberwirth, I.; Schilling, C.; Fischer, S.; Barth, H.; Knöll, B.; Synatschke, C.V. Autonomous Ultrafast Self-Healing Hydrogels by pH-Responsive Functional Nanofiber Gelators as Cell Matrices. Adv. Mater. 2019, 31, 1805044. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cheng, M.; Liang, Y.; Zhu, H.; Sun, Y.; Dong, D.; Wang, S. Intelligent Cellulose Nanofibers with Excellent Biocompatibility Enable Sustained Antibacterial and Drug Release via a pH-Responsive Mechanism. J. Agric. Food Chem. 2020, 68, 3518–3527. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Abdalkarim, S.Y.H.; Yu, H.; Wang, C.; Chen, Y.; Zou, Z.; Han, L.; Yao, J.; Tam, K.C. Thermo and light-responsive phase change nanofibers with high energy storage efficiency for energy storage and thermally regulated on–off drug release devices. Chem. Eng. J. 2019, 375, 121979. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Sun, Z.; Yang, C.; Tang, D. Electrospun P (NVCL-co-MAA) nanofibers and their pH/temperature dual-response drug release profiles. Colloid Polym. Sci. 2020, 298, 629–636. [Google Scholar] [CrossRef]
- Hoque, J.; Sangaj, N.; Varghese, S. Stimuli-Responsive Supramolecular Hydrogels and Their Applications in Regenerative Medicine. Macromol. Biosci. 2019, 19, 1800259. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.H.; Park, S.B.; Hong, J.S.; Lee, H.L.; Song, Y.H.; Kim, J.; Jung, Y.H.; Kim, C.; Kim, D.-M.; Lee, S.E.; et al. Piperlongumine-Eluting Gastrointestinal Stent Using Reactive Oxygen Species-Sensitive Nanofiber Mats for Inhibition of Cholangiocarcinoma Cells. Nanoscale Res. Lett. 2019, 14, 58. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Nagarajan, S.; Bechelany, M.; Kalkura, N.S.; Miele, P.; Bohatier, C.P.; Balme, S. Chapter 20—Electrospun Nanofibers for Drug Delivery in Regenerative Medicine. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 595–625. [Google Scholar]
- Leung, C.M.; Dhand, C.; Mayandi, V.; Ramalingam, R.; Lim, F.P.; Barathi, V.A.; Dwivedi, N.; Orive, G.; Beuerman, R.W.; Ramakrishna, S.; et al. Wound healing properties of magnesium mineralized antimicrobial nanofibre dressings containing chondroitin sulphate—A comparison between blend and core–shell nanofibres. Biomater. Sci. 2020, 8, 3454–3471. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Chen, J.; Li, D.; Zhang, K.; Liu, T.; Ding, J.; Chen, X. Highly Bioadhesive Polymer Membrane Continuously Releases Cytostatic and Anti-Inflammatory Drugs for Peritoneal Adhesion Prevention. ACS Biomater. Sci. Eng. 2018, 4, 2026–2036. [Google Scholar] [CrossRef]
- Sofi, H.S.; Ashraf, R.; Khan, A.H.; Beigh, M.A.; Majeed, S.; Sheikh, F.A. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater. Sci. Eng. C 2019, 94, 1102–1124. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, S.; Timofeeva, V.; Permyakova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-coated polycaprolactone nanofibers with covalently bonded platelet-rich plasma enhance adhesion and growth of human fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092. [Google Scholar] [CrossRef]
- Alippilakkotte, S.; Kumar, S.; Sreejith, L. Fabrication of PLA/Ag nanofibers by green synthesis method using Momordica charantia fruit extract for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 771–782. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, F.; Zhang, W.; Yang, Y.; You, D.; Li, L. Toward improved wound dressings: Effects of polydopamine-decorated poly (lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019, 9, 33038–33051. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In vivo biocompatibility of electrospun biodegradable dual carrier (antibiotic+ growth factor) in a mouse model—Implications for rapid wound healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seonwoo, H.; Shin, B.; Jang, K.-J.; Lee, M.; Choo, O.-S.; Park, S.-B.; Kim, Y.C.; Choi, M.-J.; Kim, J.; Garg, P.; et al. Epidermal Growth Factor–Releasing Radially Aligned Electrospun Nanofibrous Patches for the Regeneration of Chronic Tympanic Membrane Perforations. Adv. Healthc. Mater. 2019, 8, 1801160. [Google Scholar] [CrossRef]
