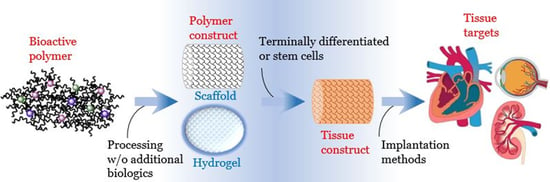
Bioactive Polymeric Materials for the Advancement of Regenerative Medicine
Abstract
1. Introduction
2. Polymeric Materials: Naturally Derived Polymers
2.1. Collagen
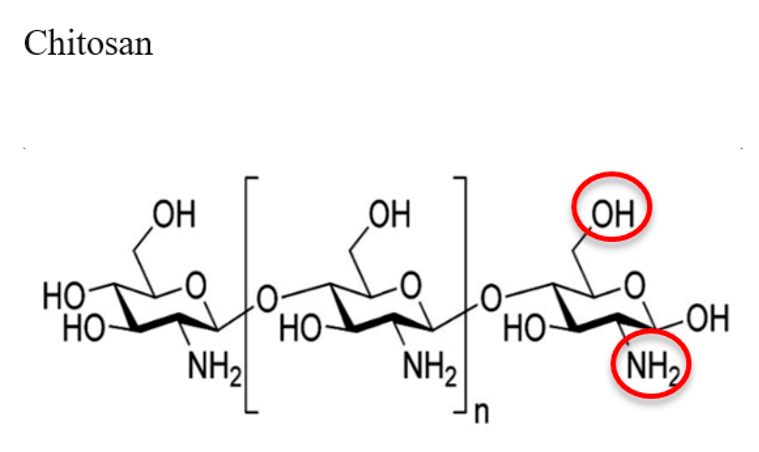
2.2. Chitosan
2.3. Alginate
2.4. Hyaluronic Acid
2.5. Silk Fibroin
3. Polymeric Materials: Synthetically Derived Polymers
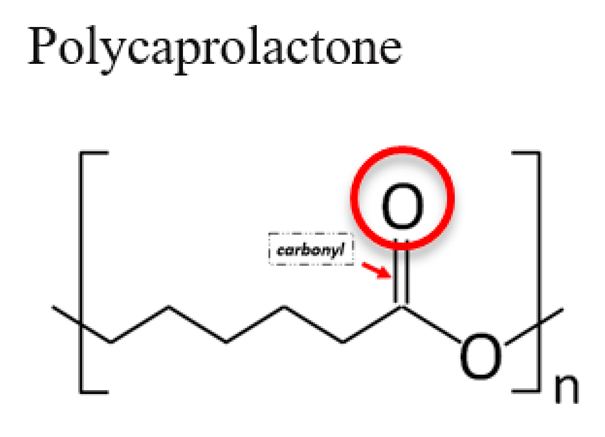
3.1. Polycaprolactone (PCL)
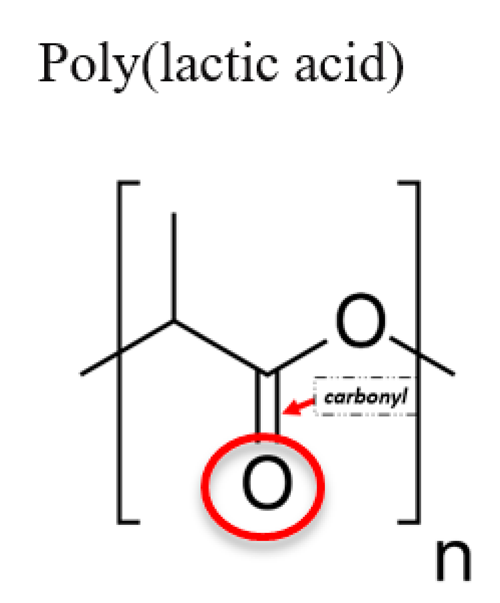
3.2. Poly (Lactic Acid) (PLA)
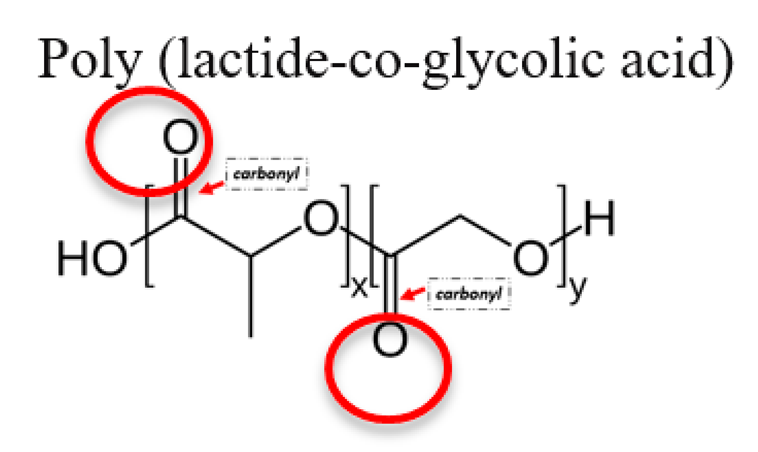
3.3. Poly (Lactide-Co-Glycolic) Acid (PLGA)
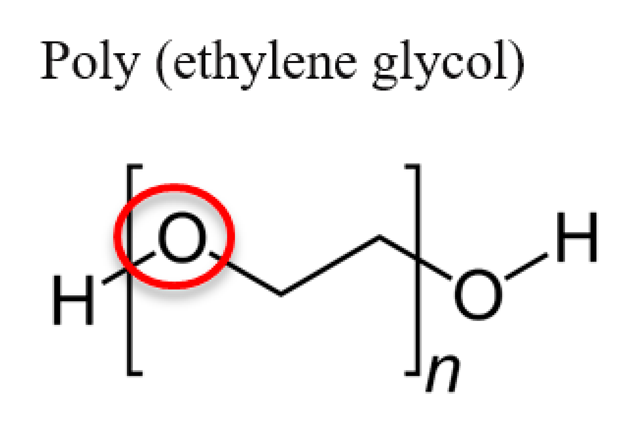
3.4. Poly(Ethylene Glycol) (PEG)
4. Advantages and Disadvantages of Naturally Derived Polymers
5. Advantages and Disadvantages of Synthetically Derived Polymers
6. Applications of Polymeric Materials in Regenerative Medicine
6.1. Mechanical and Porosity Effects on Tissue Engineering Scaffolding Functions
6.2. Regenerative Medicine
6.3. Skin Regeneration
6.4. Cartilage Regeneration
6.5. Bone Regeneration
6.6. Tendon Regeneration
7. Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Jiang, T.; Nair, L.; Laurencin, C. Chitosan bone and cartilage for regenerative engineering. In Chitosan Based Biomaterials; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 33–72. [Google Scholar]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Mouthuy, P.A.; El-Sherbini, Y.; Cui, Z.; Ye, H. Layering PLGA-based electrospun membranes and cell sheets for engineering cartilage–bone transition. J. Tissue Eng. Regen. Med. 2016, 10, E263–E274. [Google Scholar] [CrossRef]
- Ranjbarvan, P.; Soleimani, M.; Samadi Kuchaksaraei, A.; Ai, J.; Faridi Majidi, R.; Verdi, J. Skin regeneration stimulation: The role of PCL-platelet gel nanofibrous scaffold. Microsc. Res. Tech. 2017, 80, 495–503. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly (lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Geuze, R.E.; Theyse, L.F.; Kempen, D.H.; Hazewinkel, H.A.; Kraak, H.Y.; Öner, F.C.; Dhert, W.J.; Alblas, J. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng. Part A 2012, 18, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, L.G.; Crippa, G.E.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Pore size regulates cell and tissue interactions with PLGA–CaP scaffolds used for bone engineering. J. Tissue Eng. Regen. Med. 2012, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Anseth, K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, 4315–4323. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Harmon, M.D.; James, R.; Deng, M.; Laurencin, C.T.; Yu, X.; Kumbar, S.G. Cellulose and collagen derived micro-nano structured scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 2013, 9, 719–731. