Silver Nanoparticles: Mechanism of Action and Probable Bio-Application
Abstract
1. Introduction
2. Mechanism of Silver Nanoparticles (AgNPs) Biosynthesis
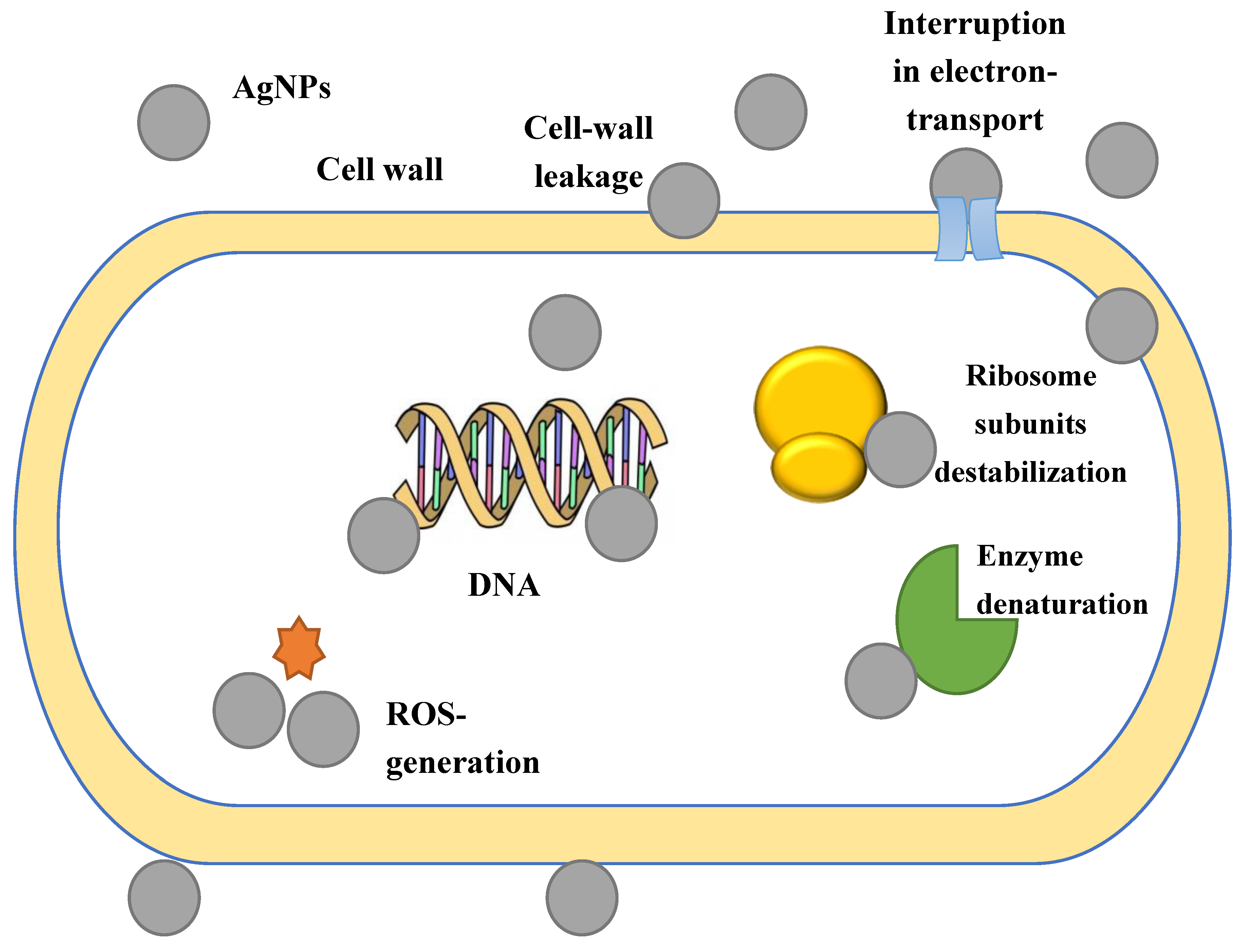
3. Mechanism of AgNP Action on Cells
4. Biomedical Application of AgNPs
4.1. Antibacterial and Antifungal Activity
4.2. Antiviral Activity
4.3. Larvicidal Activity and Antiplasmodial Activity
4.4. Anthelmintic Activity
4.5. Leishmanicidal Activity
4.6. Antioxidant Activity
4.7. Anti-Cancer Activity
4.8. Other Bioactivities
4.8.1. Anti-Diabetic Activity
4.8.2. Anti-Inflammatory Activity
4.8.3. Anti-Alzheimer Activity
4.8.4. Application in Medical Equipment
5. Conclusions
Funding
Conflicts of Interest
References
- Henglein, A. Small-particle research: Physicochemical properties of extremely small colloidal metal and semiconductor particles. Chem. Rev. 1989, 89, 1861–1873. [Google Scholar] [CrossRef]
- Shahverdy, A.R.; Fakhimi, A.; Minaian, S. Synthesis and effect of silver nanopracles on the antibacterial activity of different antibiotics against Staphylococcus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Landsdown, A.B.G. Silver in Healthcare: Its Antimicrobial Efficacy and Safety in Use; Royal Society of Chemistry: Cambridge, UK, 2010; p. 217. [Google Scholar]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Mikhailova, E.O. Elemental silver nanoparticles. Biosynthesis and bio application. Materials 2019, 12, 3177. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano-and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, D.; Hao, F.; Liu, R. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 2015, 292, 98–107. [Google Scholar] [CrossRef]
- Abdelmonem, A.M. Charge and agglomeration dependent in vitro uptake and cytotoxicity of zinc oxide nanoparticles. J. Inorg. Biochem. 2015, 153, 334–338. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods 2011, 21, 13–24. [Google Scholar] [CrossRef]
- Park, E.-J. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. Vitr. 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Luoma, S.N. Silver nanotechnologies and the environment. Proj. Emerg. Nanotechnol. Rep. 2008, 15, 12–13. [Google Scholar]
- Mohamed El Mahdy, M.; Salah, T.; Sayed Aly, H.; Mohammed, F.; Shaalan, M. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp. Toxicol. Pathol. 2015, 67, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moaca, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable peg-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; Tonk, E.C.M.; de la Fonteyne-Blankestijn, L.J.; Gremmer, E.R.; Verharen, H.W.; van der Ven, L.T.; van Loveren, H.; de Jong, W.H. Immunotoxicity of silver nanoparticles in an intravenous 28-day repeated-dose toxicity study in rats. Part. Fibre Toxicol. 2014, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Imam, M.S.; Paredes, A.M.; Bryant, M.S.; Cunningham, C.K.; Felton, R.P.; Jones, M.Y.; Davis, K.J.; Olson, G.R. Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the Sprague Dawley rat following daily oral gavage administration for 13 weeks. Toxicol. Sci. 2016, 150, 131–160. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological studies on silver nanoparticles: Challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 2011, 6, 879–898. [Google Scholar] [CrossRef]
- Burdus, C.; Gherasim, O.; Grumezescu, A.M.; Mogoanta, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef]
- Elbeshehy, E.K.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef]
- Mokhtari, N.; Daneshpajouh, S.; Seyedbagheri, S.; Atashdehghan, R.; Abdi, K.; Sarkar, S.; Minaian, S.; Shahverdi, H.R.; Shahverdi, A.R. Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: The effects of visible-light irradiation and the liquid mixing process. Mater. Res. Bull. 2009, 44, 1415–1421. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, A.; Abraham, J. A biological approach to synthesis of silver nanoparticles with Streptomyces sp. JAR1 and its antimicrobial activity. Sci. Pharm. 2013, 81, 607–621. [Google Scholar] [CrossRef]
- Otari, S.; Patil, R.; Nadaf, N.; Ghosh, S.; Pawar, S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Mater. Lett. 2012, 72, 92–94. [Google Scholar] [CrossRef]
- Natarajan, K.; Selvaraj, S.; Murty, V.R. Microbial production of silver nanoparticles. Dig. J. Nanomater. Biostr. 2010, 5, 135–140. [Google Scholar]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 4, 581890. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277–4290. [Google Scholar]
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J.; Ul-Haq, M. Growth Kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli. Biomed. Res. Int. 2014, 2014, 753419. [Google Scholar] [CrossRef]
- Rathod, V.; Banu, A.; Ranganath, E. Biosynthesis of highly stabilized silver nanoparticles by Rhizopus stolonifer and their Anti-fungal efficacy. Int. J. Mol. Clin. Microbiol. 2011, 1, 65–70. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Abdel Hafez, E.H.; Ahmed, E.A.; Abbas, H.S.; Salah El Din, R.A. Efficacy of Antibiotics Combined with Biosynthesized Silver Nanoparticles on some Pathogenic Bacteria. IJSR 2017, 6, 1294–1303. [Google Scholar]
- El-Baghdady, K.Z.; El-Shatoury, E.H.; Abdullah, O.M.; Khalil, M.M.H. Biogenic production of silver nanoparticles by Enterobacter cloacae Ism26. Turk. J. Biol. 2018, 42, 319–328. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Lu, Y.; Sun, D.; Lin, X.; Deng, X.; He, N.; Zheng, S. Biosorption and bioreduction of diamine silver complex by Corynebacterium. J. Chem. Technol. Biotechnol. 2005, 80, 285–290. [Google Scholar] [CrossRef]