- Balakrishnan, S.B.; Thambusamy, S. Preparation of silver nanoparticles and riboflavin embedded electrospun polymer nanofibrous scaffolds for in vivo wound dressing application. Process Biochem. 2020, 88, 148–158. [Google Scholar] [CrossRef]
- Luef, K.P.; Stelzer, F.; Wiesbrock, F. Poly (hydroxy alkanoate) s in medical applications. Chem. Biochem. Eng. Q. 2015, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kakoria, A.; Sinha-Ray, S. A review on biopolymer-based fibers via electrospinning and solution blowing and their applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-driven therapeutic interventions in wound healing: Potential uses and applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Thakur, M.K.; Kessler, M.R. Handbook of Composites from Renewable Materials, Biodegradable Materials; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 5. [Google Scholar]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, R.; Nageswaran, S.; Thakur, V.K.; Ahn, J.-H. Electrospinning of cellulose: Process and applications . In Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; Thakur, V.K., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 311–340. [Google Scholar]
- Kucińska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Bodin, A.; Concaro, S.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007, 1, 406–408. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Muangman, P.; Opasanon, S.; Suwanchot, S.; Thangthed, O. Efficiency of microbial cellulose dressing in partial-thickness burn wounds. J. Am. Coll. Certif. Wound Spec. 2011, 3, 16–19. [Google Scholar]
- Sahana, T.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Henriksson, M.W.; Walkenström, P. Electrospinning of cellulose-based nanofibers. J. Appl. Polym. Sci. 2007, 103, 1473–1482. [Google Scholar] [CrossRef]
- He, X.; Cheng, L.; Zhang, X.; Xiao, Q.; Zhang, W.; Lu, C. Tissue engineering scaffolds electrospun from cotton cellulose. Carbohydr. Polym. 2015, 115, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Frey, M.W. Electrospinning cellulose and cellulose derivatives. Polym. Rev. 2008, 48, 378–391. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Gao, Y.; Xu, Z.; Huang, C.; Yao, G.; Jiang, L.; Tang, Y.; Wang, X. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1556–1565. [Google Scholar] [CrossRef]
- Park, T.-J.; Jung, Y.J.; Choi, S.-W.; Park, H.; Kim, H.; Kim, E.; Lee, S.H.; Kim, J.H. Native chitosan/cellulose composite fibers from an ionic liquid via electrospinning. Macromol. Res. 2011, 19, 213–215. [Google Scholar] [CrossRef]
- Rao, S.S.; Jeyapal, S.G.; Rajiv, S. Biodegradable electrospun nanocomposite fibers based on Poly (2-hydroxy ethyl methacrylate) and bamboo cellulose. Compos. Part B Eng. 2014, 60, 43–48. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2012, 87, 1065–1072. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellulose 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Min, B.-M.; Lee, S.W.; Lim, J.N.; You, Y.; Lee, T.S.; Kang, P.H.; Park, W.H. Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer 2004, 45, 7137–7142. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.H.; Wang, P.; Kim, H.J. Blend fibers of chitosan–agarose by electrospinning. Mater. Lett. 2009, 63, 2510–2512. [Google Scholar]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Ardila, N.; Medina, N.; Arkoun, M.; Heuzey, M.-C.; Ajji, A.; Panchal, C.J. Chitosan–bacterial nanocellulose nanofibrous structures for potential wound dressing applications. Cellulose 2016, 23, 3089–3104. [Google Scholar] [CrossRef]
- Datta, S.; Rameshbabu, A.P.; Bankoti, K.; Maity, P.P.; Das, D.; Pal, S.; Roy, S.; Sen, R.; Dhara, S. Oleoyl-chitosan-based nanofiber mats impregnated with amniotic membrane derived stem cells for accelerated full-thickness excisional wound healing. ACS Biomater. Sci. Eng. 2017, 3, 1738–1749. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Sandeep, K.; Singh, M.; Singh, G.P.; Lee, J.-K.; Bhatia, S.K.; Kalia, V.C.; de Souza, L.; Shivakumar, S. Biotechnological Application of Polyhydroxyalkanoates and Their Composites as Anti-microbials Agents. In Biotechnological Applications of Polyhydroxyalkanoates; Kalia, V.C., Ed.; Springer: Singapore, 2019; pp. 227–290. [Google Scholar]