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.L.; et al. The effect of thick fibers and large pores of electrospun poly (ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Leslie-Barbick, J.E.; Saik, J.E.; Gould, D.J.; Dickinson, M.E.; West, J.L. The promotion of microvasculature formation in poly (ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials 2011, 32, 5782–5789. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.K.; Han, T.T.Y.; Marecak, D.M.; Watkins, J.F.; Amsden, B.G.; Flynn, L.E. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials 2014, 35, 1914–1923. [Google Scholar] [CrossRef]
- Naderi-Meshkin, H.; Andreas, K.; Matin, M.M.; Sittinger, M.; Bidkhori, H.R.; Ahmadiankia, N.; Bahrami, A.R.; Ringe, J. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol. Int. 2014, 38, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tare, R.S.; Oreffo, R.O.; Bradley, M. Versatile biocompatible polymer hydrogels: Scaffolds for cell growth. Angew. Chem. 2009, 121, 996–1000. [Google Scholar] [CrossRef]
- Ghidoni, I.; Chlapanidas, T.; Bucco, M.; Crovato, F.; Marazzi, M.; Vigo, D.; Torre, M.L.; Faustini, M. Alginate cell encapsulation: New advances in reproduction and cartilage regenerative medicine. Cytotechnology 2008, 58, 49–56. [Google Scholar] [CrossRef]
- Yin, H.; Gong, C.; Shi, S.; Liu, X.; Wei, Y.; Qian, Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-PEG hydrogel as a potential in situ sustained ophthalmic drug delivery system. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hiep, N.T.; Lee, B.-T. Electro-spinning of PLGA/PCL blends for tissue engineering and their biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 1969–1978. [Google Scholar] [CrossRef]
- Barbarisi, M.; Marino, G.; Armenia, E.; Vincenzo, Q.; Rosso, F.; Porcelli, M.; Barbarisi, A. Use of polycaprolactone (PCL) as scaffolds for the regeneration of nerve tissue. J. Biomed. Mater. Res. Part A 2015, 103, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Dessi, M.; Borzacchiello, A.; Mohamed, T.H.; Abdel-Fattah, W.I.; Ambrosio, L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Sá-Lima, H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010, 6, 5184–5195. [Google Scholar] [CrossRef]
- Marsich, E.; Borgogna, M.; Donati, I.; Mozetic, P.; Strand, B.L.; Salvador, S.G.; Vittur, F.; Paoletti, S. Alginate/lactose-modified chitosan hydrogels: A bioactive biomaterial for chondrocyte encapsulation. J. Biomed. Mater. Res. Part A 2008, 84, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2010, 12, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly (lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Swarnalatha, B.; Nair, S.L.; Shalumon, K.T.; Milbauer, L.C.; Jayakumar, R.; Paul-Prasanth, B.; Menon, K.K.; Hebbel, R.P.; Somani, A.; Nair, S.V. Poly (lactic acid)–chitosan–collagen composite nanofibers as substrates for blood outgrowth endothelial cells. Int. J. Biol. Macromol. 2013, 58, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 325–338. [Google Scholar] [CrossRef]