- Alani, F.; Moo-Young, M.; Anderson, W. Biosynthesis of silver nanoparticles by a new strain of Streptomyces sp. compared with Aspergillus fumigatus. World J. Microbiol. Biotechnol. 2012, 28, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Ahmad, A.; Islam, N.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Das, V.L.; Thomas, R.; Varghese, R.T.; Soniya, E.V.; Mathew, J.; Radhakrishnan, E.K. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. 3 Biotech 2013, 4, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Golkaran, D.; Eslamifar, A.; Jamalifar, H.; Fazeli, M.R.; Mohseni, F.A. Intra/extracellular biosynthesis of silver nanoparticles by an autochthonous strain of Proteus mirabilis isolated from photographic waste. J. Biomed. Nanotechnol. 2009, 5, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Jeevan, P.; Ramya, K.; Edith Rena, A. Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas Aeruginosa. Indian J. Biotechnol. 2012, 11, 72–76. [Google Scholar]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Ali, J.; Hameed, A.; Ahmed, S.; Ali, M.I.; Zainab, S.; Ali, N. Role of catalytic protein and stabilising agents in the transformation of Ag ions to nanoparticles by Pseudomonas aeruginosa. IET Nanobiotechnol. 2016, 10, 295–300. [Google Scholar] [CrossRef]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Zomorodian, K.; Pourshahid, S.; Sadatsharifi, A. Biosynthesis and characterization of silver nanoparticles by Aspergillus species. Biomed Res. Int. 2016, 2016, 5435397. [Google Scholar] [CrossRef]
- Deepak, V.; Kalishwaralal, K.; Pandian, S.R.K. An insight into the bacterial biogenesis of silver nanoparticles, industrial production and scale-up. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–35. [Google Scholar]
- Stewart, V.; Lu, Y.; Darwin, A.J. Periplasmic nitrate reductase (Napabc eEnzyme) supports anaerobic respiration by Escherichia coli K-12. J. Bacteriol. 2002, 184, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.; Vig Slenters, T.N.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver coordination polymers for prevention of implant infection: Thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2009, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, B.; Huang, P.J.; Liu, J. Rationally designed nucleobase and nucleotide coordinated nanoparticles for selective DNA adsorption and detection. Anal. Chem. 2013, 85, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Hahn, H.J.; Nam, K.; Choi, W.H.; Jeong, Y.; Kim, D.E.; Park, J.S. Evaluation of generations 2, 3 and 4 arginine modified PAMAM dendrimers for gene delivery. Int. J. Pharm. 2008, 363, 199–205. [Google Scholar] [CrossRef]
- Graf, P.; Mantion, A.; Foelske, A.; Shkilnyy, A.; Masic, A.; Thunemann, A.F.; Taubert, A. Peptide-coated silver nanoparticles: Synthesis, surface chemistry, and pH-triggered, reversible assembly into particle assemblies. Chemistry 2009, 15, 5831–5844. [Google Scholar] [CrossRef] [PubMed]
- Prasad, G.K.; Ramacharyulu, P.V.; Merwyn, S.; Agarwal, G.S.; Srivastava, A.R.; Singh, B.; Rai, G.P.; Vijayaraghavan, R. Photocatalytic inactivation of spores of Bacillus anthracis using titania nanomaterials. J. Hazard. Mater. 2010, 185, 977–982. [Google Scholar] [CrossRef]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chemistry 2007, 13, 3160–3168. [Google Scholar] [CrossRef]
- Nam, K.T.; Lee, Y.J.; Krauland, E.M.; Kottmann, S.T.; Belcher, A.M. Peptide-mediated reduction of silver ions on engineered biological scaffold. ACS Nano 2008, 2, 1480–1486. [Google Scholar] [CrossRef]
- Wei, X.; Luo, M.; Li, W.; Yang, L.; Liang, X.; Xu, L. Bioresource technology synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour. Technol. 2012, 103, 273–278. [Google Scholar] [CrossRef]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid antimicrobial enzyme and silver nanoparticle coatings for medical instruments. ACS Appl. Mater. Inter. 2009, 1, 1553–1560. [Google Scholar] [CrossRef]
- Javaid, A.; Folarin Oloketuyi, S.; Khan, M.M.; Khan, F. Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Synthesis of AgNPs by Bacillus cereus bacteria and their antimicrobial potential. JBNB 2011, 2, 155–161. [Google Scholar] [CrossRef]
- Revathi, K.; Shaifali, S.; Mohd, A.K.; Suneetha, V. A potential strain of keratinolytic bacteria VIT RSAS2 from katpadi and its pharmacological benefits. Int. J. Pharm. Sci. Res. 2013, 20, 89–92. [Google Scholar]
- Lateef, A.; Adelere, I.A.; Gueguim-Kana, E.B.; Asafa, T.B.; Beukes, L.S. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13 A. Int. Nano Lett. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, J.; Chattopadhayay, D.; Patra, S.; Chakraborty, A.; Acharya, K. Production of silver nanoparticles by a phytopathogenic fungus Bipolaris nodulosa and its antimicrobial activity. Dig. J. Nanomater. Biostruct. 2010, 5, 887–895. [Google Scholar]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Kumar, C.G.; Mamidyala, S.K.; Das, B.; Sridhar, B.; Sarala Devi, G.; Karuna, M.S. Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa Bs-161R. J. Microbiol. Biotechnol. 2010, 20, 1061–1068. [Google Scholar] [CrossRef]
- Mandal, S.; Phadtare, S.; Sastry, M. Interfacing biology with nanoparticles. Curr. Appl. Phys. 2005, 5, 118–127. [Google Scholar] [CrossRef]
- Jayalakashmi, S.; Dinakaran, S. Biosynthesis and characterization of silver nanoparticles by a marine strain Escherichia coli SJ101. Int. J. Innov. Res. 2013, 1, 1–35. [Google Scholar]
- Kumar, C.G.; Mamidyala, S.K. Extracellular synthesis of silver nanoparticles using culture supernatant of Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2011, 84, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.K.; Kumar, V.; Bera, T.; Saxena, P.S.; Nath, G.; Srivastava, S.K.; Giri, R.; Srivastava, A. Study of mechanism of enhanced antibacterial activity by green synthesis of silver nanoparticles. Nanotechnology 2011, 22, 415104. [Google Scholar] [CrossRef] [PubMed]
- Carmel Mary, R.; Panneerselvam, A.; Arokia Vijaya Anand, M.; Ernest, D. Mycosynthesized Silver Nanoparticles from marine derived Aspergillus terreus and its bactericidal activity against clinical pathogens. Int. J. S. Res. Sci. Tech. 2018, 4, 922–932. [Google Scholar]