- Osswald, T.A.; Garcia-Rodriguez, S.; Teramoto, N.; Rai, R.; Roy, I.; Visakh, P.; Thomas, S.; Pothan, L.A.; Fukushima, K.; Tabuani, D. A Handbook of Applied Biopolymer Technology: Synthesis, Degradation and Applications; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Wu, Q.; Wang, Y.; Chen, G.Q. Medical application of microbial biopolyesters polyhydroxyalkanoates. Artif. Cells Blood Substit. Immobil. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Junyu, Z.; Ekaterina, I.S.; Tatiana, G.V.; Luiziana, F.D.S.; Guo-Qiang, C. Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater. Sci. Eng. 2018, 86, 144–150. [Google Scholar]
- Chen, G.-Q.; Zhang, J. Microbial polyhydroxyalkanoates as medical implant biomaterials. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, Q.; Yang, F.; Xu, M.; Leski, M.; Chen, G.-Q. Influence of dl-β-Hydroxybutyric Acid on Cell Proliferation and Calcium Influx. Biomacromolecules 2005, 6, 593–597. [Google Scholar] [CrossRef]
- Yang, X.-D.; Zou, X.-H.; Dai, Z.-W.; Luo, R.-C.; Wei, C.-J.; Chen, G.-Q. Effects of Oligo(3-hydroxyalkanoates) on the Viability and Insulin Secretion of Murine Beta Cells. J. Biomater. Sci. Polym. Ed. 2009, 20, 1729–1746. [Google Scholar] [CrossRef] [PubMed]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Volova, T.; Goncharov, D.; Sukovatyi, A.; Shabanov, A.; Nikolaeva, E.; Shishatskaya, E. Electrospinning of polyhydroxyalkanoate fibrous scaffolds: Effects on electrospinning parameters on structure and properties. J. Biomater. Sci. Polym. Ed. 2014, 25, 370–393. [Google Scholar] [CrossRef]
- Li, W.; Cicek, N.; Levin, D.B.; Liu, S. Enabling electrospinning of medium-chain length polyhydroxyalkanoates (PHAs) by blending with short-chain length PHAs. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 499–509. [Google Scholar] [CrossRef]
- Zhu, M.; Zuo, W.; Yu, H.; Yang, W.; Chen, Y. Superhydrophobic surface directly created by electrospinning based on hydrophilic material. J. Mater. Sci. 2006, 41, 3793–3797. [Google Scholar] [CrossRef]
- Shishatskaya, E.I.; Nikolaeva, E.D.; Vinogradova, O.N.; Volova, T.G. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwantong, O.; Waleetorncheepsawat, S.; Sanchavanakit, N.; Pavasant, P.; Cheepsunthorn, P.; Bunaprasert, T.; Supaphol, P. In vitro biocompatibility of electrospun poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fiber mats. Int. J. Biol. Macromol. 2007, 40, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Han, I.; Shim, K.J.; Kim, J.Y.; Im, S.U.; Sung, Y.K.; Kim, M.; Kang, I.-K.; Kim, J.C. Effect of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Nanofiber Matrices Cocultured With Hair Follicular Epithelial and Dermal Cells for Biological Wound Dressing. Artif. Organs 2007, 31, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, P.; Vasanthan, K.S.; Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Development of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibers for Skin Tissue Engineering: Effects of Topography, Mechanical, and Chemical Stimuli. Biomacromolecules 2011, 12, 3156–3165. [Google Scholar] [CrossRef]
- Abdul, M.; Kasturi, M.; Sivakumar, M.; Kumar, S.; Syed, S.; Rahman, S.; Noor, A.; Nanthini, S. Fabrication and Characterization of an Electrospun PHA/Graphene Silver Nanocomposite Scaffold for Antibacterial Applications. Materials 2018, 11, 15. [Google Scholar]
- Yuan, J.; Geng, J.; Xing, Z.; Shim, K.J.; Han, I.; Kim, J.C.; Kang, I.K.; Shen, J. Novel wound dressing based on nanofibrous PHBV–keratin mats. J. Tissue Eng. Regen. Med. 2015, 9, 1027–1035. [Google Scholar] [CrossRef]