- Soscia, D.A.; Lam, D.; Tooker, A.C.; Enright, H.A.; Triplett, M.; Karande, P.; Peters, S.K.; Sales, A.P.; Wheeler, E.K.; Fischer, N.O. A flexible 3-dimensional microelectrode array for in vitro brain models. Lab A Chip 2020, 20, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Aydin, R.S.; Abu-Laban, M.; Heo, D.N.; Rizk, E.; Tucker, S.M.; Lewis, G.S.; Hayes, D.; Ozbolat, I.T. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater. Sci. Eng. C 2019, 105, 110128. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Sapudom, J.; Kalbitzer, L.; Anderegg, U.; Pompe, T. Topologically defined composites of collagen types I and V as in vitro cell culture scaffolds. Acta Biomater. 2014, 10, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Mohamed, W.K.E.; Garcia-Sabaté, A.; Alatoom, A.; Karaman, S.; Mahtani, N.; Teo, J.C.M. Collagen Fibril Density Modulates Macrophage Activation and Cellular Functions during Tissue Repair. Bioengineering 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lewin Mejia, D.; Chiang, B.; Luker, K.E.; Luker, G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ding, M.; Saadoon, O.; Vess, E.; Fernandez, A.; Zhao, P.; Jin, L.; Li, X. A novel culture platform for fast proliferation of human annulus fibrosus cells. Cell Tissue Res. 2016, 367, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ge, L.; Zhou, Q.; Mokabber, T.; Pei, Y.; Bron, R.; Rijn, P. Biomimetic Multiscale Hierarchical Topography Enhances Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. Interfaces 2020, 2000385. [Google Scholar] [CrossRef]
- Kang, L.; Jia, W.; Li, M.; Wang, Q.; Wang, C.; Liu, Y.; Wang, X.; Jin, L.; Jiang, J.; Gu, G.; et al. Hyaluronic acid oligosaccharide-modified collagen nanofibers as vascular tissue-engineered scaffold for promoting endothelial cell proliferation. Carbohydr. Polym. 2019, 223, 115106. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked Collagen Hydrogel Matrix Resisting Contraction To Facilitate Full-Thickness Skin Equivalents. ACS Appl. Mater. Interfaces 2017, 9, 20417–20425. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Gao, C. Facile fabrication of the glutaraldehyde cross-linked collagen/chitosan porous scaffold for skin tissue engineering. Mater. Sci. Eng. C 2012, 32, 2361–2366. [Google Scholar] [CrossRef]
- Kew, S.J.; Gwynne, J.H.; Enea, D.; Abu-Rub, M.; Pandit, A.; Zeugolis, D.; Brooks, R.A.; Rushton, N.; Best, S.M.; Cameron, R.E. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011, 7, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Song, D.K.; Oh, S.H.; Lee-Yoon, D.S.; Bae, E.H.; Lee, J.H. In vitro and in vivo degradation behavior of acetylated chitosan porous beads. J. Biomater. Sci. Polym. Ed. 2008, 19, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, C.; Heinemann, S.; Lode, A.; Bernhardt, A.; Worch, H.; Hanke, T. In vitro evaluation of textile chitosan scaffolds for tissue engineering using human bone marrow stromal cells. Biomacromolecules 2009, 10, 1305–1310. [Google Scholar] [CrossRef]