- Abdel Ghany, T.M.; Kasem, W.T.; Nabih, M.A.; Mabrouk, A.S. Dual synergistic actions of silver nanoparticles with natural products on Ochratoxin A production. Life Sci. J. 2017, 14, 65–71. [Google Scholar]
- Paul, D.; Sinha, S.N. Extracellular synthesis of silver nanoparticles using Pseudomonas aeruginosa KUPSB12 and its antibacterial activity. JJBS 2014, 7, 245–250. [Google Scholar] [CrossRef]
- Busi, S.; Rajkumari, J.; Ranjan, B.; Karuganti, S. Green rapid biogenic synthesis of bioactive silver nanoparticles (AgNPs) using Pseudomonas aeruginosa. IET Nanobiotechnol. 2014, 8, 267–274. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Binti Zahrudin, A.; Dash, G. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int. J. Mod. Sci. 2018, 4, 86–92. [Google Scholar] [CrossRef]
- Sarsar, V.; Selwal, M.K.; Selwal, K.K. Biofabrication, characterization and antibacterial efficacy of extracellular silver nanoparticles using novel fungal strain of Penicillium atramentosum. J. Saudi Chem. Soc. 2015, 19, 682–688. [Google Scholar] [CrossRef]
- Kumar, P.S.; Balachandran, C.; Duraipandiyan, V.; Ramasamy, D.; Ignacimuthu, S.; Al-Dhabi, N.A. Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties. Appl. Nanosci. 2015, 5, 169–180. [Google Scholar] [CrossRef]
- Prasad, R. Synthesis of Silver Nanoparticles in Photosynthetic Plants. J. Nanoparticles 2014, 2014, 963961. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Prasad, R. Nanobiotechnology Applications in Plant Protection; Springer International Publishing: Basel, Switzerland, 2015; pp. 271–288. [Google Scholar]
- Tripathi, R.M.; Rana, D.; Shrivastava, A.; Singh, R.P.; Shrivastav, B.R. Biogenic synthesis of silver nanoparticles using Saraca indica leaf extract and evaluation of their antibacterial activity. Nano Biomed. Eng. 2013, 5, 50–56. [Google Scholar] [CrossRef]
- Mashwani, Z.R.; Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, A.; Vigneshwaran, K.; Loganathan, K. Green synthesis of silver nanoparticles from Ficus racemosa. L extract and their antifungal activity. Nano Biomed. Eng. 2014, 10, 93–96. [Google Scholar]
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-biofilm effects of gold and silver nanoparticles synthesized by the Rhodiola rosea rhizome extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, 886–899. [Google Scholar] [CrossRef]
- Kasithevar, M.; Saravanan, M.; Prakash, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z.K. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J. Interdiscip. Nanomed. 2017, 2, 131–141. [Google Scholar] [CrossRef]
- Benakashani, F.; Allafchian, A.R.; Jalali, S.A.H. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int. J. Mod. Sci. 2016, 2, 251–258. [Google Scholar] [CrossRef]
- Mandal, P.; Babu, S.S.; Mandal, N. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia 2005, 76, 462–465. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Kirthi, A.V.; Jayaseelan, C.; Bagavan, A.; Zahir, A.A.; Elango, G.; Kamaraj, C. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol. Res. 2011, 108, 1541–1549. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, T.; Nadhman, A. Applications of plant terpenoids in the synthesis of colloidal silver nanoparticles. Adv. Colloid Interface Sci. 2016, 234, 132–141. [Google Scholar]
- Ebrahiminezhad, A.; Taghizadeh, S.; Ghasemi, Y. Green synthesis of silver nanoparticles using mediterranean cypress (Cupressus sempervirens) Leaf Extract. Am. J. Biochem. Biotechnol. 2017, 13, 1–6. [Google Scholar] [CrossRef]
- VonWhite, G.; Kerscher, P.; Brown, R.M.; Morella, J.D.; McAllister, W.; Dean, D.; Kitchens, C.L. Green Synthesis of Robust, Biocompatible Silver Nanoparticles Using Garlic Extract. J. Nanomater. 2012, 2012, 730746. [Google Scholar]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess. Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Chitte, H.K.; Bhat, N.V.; Karmakar, N.S.; Kothari, B.C.; Shinde, G.N. Tuning of refractive index of poly(vinyl alcohol): Effect of embedding Cu and Ag nanoparticles tuning of refractive index of poly(vinyl alcohol): Effect of embedding Cu and Ag nanoparticles. World J. Nanosci. Eng. 2012, 2, 19–24. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Peng, H.C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Kumar Mandal, A.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- El-Rafie, M.H.; El-Naggar, M.E.; Ramadan, M.A.; Fouda, M.M.G.; Al-Dey, S.S.; Hebeish, A. Environmental synthesis of silver nanoparticles using hydroxypropyl starch and their characterization. Carbohydr. Polym. 2011, 86, 630–635. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Chauhan, N.; Tyagi, A.K.; Kumar, P.; Malik, A. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 27, 1712–1720. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Nagam, V.; Bobbu, P.; Ghosh, S.B.; Tartte, V. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl. Nanosci. 2014, 5, 123–129. [Google Scholar] [CrossRef]
- Rashid, M.M.O.; Akhter, K.N.; Chowdhury, J.A.; Hossen, F.; Hussain, M.S.; Hossain, M.T. Characterization of phytoconstituents and evaluation of antimicrobial activity of silver extract nanoparticles synthesized from Momordica charantia fruit extract. BMC Complement. Altern. Med. 2017, 17, 336–341. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Kaur, P.; Bajaj, R.; Rawat, M. A review on green synthesis and characterization of silver nanoparticles and their application: A green nanoworld. World J. Pharm. Pharm. Sci. 2016, 6, 730–762. [Google Scholar]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; Bispo de Jesus, M.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, J.S.; Groena Infante, H.; Stokes, E.; Shaw, A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electro-chemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; AbdAllah, E.F.; Mohanta, T.K. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-M.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 1–17. [Google Scholar] [CrossRef]