- Salvatore, L.; Carofiglio, V.E.; Stufano, P.; Bonfrate, V.; Calò, E.; Scarlino, S.; Nitti, P.; Centrone, D.; Cascione, M.; Leporatti, S. Potential of electrospun poly (3-hydroxybutyrate)/collagen blends for tissue engineering applications. J. Healthc. Eng. 2018, 2018, 6573947. [Google Scholar] [CrossRef]
- Meng, W.; Kim, S.-Y.; Yuan, J.; Kim, J.C.; Kwon, O.H.; Kawazoe, N.; Chen, G.; Ito, Y.; Kang, I.-K. Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 81–94. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Perumal, S.; Madhan, B.; Umamaheswari, N.; Banday, J.A.; Perumal, P.T.; Santhanakrishnan, V.P. Synthesis and Fabrication of Collagen-Coated Ostholamide Electrospun Nanofiber Scaffold for Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 8556–8568. [Google Scholar] [CrossRef]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu Altan, O.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Applications. J. Funct. Biomater 2020, 11, 62. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditaranto, N.; Basoli, F.; Trombetta, M.; Cioffi, N.; Rainer, A. Electrospun nanomaterials implementing antibacterial inorganic nanophases. Appl. Sci. 2018, 8, 1643. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.M.; Lagaron, J. Electrospun antimicrobial films of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) containing eugenol essential oil encapsulated in mesoporous silica nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, S.; Ahmad, M.; Mahdavi, M.; Abdolmohammadi, S. Preparation, characterization, and antimicrobial activities of ZnO nanoparticles/cellulose nanocrystal nanocomposites. BioResources 2013, 8, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun active biopapers of food waste derived poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with short-term and long-term antimicrobial performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef] [Green Version]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.-Y.; Wang, D.; Yao, J. Electrospun poly(3-hydroxybutyrate-co-3-hydroxy-valerate)/cellulose reinforced nanofibrous membranes with ZnO nanocrystals for antibacterial wound dressings. Cellulose 2017, 24, 2925–2938. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Gordillo, G.; Bagchi, D.; Bagchi, M.; Roy, S. Oxygen, oxidants, and antioxidants in wound healing: An emerging paradigm. Ann. NY Acad. Sci. 2002, 957, 239–249. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.E.A. Cerium Oxide Nanoparticle Incorporated Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Membranes for Diabetic Wound Healing Applications. ACS Biomater. 2020, 6, 58–70. [Google Scholar] [CrossRef]
- Cristallini, C.; Danti, S.; Azimi, B.; Tempesti, V.; Ricci, C.; Ventrelli, L.; Cinelli, P.; Barbani, N.; Lazzeri, A. Multifunctional Coatings for Robotic Implanted Device. Int. J. Mol. Sci. 2019, 20, 5126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Cicek, N.; Levin, D.B.; Logsetty, S.; Liu, S. Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 394–406. [Google Scholar] [CrossRef]
- Fattahi, F.-S.; Khoddami, A.; Avinc, O. Poly(lactic acid) (PLA) Nanofibers for Bone Tissue Engineering. J. Text. Polym. 2019, 7, 47. [Google Scholar]
- Kian, L.; Saba, N.; Jawaid, M.; Sultan, M. A review on processing techniques of bast fibers nanocellulose and its polylactic acid (PLA) nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef]
- Li, W.; Fan, X.; Wang, X.; Shang, X.; Wang, Q.; Lin, J.; Hu, Z.; Li, Z. Stereocomplexed micelle formation through enantiomeric PLA-based Y-shaped copolymer for targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 688–695. [Google Scholar] [CrossRef]
- Maleki, H.; Barani, H. Stereocomplex electrospun fibers from high molecular weight of poly (L-lactic acid) and poly (D-lactic acid). J. Polym. Eng. 2020, 40, 136–142. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Hasan, A.; Wang, M.; Webster, T.J. CTGF loaded electrospun dual porous core-shell membrane for diabetic wound healing. Int. J. Nanomed. 2019, 14, 8573. [Google Scholar] [CrossRef] [Green Version]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly(L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Zou, F.; Sun, X.; Wang, X. Elastic, hydrophilic and biodegradable poly (1, 8-octanediol-co-citric acid)/polylactic acid nanofibrous membranes for potential wound dressing applications. Polym. Degrad. Stab. 2019, 166, 163–173. [Google Scholar] [CrossRef]