- Custódio, C.A.; Alves, C.; Reis, R.; Mano, J. Immobilization of fibronectin in chitosan substrates improves cell adhesion and proliferation. J. Tissue Eng. Regen. Med. 2010, 4, 316–323. [Google Scholar] [CrossRef]
- Walker, K.J.; Madihally, S.V. Anisotropic temperature sensitive chitosan-based injectable hydrogels mimicking cartilage matrix. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Ishihara, M.; Ishizuka, T.; Fujita, M.; Ozeki, Y.; Maehara, T.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 2003, 24, 3437–3444. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Z.; Kuwahara, K.; Nimni, M.E.; Wan, C.; Han, B. Delivery of demineralized bone matrix powder using a thermogelling chitosan carrier. Acta Biomater. 2012, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Valmikinathan, C.M.; Mukhatyar, V.J.; Jain, A.; Karumbaiah, L.; Dasari, M.; Bellamkonda, R.V. Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter 2012, 8, 1964–1976. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, M.; Almazan, G.; Tabrizian, M. Purine-crosslinked injectable chitosan sponges promote oligodendrocyte progenitor cells’ attachment and differentiation. Biomater. Sci. 2015, 3, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.; Fan, D.; Zhu, C. New suitable for tissue reconstruction injectable chitosan/collagen-based hydrogels. Soft Matter 2012, 8, 3781–3790. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable iron (III) cross-linked alginate gels. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Klöck, G.; Pfeffermann, A.; Ryser, C.; Gröhn, P.; Kuttler, B.; Hahn, H.J.; Zimmermann, U. Biocompatibility of mannuronic acid-rich alginates. Biomaterials 1997, 18, 707–713. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Vowden, P.; Romanelli, M.; Peter, R.; Boström, Å.; Josefsson, A.; Stege, H. The effect of amelogenins (Xelma™) on hard-to-heal venous leg ulcers. Wound Repair Regen. 2006, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Moyer, H.R.; Kinney, R.C.; Singh, K.A.; Williams, J.K.; Schwartz, Z.; Boyan, B.D. Alginate microencapsulation technology for the percutaneous delivery of adipose-derived stem cells. Ann. Plast. Surg. 2010, 65, 497–503. [Google Scholar] [CrossRef]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2019, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Bray, D.; Hopin, K.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Tissues and cancer. In Essential Cell Biology; Garland Science: New York, NY, USA, 2004; Volume 2. [Google Scholar]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.; Vo, D.V.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, properties, and applications. Encycl. Polym. Sci. Technol. 2002, 1–36. [Google Scholar] [CrossRef]