- Jena, P.; Bhattacharya, M.; Bhattacharjee, G.; Satpati, B.; Mukherjee, P.; Senapati, D.; Srinivasan, R. Bimetallic gold-silver nanoparticles mediate bacterial killing by disrupting the actin cytoskeleton MreB. Nanoscale 2020, 12, 3731–3749. [Google Scholar] [CrossRef]
- Cakic, M.; Glisic, S.; Nikolic, G. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. J. Mol. Struct. 2016, 1110, 156–161. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Gamboa, S.M.; Rojas, E.R.; Martínez, V.V.; Vega-Baudrit, J. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosen. Bioelectron. 2019, 5, 166–173. [Google Scholar]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Mosleh, A.M.; Abd Elghany, A.; Shams-Eldin, E.; Serea, E.S.A.; Alia, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef]
- Bao, H.; Yu, X.; Xu, C.; Li, X.; Li, Z.; Wei, D.; Liu, Y. New Toxicity Mechanism of Silver Nanoparticles: Promoting Apoptosis and Inhibiting Proliferation. PLoS ONE 2015, 10, e0122535. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Gaddam, S.A.; Rao, Y.S.; Prasad, T.N.V.K.V.; Reddy, A.V.; Gopal, D.V.R.S. Biofabrication of silver nanoparticles by Andrographis paniculata. Eur. J. Chem. 2014, 73, 135–140. [Google Scholar] [CrossRef]
- Shanmugam, C.; Sivasubramanian, G.; Parthasarathi, B.; Baskaran, K.; Balachander, R.; Parameswaran, V.R. Antimicrobial, free radical scavenging activities and catalytic oxidation of benzyl alcohol by nano-silver synthesized from the leaf extract of Aristolochia indica L.: A promenade towards sustainability. Appl. Nanosci. 2016, 6, 711–723. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ion son Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103–225111. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The role of p53 in apoptosis. Discov. Med. 2010, 9, 145–152. [Google Scholar] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Ruíz-Baltazar, A.J.; Reyes-López, S.Y.; Larrañaga, D.; Estévez, M.; Pérez, R. Green synthesis of silver nanoparticles using a Melissa officinalis leaf extract with antibacterial properties. Results Phys. 2017, 7, 2639–2643. [Google Scholar] [CrossRef]
- Alharbi, F.A.; Alarfaj, A.A. Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J. King Saud Univ. Sci. 2020, 32, 1346–1352. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, N.A.; Ros, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biró, D.; et al. Antibacterial and antioxidant potential of silver nanoparticles biosynthesized using the spruce bark extract. Nanomaterials 2019, 9, 1541. [Google Scholar] [CrossRef]
- Rodríguez-Luis, O.E.; Hernandez-Delgadillo, R.; Sánchez-Nájera, R.I.; Martínez-Castañón, G.A.; Niño-Martínez, N.; del Carmen Sánchez Navarro, M.; Ruiz, F.; Cabral-Romero, C. Green synthesis of silver nanoparticles and their bactericidal and antimycotic activities against oral microbes. J. Nanomater. 2016, 2016, 9204573. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Prasad, T.N.V.K.V.; Shiekh, R.A.; Balam, S.K.; Narasimhulu, G.; Reddy, C.S.; Ab Rahman, I.; Gan, S.H. Biogenic silver nanoparticles using Rhinacanthus nasutus leaf extract: Synthesis, spectral analysis, and antimicrobial studies. Int. J. Nanomed. 2013, 8, 3355–3364. [Google Scholar] [CrossRef]
- Puiso, J.; Jonkuviene, D.; Macioniene, I.; Salomskiene, J.; Jasutiene, I.; Kondrotas, R. Biosynthesis of silver nanoparticles using lingonberry and cranberry juices and their antimicrobial activity. Colloids Surf. B Biointerfaces 2014, 121, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.L.; Savithramma, N. Antimicrobial activity of silver nanoparticles synthesized by using stem extract of Svensonia hyderobadensis (Walp.) Mold–A rare medicinal plant. Res. Biotech. 2010, 3, 41–47. [Google Scholar]
- Vankar, S.P.; Shukla, D. Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl. Nanosci. 2012, 2, 163–168. [Google Scholar] [CrossRef]
- Elumalai, E.K.; Prasad, T.N.V.K.V.; Kambala, V.; Nagajyothi, P.C.; David, E. Green synthesis of silver nanoparticle using Euphorbia hirta L. and their antifungal activities. Arch. Appl. Sci. Res. 2010, 2, 76–81. [Google Scholar]
- Latha, M.; Priyanka, M.; Rajasekar, P.; Manikandan, R.; Prabhu, N.M. Biocompatibility and antibacterial activity of the Adathoda vasica Linn extract mediated silver nanoparticles. Microb. Pathog. 2016, 93, 88–94. [Google Scholar] [CrossRef]
- Phull, A.-R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliate. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Agarwal, P.; Mehta, A.; Kachhwaha, S.; Kothari, S.L. Green Synthesis of Silver Nanoparticles and Their Activity Against Mycobacterium tuberculosis. Adv. Sci. Eng. Med. 2013, 5, 1–6. [Google Scholar] [CrossRef]
- Hussein, R.R.S.; Farghali, A.A.; Hassanein, A.H.A.; Ibraheem, I.B.M. Biosynthesis of silver nanoparticles by using of the marine alga Gracilaria parvispora and its antagonistic efficacy against some common skin infecting pathogens. Aust. J. Basic Appl. Sci. 2017, 11, 219–227. [Google Scholar]
- Rajeshkumar, S.; Malarkodi, C.; Paulkumar, K.; Vanaja, M.; Gnanajobitha, G.; Annadurai, G. Algae Mediated Green Fabrication of Silver Nanoparticles and Examination of Its Antifungal Activity against Clinical Pathogens International. J. Met. 2014, 2014, 692643. [Google Scholar]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.; Husen, A.; Rehman, S. Biogenic fabrication and characterization of silver nanoparticles using aqueousethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 1–9. [Google Scholar] [CrossRef]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Samundeeswari, A.; Dhas, S.P.; Nirmala, J.; John, S.P.; Mukherjee, A.; Chandrasekaran, N. Biosynthesis of silver nanoparticles using actinobacterium Streptomyces albogriseolus and its antibacterial activity. Biotechnol. Appl. Biochem. 2012, 9, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, H.R. Biosynthesis of silver nanoparticles using Salmonella typhirium. J. Nano. Chem. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Horbani, H.R.; Soltani, S. Antibacterial Effects of Silver Nanoparticles on Escherichia coli and Bacillus subtilis. Orient. J. Chem. 2015, 31, 341–344. [Google Scholar] [CrossRef]