- Yang, C.; Yan, Z.; Lian, Y.; Wang, J.; Zhang, K. Graphene oxide coated shell-core structured chitosan/PLLA nanofibrous scaffolds for wound dressing. J. Biomater. Sci. Polym. Ed. 2020, 31, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Majchrowicz, A.; Roguska, A.; Krawczyńska, A.; Lewandowska, M.; Martí-Muñoz, J.; Engel, E.; Castano, O. In vitro evaluation of degradable electrospun polylactic acid/bioactive calcium phosphate ormoglass scaffolds. Arch. Civ. Mech. Eng. 2020, 20, 50. [Google Scholar] [CrossRef] [Green Version]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S.; Cascone, M.; Baldassare, A.; Lazzeri, L. Application of the dry-spinning method to produce poly (ε-caprolactone) fibers containing bovine serum albumin laden gelatin nanoparticles. J. Appl. Polym. Sci. 2016, 133, 44233. [Google Scholar] [CrossRef]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2016, 112, 1–17. [Google Scholar] [CrossRef]
- Spolenak, R.; Gorb, S.; Arzt, E. Adhesion design maps for bio-inspired attachment systems. Acta Biomater. 2005, 1, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Bolgen, N.; Vargel, I.; Korkusuz, P.; Menceloglu, Y.Z.; Piskin, E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81B, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lv, G.; Pan, C.; Song, M.; Wu, C.H.; Guo, D.D.; Wang, X.M.; Chen, B.A.; Gu, Z.Z. Poly(lactic acid) (PLA) based nanocomposites—A novel way of drug-releasing. Biomed. Mater. 2007, 2, L1–L4. [Google Scholar] [CrossRef]
- Conway, J.; Whettam, J. Adverse reactions to wound dressings. Nurs. Stand. 2002, 16, 52–60. [Google Scholar] [CrossRef]
- Stoddard, R.J.; Steger, A.L.; Blakney, A.K.; Woodrow, K.A. In pursuit of functional electrospun materials for clinical applications in humans. Ther. Deliv. 2016, 7, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing—Production, characterization, and in vivo behavior. J. Biomed. Mater. Res. 2011, 97B, 20–29. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Front. Bioeng. Biotech. 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wijeyaratne, S.M.; Kannangara, L. Safety and Efficacy of Electrospun Polycarbonate-Urethane Vascular Graft for Early Hemodialysis Access: First Clinical Results in Man. JAVA 2011, 12, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.T.; Rothwell, S.W.; Risdahl, J.; Martin, R.; Olson, C.; Rose, N. Salmon thrombin-fibrinogen dressing allows greater survival and preserves distal blood flow compared with standard kaolin gauze in coagulopathic swine with a standardized lethal femoral artery injury. J. Spec. Oper. Med. 2012, 12, 16–26. [Google Scholar]
- Silva, S.Y.; Rueda, L.C.; López, M.; Vélez, I.D.; Rueda-Clausen, C.F.; Smith, D.J.; Muñoz, G.; Mosquera, H.; Silva, F.A.; Buitrago, A.; et al. Double blind, randomized controlled trial, to evaluate the effectiveness of a controlled nitric oxide releasing patch versus meglumine antimoniate in the treatment of cutaneous leishmaniasis [NCT00317629]. Trials 2006, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.Y.; Rueda, L.C.; Márquez, G.A.; López, M.; Smith, D.J.; Calderón, C.A.; Castillo, J.C.; Matute, J.; Rueda-Clausen, C.F.; Orduz, A.; et al. Double blind, randomized, placebo controlled clinical trial for the treatment of diabetic foot ulcers, using a nitric oxide releasing patch: Pathon. Trials 2007, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-activated nanofibre textiles exert antibacterial effects in the setting of chronic wound healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Safety and Performance Study of a Novel Fibrin Dressing for Cancellous Bone Bleeding. Available online: https://stteresamedical.com/PDFS/SRS_2016_Abstr.pdf (accessed on 7 September 2020).