- Dai, N.-T.; Williamson, M.R.; Khammo, N.; Adams, E.F.; Coombes, A.G. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Pok, S.; Benavides, O.M.; Hallal, P.; Jacot, J.G. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng. Part A 2014, 20, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Han, J.; Yu, Y.; Maysinger, D.; Eisenberg, A. Polycaprolactone–b-poly (ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosterone. J. Control. Release 2000, 63, 275–286. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, L.; Hu, C.; Li, H.; Zhang, Y.; Chang, J. Electrospun poly (L-lactide) fiber with ginsenoside rg3 for inhibiting scar hyperplasia of skin. PLoS ONE 2013, 8, e68771. [Google Scholar] [CrossRef]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the treatment of chronic wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lou, W. Regeneration of facial nerve defects with xenogeneic acellular nerve grafts in a rat model. Head Neck 2014, 36, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, H.H.; Knowles, J. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly (lactic acid) for bone regeneration. J. Biomed. Mater. Res. Part A 2006, 79, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Nassif, L.; El Sabban, M. Mesenchymal stem cells in combination with scaffolds for bone tissue engineering. Materials 2011, 4, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Ergenç, T.I.; Kizilel, S. Recent advances in the modeling of PEG hydrogel membranes for biomedical applications. In Biomedical Engineering, Trends in Materials Science; InTechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Kumbar, S.G.; Rudzinski, W. Stimulus-responsive “smart” hydrogels as novel drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Tessmar, J.K.; Göpferich, A.M. Customized PEG-derived copolymers for tissue-engineering applications. Macromol. Biosci. 2007, 7, 23–39. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef]
- Harris, J.M. Poly (Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Springer Science & Business Media: Berlin, Germany, 2013; pp. 7–12. [Google Scholar]
- Nguyen, L.H.; Kudva, A.K.; Guckert, N.L.; Linse, K.D.; Roy, K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials 2011, 32, 1327–1338. [Google Scholar] [CrossRef]
- Mahoney, M.J.; Anseth, K.S. Three-dimensional growth and function of neural tissue in degradable polyethylene glycol hydrogels. Biomaterials 2006, 27, 2265–2274. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Salehi-Nik, N.; Rad, M.R.; Nazeman, P.; Khojasteh, A. Polymers for oral and dental tissue engineering. Biomater. Oral Dent. Tissue Eng. 2017, 25–46. [Google Scholar] [CrossRef]