- Saravanan, M.; Jacob, V.; Arockiaraj, J.; Prakash, P. Extracellular biosynthesis, characterization and antibacterial activity of silver nanoparticles synthesized by Bacillus subtilis (NCIM-2266). J. Bionanosci. 2014, 8, 21–27. [Google Scholar] [CrossRef]
- Nanda, A.; Majeed, S. Enhanced antibacterial efficacy of biosynthesized AgNPs from Penicillium glabrum (MTCC1985) pooled with different drugs. Int. J. PharmTech Res. 2014, 6, 217–223. [Google Scholar]
- Majeed, S.; Abdullah, M.S.; Nanda, A.; Ansari, M.T. In vitro study of the antibacterial and anticancer activities of silver nanoparticles synthesized from Penicillium brevicompactum (MTCC-1999). J. Taibah Univ. Sci. 2016, 10, 614–620. [Google Scholar] [CrossRef]
- Wang, C.; Kim, Y.J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1127–1132. [Google Scholar]
- Singh, H.; Du, J.; Yi, T.H. Biosynthesis of silver nanoparticles using Aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif. Cells Nanomed. Biotechnol. 2017, 45, 584–590. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; Mubarak, A.D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathogen. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Gupta, R.; Kumar, G.; Kumari, S.; Biswas, S.; Padmanabhan, P. Synthesis of silver nanoparticles by Bacillus clausii and computational profiling of nitrate reductase enzyme involved in production. J. Genet. Eng. Biotechnol. 2018, 16, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Sanjivkumar, M.; Vaishnavi, R.; Neelakannan, M.; Kannan, D.; Silambarasan, T.; Immanuel, G. Investigation on characterization and biomedical properties of silver nanoparticles synthesized by an actinobacterium Streptomyces olivaceus (MSU3). Biocatal. Agric. Biotechnol. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Gu, X.; Li, J.; Lin, X. Biosynthesis, characterization and antibacterial activity of silver nanoparticles by the Arctic anti-oxidative bacterium Paracoccus sp. Arc7-R13. Artif. Cells Nanomedi. Biotechnol. 2019, 47, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Chandrakanth, K.R.; Ashajyothi, C.; Oli, A.K.; Prabhurajeshwar, C. Potential bactericidal effect of silver nanoparticles synthesized from Enterococcus species. Orient. J. Chem. 2014, 30, 1253–1262. [Google Scholar]
- Shaker, M.A.; Shaaban, M.I. Synthesis of silver nanoparticles with antimicrobial and anti-adherence activities against multidrug-resistant isolates from Acinetobacter baumannii. J. Taibah Univ. Med. Sci. 2017, 12, 291–297. [Google Scholar] [CrossRef]
- Mohamedin, A.; El-Naggar, N.E.; Hamza, S.S.; Sherief, A.A. Green synthesis, characterization and antimicrobial activities of silver nanoparticles by Streptomyces viridodiastaticus SSHH-1 as a living nanofactory: Statistical optimization of process variables. Curr. Nanosci. 2015, 11, 640–654. [Google Scholar] [CrossRef]
- Al-Khafaji, M.H.M.; Majeed, S.M.A.; Basi, R.Q. Biosynthesis of Silver Nanoparticles by Food-Origin E. coli and Candida Species and Testing Its Antimicrobial Activity against Pathogenic Bacteria and Fungi. IOSR J. Pharm. Biol. Sci. 2017, 12, 29–34. [Google Scholar] [CrossRef]
- Singha, H.; Du, J.; Singh, P.; Tae Hoo, Y. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1.4 and their antimicrobial application. J. Pharm. Anal. 2018, 8, 258–264. [Google Scholar] [CrossRef]
- Abdeen, S.; Geo, S.; Praseetha, P.K.; Dhanya, R.P. Biosynthesis of Silver nanoparticles from Actinomycetes for therapeutic applications. Int. J. Nano Dimens. 2014, 5, 155–162. [Google Scholar]
- Ranjan, R.; Nilotpala, N.; Behera, D.; Pradhan, K.M.; Mishra, S.; Sukla, B.L.; Mishra, B.K. Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: The process and optimization. J. Nanopart. Res. 2011, 13, 3129–3137. [Google Scholar]
- Singh, D.; Rathod, V.; Ninganagouda, S.; Herimath, J.; Kulkarni, P. Biosynthesis of silver nanoparticle by endophytic fungi Pencillium sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram-negative bacteria. J. Pharm. Res. 2013, 7, 448–453. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2017, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Majeed, S.; Abdullah, M.S.; Dash, G.K.; Ansari, M.T.; Nanda, A. Biochemical synthesis of silver nanoparticles using filamentous fungi Penicillium decumbens (MTCC-2494) and its efficacy against A-549 lung cancer cell line. Chin. J. Nat. Med. 2016, 14, 615–620. [Google Scholar]
- Shelar, G.B.; Chavan, A.M. Fusarium semitectum mediated extracellular synthesis of silver nanoparticles and their antibacterial activity. IJBAR 2014, 5, 348–351. [Google Scholar]
- Abd, F.G.; Mohsen, L.Y.; Al Shalah, L.A.M.; Alkaim, A.F. Silver nanoparticles synthesized by using Pseudomonas aeruginosa synergistically act with antibiotic. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 20, 50–52. [Google Scholar]
- Vidya, P.; Subramani, G. Fungus Mediated Synthesis of Silver Nanoparticles Using Aspergillus flavus and Its Antibacterial Activity against Selective Food Borne Pathogens. Indo Am. J. Pharm. Sci. 2017, 4, 4627–4634. [Google Scholar]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Keskin, N.O.S.; Kılıç, N.K.; Dönmez, G.; Tekina, T. Green synthesis of silver nanoparticles using cyanobacteria and evaluation of their photocatalytic and antimicrobial activity. J. Nano Res. 2016, 40, 120–127. [Google Scholar] [CrossRef]