- Balain, B.; Craig, N.; Gnanalingham, K.; Madan, S.; Sharma, H.; Wynne-Jones, G.; Hoseth, J.; Øsytein, L.; Punsvik, V.; Sura, S.; et al. Safety and Efficacy of a Novel Fibrin Dressing on Bleeding Cancellous Bone. J Clin. Exp. Orthop. 2018, 4, 50. [Google Scholar] [CrossRef]
- Floyd, C.T.; Padua, R.A.; Olson, C.E. Hemostasis and Safety of a Novel Fibrin Dressing Versus Standard Gauze in Bleeding Cancellous Bone in a Caprine Spine Surgery Model. Spine Deform. 2017, 5, 310–313. [Google Scholar] [CrossRef]
- Performance of a Dextran-only DressingVersus SurgiClot®to Achieve Hemostasis in a Swine Injury ModelofCancellous BoneBleeding. Available online: https://stteresamedical.com/PDFS/170-19-ORS_2016_FINAL-copy.pdf (accessed on 7 September 2020).
- Spincare™. Available online: https://nanomedic.com/ (accessed on 7 September 2020).
- Case Report 1: Using Spincare™ for Donor Site Wounds. Available online: https://nanomedic.com/research/donor-site-wounds/ (accessed on 7 September 2020).
- Case Report 2: Using Spincare™ for Partial Thickness Wounds. Available online: https://nanomedic.com/research/partial-thickness-burns/ (accessed on 7 September 2020).
- Clinical Trials Using Electrospinning. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=electrospinning&cntry=&state=&city=&dist= (accessed on 7 September 2020).
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Bosworth, L.A. Travelling along the Clinical Roadmap: Developing Electrospun Scaffolds for Tendon Repair. Conf. Pap. Sci. 2014, 304974. [Google Scholar] [CrossRef] [Green Version]
- Paul, R. High Performance Technical Textiles: An Overview. In High Performance Technical Textiles: 2019; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 1–10. [Google Scholar]
- Gizaw, M.T.J.; Faglie, A.; Lee, S.; Neuenschwander, P.; Chou, S. Electrospun Fibers as a Dressing Material for Drug and Biological Agent Delivery in Wound Healing Applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing–a review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasouri, K. Novel estimation of morphological behavior of electrospun nanofibers with artificial intelligence system (AIS). Polym. Test. 2018, 69, 499–507. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Salsano, J.E.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste autochthonous tuscan olive leaves (Olea europaea var. olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef] [Green Version]
- Orlando, I.; Roy, I. Cellulose-Based Hydrogels for Wound Healing. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A Reference Series; Mondal, M., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–18. [Google Scholar] [CrossRef]








| Electrospun Mesh | Incorporated Therapeutics | Function & Wound Type | References |
|---|---|---|---|
| Cellulose acetate (CA)/Manuka honey (MH) | - | Antibacterial activity for infection in the burn wounds | [37] |
| CA/polyester urethane | Polyhexamethylene biguanide (PHMB) | Antimicrobial activity | [110] |
| Chitosan/bacterial nano cellulose | - | Antimicrobial properties | [122] |
| Chitosan/silk fibroin | - | Antibacterial properties Acute wounds | [121] |
| Chitosan/sericin | - | Biocompatibility and antibacterial properties | [120] |
| Chitosan/Poly (l-lactide) (PLLA) | Graphene oxide | Antimicrobial activities for chronic infected wounds | [50] |
| Chitosan/keratin/polycaprolactone (PCL) | Aloevera extract | Anti-inflammatory, antibacterial, antiviral, and antioxidant properties for acute and burn wounds | [35] |
| Chitosan/Polyvinyl alcohol (PVA) | Nanobioglass (nBG) | Biocompatibility, antibacterial activity and regeneration promotion effect for chronic wound | [5] |