- Allen, A.B.; Priddy, L.B.; Li, M.T.; Guldberg, R.E. Functional augmentation of naturally-derived materials for tissue regeneration. Ann. Biomed. Eng. 2015, 43, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012, 8, 3714–3722. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110698. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 29, 863–893. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Wu, J.C. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev. Cardiovasc. Therapy 2012, 10, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.H.; Sarmadi, M.; Helman, J.I.; Krebsbach, P.H. Effects of pH on human bone marrow stromal cellsin vitro: Implications for tissue engineering of bone. J. Biomed. Mater. Res. 2002, 60, 292–299. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Shah, A.; Brugnano, J.; Sun, S.; Vase, A.; Orwin, E. The Development of a Tissue-Engineered Cornea: Biomaterials and Culture Methods. Pediatr. Res. 2008, 63, 535–544. [Google Scholar] [CrossRef]
- Griffith, M. Functional Human Corneal Equivalents Constructed from Cell Lines. Science 1999, 286, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.A.; Bokhari, M.A. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, W. A novel comby scaffold with improved mechanical strength for bone tissue engineering. Mater. Lett. 2017, 194, 220–223. [Google Scholar] [CrossRef]
- Sartuqui, J.; D’Elía, N.; Gravina, A.N.; Messina, P.V. Analyzing the hydrodynamic and crowding evolution of aqueous hydroxyapatite-gelatin networks: Digging deeper into bone scaffold design variables. Biopolymers 2015, 103, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Stegemann, J.P. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J. Biomater. Sci. Polym. Ed. 2008, 19, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Tsang, V.L.; Bhatia, S.N. Three-dimensional tissue fabrication. Adv. Drug Deliv. Rev. 2004, 56, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Ruso, J.; Messina, P. Biopolymers in Regenerative Medicine: Overview, Current Advances and Future Trends; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Mani, G.; Feldman, D.M.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Melek, L.N. Tissue engineering in oral and maxillofacial reconstruction. Tanta Dent. J. 2015, 12, 211–223. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Reuse, recycling, and disposal. In William Andrew, 1st ed.; Imprint of Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Patel, D. Regenerative medicine using nanotechnology: A review. Int. J. Pharm. Biol. Arch. 2011, 2, 1033–1039. [Google Scholar]
- Rathenow, J.; Ban, A.; Kunstmann, J.; Mayer, B.; Asgari, S. Biocompatible Coated Medical Implants. EP1982772, 22 October 2008. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: New York, NY, USA, 2004; p. 10003. [Google Scholar]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Lim, G.T.; Valente, S.A.; Hart-Spicer, C.R.; Evancho-Chapman, M.M.; Puskas, J.E.; Horne, W.I.; Schmidt, S.P. New biomaterial as a promising alternative to silicone breast implants. J. Mech. Behav. Biomed. Mater. 2013, 21, 47–56. [Google Scholar]
- Terzic, A.; Nelson, T.J. Regenerative medicine: Advancing healthcare 2020. J. Am. Coll. Cardiol. 2010, 55, 2254–2257. [Google Scholar] [CrossRef][Green Version]