- Al Rashed, S.; Al Shehri, S.; Moubayed, N.M.S. Extracellular biosynthesis of silver nanoparticles from Cyanobacteria. Biomed. Res. 2018, 29, 2859–2862. [Google Scholar] [CrossRef]
- Tăbăran, A.-F.; Matea, C.T.; Mocan, T.; Tăbăran, A.; Mihaiu, M.; Iancu, C.; Mocan, L. Silver nanoparticles for the therapy of tuberculosis. Int. J. Nanomed. 2020, 15, 2231–2258. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Benelli, G. Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J. Clust. Sci. 2017, 28, 1709–1727. [Google Scholar] [CrossRef]
- Hwang, I.-S.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silvernanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Lv, X.; Wang, P.; Bai, R.; Cong, Y.; Suo, S.; Ren, X.; Chen, C. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 2014, 35, 4195–4203. [Google Scholar] [CrossRef]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 1–9. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 20, 1–6. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Itxtepan-Turren, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.S. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. J. Curr. Med. Res. Opin. 2020, 3, 449–450. [Google Scholar] [CrossRef]
- Nakamura, S.; Sato, M.; Sato, Y.; Ando, N.; Takayama, T.; Fujita, M.; Ishihara, M. Synthesis and application of silver nanoparticles (Ag-NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019, 20, 3620. [Google Scholar] [CrossRef] [PubMed]
- Nalini, M.; Lena, M.; Sumathi, P.; Sundaravadivelan, C. Effect of phyto-synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egypt. J. Basic Appl. Sci. 2017, 4, 212–218. [Google Scholar] [CrossRef]
- Velayutham, K.; Ramanibai, R. Larvicidal activity of synthesized silver nanoparticles using isoamyl acetate identified in Annona squamosa leaves against Aedes aegypti and Culex quinquefasciatus. J. Basic Appl. Zool. 2016, 74, 16–22. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control 2017, 2, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Jianchu, M. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef]
- Benell, G.; Caselli, A.; Canale, A. Nanoparticles for mosquito control: Challenges and constraints. J. King Saud Univ. Sci. 2017, 29, 424–435. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Kumar, P.M.; Dinesh, D.; Chandramohan, B.; Suresh, U.; Nicoletti, M.; et al. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: Towards an integrative approach? Environ. Sci. Pollut. Res. Int. 2015, 22, 20067–20083. [Google Scholar] [CrossRef]
- Benelli, G. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: A review. Parasitol. Res. 2016, 115, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef]
- Mohapatra, S.C.; Tiwari, H.K.; Singla, M.; Rathi, B.; Sharma, A.; Mahiya, K. Antimalarial evaluation of copper (II) nanohybrid solids: Inhibition of plasmepsin II, a hemoglobin-degrading malarial aspartic protease from Plasmodium falciparum. JBIC 2010, 15, 373–385. [Google Scholar] [CrossRef]
- Barbosa, A.C.M.C.; Silva, L.P.C.; Ferraz, C.M.; Tobias, F.L.; de Araújo, J.V.; Loureiro, B.; Braga, G.M.A.M.; Veloso, F.B.R.; Soares, F.E.F.; Fronza, M.; et al. Nematicidal activity of silver nanoparticles from the fungus Duddingtonia flagrans. Int. J. Nanomed. 2019, 14, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Sri, P.; Leelavathi, V.; Sree, N.; Kumar, M.A. Antihelmenthic and Antimicrobial Activity of Green Synthesized Silver Nanoparticles from Illicium verum Hook. F. Fruit. J. Pharm. Biol. Sci. 2015, 10, 61–65. [Google Scholar]
- Priya, S.; Santhi, S. Biosynthesis and in vitro anthelmintic activity of silver nanoparticles using aqueous leaf extract of Azadirachta indica. World J. Pharm. Pharm. Sci. 2015, 4, 2105–2115. [Google Scholar]
- Kumari, K.; Singh, N.; Kumar, A. Antihelmenthic potencial of crude ethanolic extracts of selected plant species. IJMPS 2017, 7, 35–40. [Google Scholar]
- Ejaz, K.; Sadia, H.; Zia, G.; Nazir, S.; Raza, A.; Ali, S.; Iqbal, T.; Andleeb, S. Biofilm reduction, cell proliferation, anthelmintic and cytotoxicity effect of green synthesised silver nanoparticle using Artemisia vulgaris extract. IET Nanobiotechnol. 2018, 12, 71–77. [Google Scholar] [CrossRef]
- Rashid, M.O.; Ferdous, J.; Banik, S.; Islam, R.; Mazbah Uddin, A.H.M.; Robel, F.N. Anthelmintic activity of silver-extract nanoparticles synthesized from the combination of silver nanoparticles and M. charantia fruit extract. BMC Complement. Altern Med. 2016, 16, 242. [Google Scholar] [CrossRef]
- Ovais, M.; Nadhman, A.; Khalil, A.T.; Raza, A.; Khuda, F.; Sohail, M.F.; Zakiullah; Islam, N.U.; Sarwar, H.S.; Shahnaz, G.; et al. Biosynthesized colloidal silver and gold nanoparticles as emerging leishmanicidal agents: An insight. Nanomedicine 2017, 12, 2807–2819. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 9, 4782–4799. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jagtap, S.; More, P.; Shete, U.J.; Maheshwari, N.O.; Rao, S.J.; Kitture, R.; Kale, S.; Bellare, J.; Patil, S.; et al. Dioscorea bulbifera mediated synthesis of novel Au core Ag shell nanoparticles with potent antibiofilm and antileishmanial activity. J. Nanomater. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Bunchez, I.R.; Barbinta Patrascu, M.E.; Badea, N.; Doncea, S.M.; Popescu, A.; Ion, R.M. Antioxidant silver nanoparticles green synthesized using ornamental plants. JOAM 2012, 14, 1016–1022. [Google Scholar]
- Salaria, S.; Bahabadia, S.E.; Samzadeh-Kermanib, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of green synthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm Res. 2019, 18, 430–445. [Google Scholar]