| Gelatin/Oleoyl chitosan | - | Large skin defects or chronic wounds | [123] |
| Polyhydroxyalkanoate (PHA) | Dodecyltrimethylammonium chloride (DTAC) biocide | Antimicrobial effects for chronic wounds | [160] |
| PHA | Graphene/decorated silver nanoparticles (GAg) | Antimicrobial activity for chronic wounds | [141] |
| Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) | Cerium Oxide Nanoparticle | Antioxidant and angiogenic properties Diabetic wounds | [8] |
| PHBV | Cerium oxide nanoparticles (nCeO2) | Antioxidant and angiogenic properties for diabetic wounds | [8] |
| PHBV | Curcumin | Antioxidant, anti-inflammatory, and antitumor properties chronic wounds including burns, diabetic foot ulcers, venous leg ulcers, and pressure ulcers | [39] |
| PHBV/cellulose | ZnO nanocrystals | Antibacterial activity for acute and infected wounds | [154] |
| Polylactides (PLA) | AgNPs | Antimicrobial activity for burn wounds and diabetic ulcers | [87] |
| PLA | Curcumin, Enrofloxacin | Antioxidant activity, antimicrobial activity, and biocompatibility | [70] |
| PLA | Doxycycline (DCH) | Antibacterial activity, Chronic wounds, diabetic wounds | [42] |
| PLA | Silver (I) metal–organic framework Ag2[HBTC][im] | Antibacterial feature | [10] |
| PLLA | Curcumin | Anti-inflammatory antioxidant effects | [166] |
| PLA/hyperbranched polyglycerol (HPG) | Curcumin | Antioxidant, anti-inflammatory and anti-infective properties for acute and chronic wound | [31] |
| PLA/PVA | Connective tissue growth factor (CTGF) | Diabetic wounds | [165] |
| poly(lactic-co-glycolic acid) (PLGA)/gelatin | Recombinant human epidermal growth factor (rhEGF), gentamicin sulfate | - Antibacterial activity and rhEGF supply - Diabetic wound | [90] |
| PLGA/polydopamine | Basic fibroblast growth factor (bFGF), ponericin G1 | Antibacterial and cell proliferation-promoting properties for skin tissue regeneration | [89] |
| Product | Polymer | Device | Current Status | Case Studie or Clinical Trial Performed/Ongoing | References |
|---|---|---|---|---|---|
| Pathon | Polyurethane | Composite mesh, NO drug delivery | Clinical trial | Two double blind, randomized controlled clinical trials | [182,183] |
| Tecophilic™ | Polyurethane-PEG | Photosensitizing-loaded mesh | Clinical trial | Comparative, 3-group based study over a total of 162 patients | [184] |
| SurgiCLOT® | Dextran | Fibrin Sealant Patch | On the market | Clinical safety and performance study in UK and Norway, Clinical safety and performance study in India, Pre-clinical comparative study of bone bleeding treated with SurgiCLOT® and standard gauze, Pre-clinical Performance study of SurgiCLOT® compared to Dextran-only dressing | [181,185,186,187,188] |
| SpinCare™ | Various electrospinnable polymers | Portable electrospinning wound dressing device | On the market | Donor site wound single case Partial thickness burns single case | [189,190,191] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. https://doi.org/10.3390/jfb11030067
Azimi B, Maleki H, Zavagna L, De la Ossa JG, Linari S, Lazzeri A, Danti S. Bio-Based Electrospun Fibers for Wound Healing. Journal of Functional Biomaterials. 2020; 11(3):67. https://doi.org/10.3390/jfb11030067
Chicago/Turabian StyleAzimi, Bahareh, Homa Maleki, Lorenzo Zavagna, Jose Gustavo De la Ossa, Stefano Linari, Andrea Lazzeri, and Serena Danti. 2020. "Bio-Based Electrospun Fibers for Wound Healing" Journal of Functional Biomaterials 11, no. 3: 67. https://doi.org/10.3390/jfb11030067
APA StyleAzimi, B., Maleki, H., Zavagna, L., De la Ossa, J. G., Linari, S., Lazzeri, A., & Danti, S. (2020). Bio-Based Electrospun Fibers for Wound Healing. Journal of Functional Biomaterials, 11(3), 67. https://doi.org/10.3390/jfb11030067