- Tseng, D.; Donahue, W.; Parsons, B.A. Polymer Coated Stent. Patent No. WO2000056247A1, 28 September 2000. [Google Scholar]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lawrence, J.G.; Bhaduri, S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: A review. Acta Biomater. 2012, 8, 1999–2016. [Google Scholar] [CrossRef]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; Schoenfeld, D.A.; Malloy, M.; Schulz, J.T.I.; Sheridan, R.L.; Tompkins, R.G. Use of integra(R) artificial skin is associated with decreased length of stay for severely injured adult burn survivors. J. Burn Care Res. 2002, 23, 311–317. [Google Scholar] [CrossRef]
- Tan, H.P.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast. Reconstr. Surg. 1996, 97, 167–178. [Google Scholar]
- Cruz, D.M.G.; Ivirico, J.L.E.; Gomes, M.M.; Ribelles, J.L.G.; Sanchez, M.S.; Reis, R.L.; Mano, J.F. Chitosan microparticles as injectable scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2008, 2, 378–380. [Google Scholar] [CrossRef]
- Ahlmann, E.; Patzakis, M.; Roidis, N.; Shepherd, L.; Holtom, P. Comparison of anterior and posterior iliac crest bone graft in terms of harvest-site morbidity and functional outcomes. J. Bone Jt. Surg. 2002, 84, 716–720. [Google Scholar] [CrossRef] [PubMed]
- St John, T.A.; Vaccaro, A.R.; Sah, A.P.; Schaefer, M.; Berta, S.C.; Albert, T.; Hilibrand, A. Physical and monetary costs associated with autogenous bone graft harvesting. Am. J. Orthop. 2003, 32, 18–23. [Google Scholar]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. 2002, 84, 454–464. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef]
- Komatsu, D.E.; Warden, S.J. The control of fracture healing and its therapeutic targeting: Improving upon nature. J. Cell. Biochem. 2010, 109, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for tendon and ligament repair: Review of the efficacy of commercial products. Expert Rev. Med Devices 2009, 6, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Lamberti, A.; Petrillo, S.; Maffulli, N.; Denaro, V. Scaffolds in tendon tissue engineering. Stem Cells Int. 2012, 2012, 517165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bogdanowicz, D.; Erisken, C.; Lee, N.M.; Lu, H.H. Review Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elb. Surg. 2012, 21, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ramanath, H.S.; Wang, D.A. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008, 26, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ding, C.; Tang, L.; Deng, F.; Yang, Q.; Wu, H.; Chen, L.; Ni, Y.; Huang, L.; Zhang, M. Novel modification of collagen: Realizing desired water solubility and thermostability in a conflict-free way. ACS Omega 2020, 5, 5772–5780. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lim, C.; Israelachvili, J.N.; Hwang, D.S. Strong adhesion and cohesion of chitosan in aqueous solutions. Langmuir 2013, 29, 14222–14229. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Singh, D. Biodegradable Nanoparticles are Excellent Vehicle for Site Directed in-vivo Delivery of Drugs and Vaccines. J. Nanobiotechnol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Kozioł, A.; Graham, E.; Jaroszewicz, J.; Chlanda, A.; Kumar, P.S.; Ivanovski, S.; Swieszkowski, W.; Vaquette, C. Surface modification of 3D printed polycaprolactone constructs via a solvent treatment: Impact on physical and osteogenic properties. ACS Biomater. Sci. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 3, 338–356. [Google Scholar] [CrossRef]
- Tasaka, F.; Miyazaki, H.; Ohya, Y.; Ouchi, T. Synthesis of comb-type biodegradable polylactide through depsipeptide–lactide copolymer containing serine residues. Macromolecules 1999, 32, 6386–6389. [Google Scholar] [CrossRef]
- Lanchero, R.; Godoy-Silva, R.; Guerero, C.A. Degradation kinetics of PLGA and PLGA conjugated with alendronate nanoparticles. In Proceedings of the 2016 AIChE Annual Meeting, Nanoscale Science and Engineering Forum, Nanotechnology for Biotechnology and Pharmaceuticals, San Francisco, CA, USA, 14 November 2016. [Google Scholar]
- Cenni, E.; Micieli, D.; Fotia, C.; Salerno, M.; Granchi, D.; Avnet, S.; Sarpietro, M.G.; Castelli, F.; Baldini, N. A novel biomaterial for osteotropic drug nanocarriers: Synthesis and biocompatibility evaluation of a PLGA–ALE conjugate Rosario Pignatello. Nanomedicine 2009, 4, 161–175. [Google Scholar]
- Han, Y.; Yuan, Z.; Zhang, P.; Jiang, S. Zwitterlation mitigates protein bioactivity loss in vitro over PEGylation. Chem. Sci. 2018, 9, 8561–8566. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Won, C.Y. PEG-modified biopharmaceuticals. Expert Opin. Drug Deliv. 2009, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
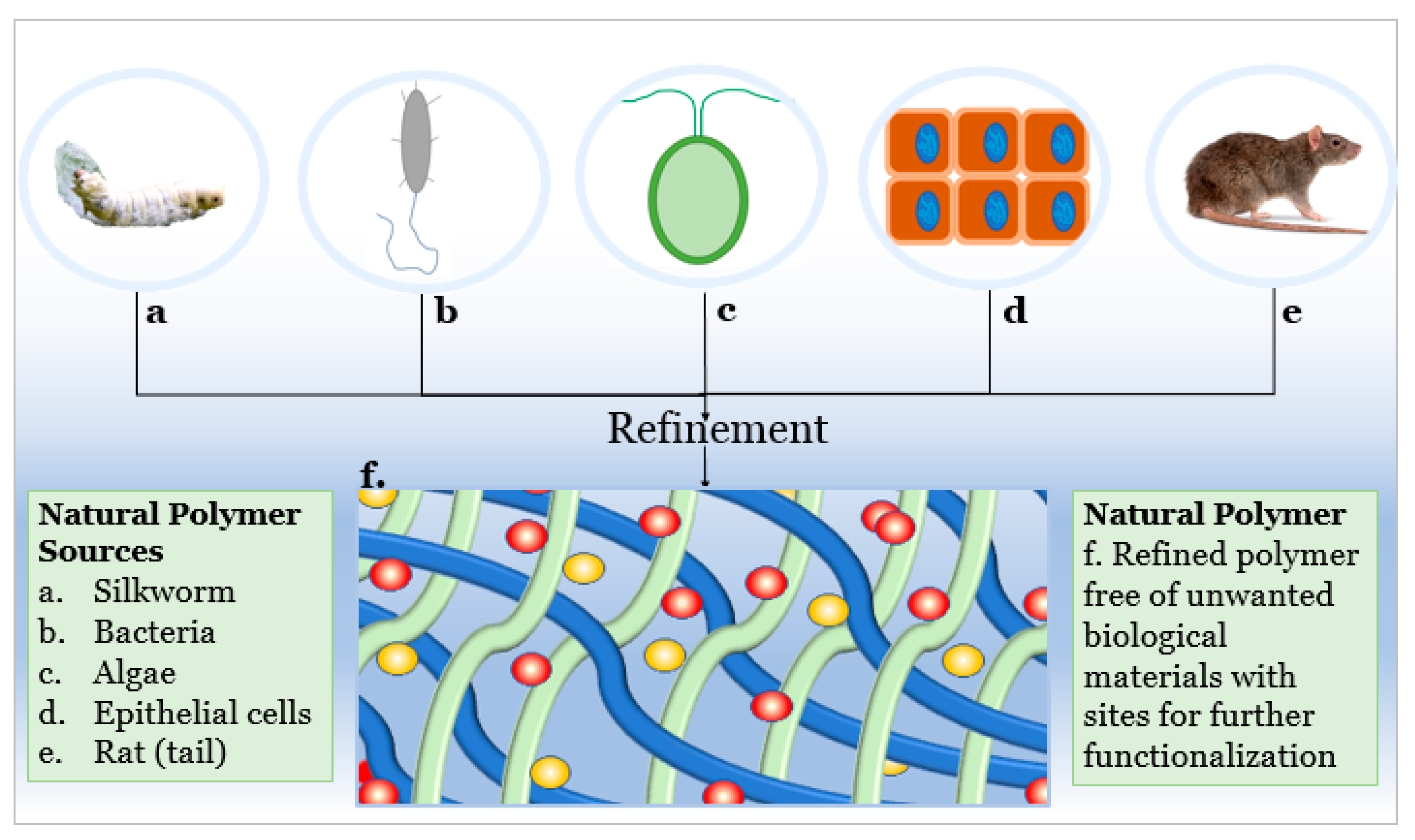

| Biopolymer (Highlighted Groups Are Modification Sites) | Material Bioactivity | Experimental Stage Modifications for Additional Bioactivity |
|---|---|---|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
|
 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iovene, A.; Zhao, Y.; Wang, S.; Amoako, K. Bioactive Polymeric Materials for the Advancement of Regenerative Medicine. J. Funct. Biomater. 2021, 12, 14. https://doi.org/10.3390/jfb12010014
Iovene A, Zhao Y, Wang S, Amoako K. Bioactive Polymeric Materials for the Advancement of Regenerative Medicine. Journal of Functional Biomaterials. 2021; 12(1):14. https://doi.org/10.3390/jfb12010014
Chicago/Turabian StyleIovene, Anthony, Yuwen Zhao, Shue Wang, and Kagya Amoako. 2021. "Bioactive Polymeric Materials for the Advancement of Regenerative Medicine" Journal of Functional Biomaterials 12, no. 1: 14. https://doi.org/10.3390/jfb12010014
APA StyleIovene, A., Zhao, Y., Wang, S., & Amoako, K. (2021). Bioactive Polymeric Materials for the Advancement of Regenerative Medicine. Journal of Functional Biomaterials, 12(1), 14. https://doi.org/10.3390/jfb12010014