- Vorobyova, V.; Vasyliev, G.; Skiba, M. Eco-friendly “green” synthesis of silver nanoparticles with the black currant pomace extract and its antibacterial, electrochemical, and antioxidant activity. Appl. Nanosci. 2020. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and antibacterial potential of silver nanoparticles: Biogenic synthesis utilizing apple extract. J. Pharm. (Cairo) 2016, 2016, 7141523. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 719–724. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.; Reddy, S.G.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.V.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Art. Cells Nanomed. Biotechnol. 2018, 46, 1138–1148. [Google Scholar] [CrossRef]
- Selvam., K.; Sudhakar., C.; Govarthanan, M.; Thiyagarajan, P.; Sengottaiyan., A.; Senthilkumar, B.; Selvankumar, T. Eco-friendly biosynthesis and characterization of silver nanoparticles using Tinospora cordifolia (Thunb.) Miers and evaluate its antibacterial, antioxidant potential. J. Radiat. Res. Appl. Sci. 2017, 10, 6–12. [Google Scholar] [CrossRef]
- Bedlovicová, Z.; Strapá, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Afolayan, A.J. In vitro antibacterial, antioxidant and toxicity profile of silver nanoparticles greensynthesized and characterized from aqueous extract of a spice blend formulation. Biotechnol. Biotechnol. Equip. 2018, 32, 724–733. [Google Scholar] [CrossRef]
- Reddy, N.J.; Nagoor Vali, D.; Rani, M. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Gu, L.; Han, T.T.; Wang, S.; Meng, F.B. Bioinspired synthesis, characterization and antibacterial activity of plant-mediated silver nanoparticles using purple sweet potato root extract. Mat. Technol. 2016, 31, 437–442. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver nanoparticles mediated by Costus afer leaf electrochemical properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [PubMed]
- Patil Shriniwas, P.; Kumbhar Subhash, T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Abdel-Fattah, W.I.; Ghareib, W.A. On the anti-cancer activities of silver nanoparticles. J. Appl. Biotechnol. Bioeng. 2018, 5, 43–46. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Al-Massarani, S.M.; Saquib, Q.; Wahab, R.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Anticancer Potential of Green Synthesized Silver Nanoparticles Using Extract of Nepeta deflersiana against Human Cervical Cancer Cells (HeLA). Bioinorg. Chem. Appl. 2018, 2018, 9390784. [Google Scholar] [CrossRef]
- Gurunathan, S.; Lee, K.J.; Kalimuthu, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Siddiqui, M.A.; Farshori, N.N.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Synthesis, characterization and toxicological evaluation of iron oxide nanoparticles in human lung alveolar epithelial cells. Colloids Surf. B Biointerfaces 2014, 122, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Faedmaleki, F.; Shirazi, F.H.; Salarian, A.A.; Ashtianid, H.A.; Rastega, H. Toxicity Effect of Silver Nanoparticles on Mice Liver Primary Cell Culture and HepG2 Cell Line. Iran. J. Pharm. Res. 2014, 13, 235–242. [Google Scholar] [PubMed]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 12, e0220950. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomedicine. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [PubMed]
- Dehghanizadea, S.; Arasteha, J.; Mirzaie, A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Art. Cells Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Devanesan, A.H.O. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale Res. Lett. 2018, 13, 315–325. [Google Scholar] [CrossRef]
- Mollick, M.R.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Bhowmick, B.; Maity, D.; Mondal, D.; Pattanayak, S.; Roy, S.; Chakraborty, M.; et al. Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications. Arab. J. Chem. 2019, 12, 2572–2584. [Google Scholar] [CrossRef]
- Lakshmanan, G.; Sathiyaseelan, A.; Kalaichelvan, P.T.; Murugesan, K. Karbala Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: Assessment of their antibacterial and anticancer activity. Int. J. Mod. Sci. 2018, 4, 61–68. [Google Scholar]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process. Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Kumar, P.S. Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and antiproliferation efficiency. Mater. Sci. Energy Technol. 2020, 3, 371–376. [Google Scholar]
- Othman, A.M.; Elsayed, M.A.; Al-Balakocy, N.G.; Hassan, M.M.; Elshafei, A.M. Biosynthesis and characterization of silver nanoparticles induced by fungal proteins and its application in different biological activities. J. Genet. Eng. Biotechnol. 2019, 17, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Bagyalakshmi, J.; Haritha, H. Green synthesis and characterization of silver nanoparticles using Pterocarpus marsupium and assessment of its in vitro antidiabetic activity. AJADD 2017, 5, 118–130. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.-S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. Antidiabetic effect of silver nanoparticles synthesized using lemongrass (Cymbopogon citratus) through conventional heating and microwave irradiation approach. J. Microbiol. Biotech. Food Sci. 2018, 7, 371–376. [Google Scholar] [CrossRef]
- Prabhu, S.; Vinodhini, S.; Elanchezhiyan, C.; Rajeswari, D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles using Pouteria sapota in streptozotocin-induced diabetic rats. J. Diabetes 2018, 10, 28–42. [Google Scholar] [CrossRef]
- Shanker, K.; Mohan, G.K.; Hussain, A.; Jayarambabu, N.; Pravallika, P.L. Green biosynthesis, characterization, in vitro antidiabetic activity, and investigational acute toxicity studies of some herbal-mediated silver nanoparticles on animal models. Pharmacogn. Mag. 2017, 13, 188–192. [Google Scholar]
- Singh, P.; Ahn, S.; Kang, J.-P.; Soshnikova, V.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Castro Aceituno, V.; Kim, Y.J.; et al. Invitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Art. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juránˇová, J.; Ulrichová, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, 137–142. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Hussein, R.H.; Saudi, A.H. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J. Biol. Sci. 2020, 27, 2410–2419. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Rai, V.R.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Li, F.; Gao, D.; Xing, B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. Chemosphere 2009, 77, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Aparna Mani, K.M.; Seethalakshmi, S.; Gopal, V. Evaluation of In-vitro Anti-Inflammatory Activity of Silver Nanoparticles Synthesised using Piper Nigrum Extract. Nanomed. Nanotechnol. 2015, 6, 1–5. [Google Scholar]
- Moldovan, B.; David, L.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Baldea, I.; Clichici, S.; Filip, G.A. In vitro and in vivo anti-inflammatory properties of green synthesized silver nanoparticles using Viburnum opulus L. fruits extract. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 720–727. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, M.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Crisan, D.; Schar etter-Kochanek, K.; Crisan, M.; Schatz, S.; Hainzl, A.; Olenic, L.; Filip, A.; Schneider, L.A.; Sindrilaru, A. Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-B activity. Exp. Derm. 2018, 27, 1166–1169. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Fereiga, S.A.; El-Zaafaranyb, G.M.; Arafaa, M.G.; Abdel-Mottaleb, M.M. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef]
- Popli, D.; Anil, V.; Subramanyam, A.B.; Namratha, M.N.; Ranjitha, V.R.; Rao, S.N.; Rai, R.V.; Govindappa, M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Art. Cells Nanomed. Biotechnol. 2018, 46, 676–683. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Shahid Rafique, M.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Art. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Halladay, L.J.; Cripps, P. The effect of silver impregnation of surgical scrub suits on surface bacterial contamination. Vet. J. 2012, 192, 489–493. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252. [Google Scholar]
- Mala, R.; Annie Aglin, A.; Ruby Celsia, A.S.; Geerthika, S.; Kiruthika, N.; VazagaPriya, C.; Srinivasa Kumar, K. Foley catheters functionalised with a synergistic combination of antibiotics and silver nanoparticles resist biofilm formation. IET Nanobiotechnol. 2017, 11, 612–620. [Google Scholar] [CrossRef]
- Thomas, R.; Mathew, S.; Nayana, A.R.; Mathews, J.; Radhakrishnan, E.K. Microbially and phytofabricated agnps with different mode of bactericidal action were identified to have comparable potential for surface fabrication of central venous catheters to combat staphylococcus aureus biofilm. J. Photochem. Photobiol. B Biol. 2017, 171, 96–103. [Google Scholar] [CrossRef]
- Pourali, P.; Yahyaei, B. Biological production of silver nanoparticles by soil isolated bacteria and preliminary study of their cytotoxicity and cutaneous wound healing efficiency in rat. J. Trace Elem. Med. Biol. 2016, 34, 22–31. [Google Scholar] [CrossRef]
- Jishma, P.; Narayanan, R.; Snigdha, S.; Thomas, R.; Radhakrishnan, E.K. Rapid degradative effect of microbially synthesized silver nanoparticles on textile dye in presence of sunlight. Biocatal. Agric. Biotechnol. 2018, 14, 410–417. [Google Scholar] [CrossRef]
- Emam, H.E.; Saleh, N.H.; Nagy, K.S.; Zahran, M.K. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int. J. Biol. Macromol. 2015, 78, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Chen, S.; Feng, C.; Chen, S.; Yin, N.; Yang, J.; Wang, H.; Xu, Y. Facilely green synthesis of silver nanoparticles into bacterial cellulose. Cellulose 2015, 22, 373–383. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]

| Test Microorganism | Resource of AgNPs | References |
|---|---|---|
| S. aureus, Escherichia coli | Melissa officinalis leaf extract | [128] |
| K. pneumoniae, Acinetobacter baumannii | Neurada procumbens leaf extract | [129] |
| Pr. vulgaris, S. marcescens, Ps. aeruginosa, B. subtilis | Picea abies L. stem bark extract | [130] |
| Ent. faecalis and C. albicans | Glycyrrhiza glabra root | [131] |
| E. coli, K. pneumoniae, S. typhimurium, S. enteritidis | pu-erh tea leaves | [132] |
| St. aureus, B. subtilis, Ps. aeruginosa, E. coli, K. pneumonia, A. niger, A. flavus | Rhinacanthus nasutus leaf extract | [133] |
| S. aureus, B. subtilis, B. cereus, C. albicans | Lingo-berry and cranberry juice | [134] |
| Fusarium, Rhizopus, Proteus, A. flavus, A. niger | Svensonia hyderobadensis leaf extract | [135] |
| F. oxysporum, Alt. brassicicola | Citrus limon leaf extract | [136] |
| C. albicans, C. kefyr | Euphorbia hirta leaf extract | [137] |
| V. parahaemolyticus | Adathoda vasica leaf extract | [138] |
| A. fumigatus, F. solani, A. niger, A. flavus, S. aureus, E. aerogenes, B. bronchiseptia | Bergenia ciliate leaf extract | [139] |
| M. tuberculosis | Cucumis sativus plant extract | [140] |
| St. aureus, Ps. aeruginosa | Gracilaria parvispora extract | [141] |
| A. fumigatus, C. albicans, Fusarium sp. | Sargassum longifolium extract | [142] |
| St. aureus, Str. pyrogenes, Str. viridans, Corynebacterium xerosis, | Usnea longissima extract | [143] |
| Pr. vulgaris, Ps. aeruginosa, Ser. marcescens, S. typhi, St. epidermidis | Parmotrema praesorediosum extract | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. https://doi.org/10.3390/jfb11040084
Mikhailova EO. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. Journal of Functional Biomaterials. 2020; 11(4):84. https://doi.org/10.3390/jfb11040084
Chicago/Turabian StyleMikhailova, Ekaterina O. 2020. "Silver Nanoparticles: Mechanism of Action and Probable Bio-Application" Journal of Functional Biomaterials 11, no. 4: 84. https://doi.org/10.3390/jfb11040084
APA StyleMikhailova, E. O. (2020). Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. Journal of Functional Biomaterials, 11(4), 84. https://doi.org/10.3390/jfb11040084




