Functional Properties of Low-Modulus PMMA Bone Cements Containing Linoleic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. 1H NMR Spectroscopy
2.3. Supercritical Fluid Chromatography-Tandem Mass Spectrometry (SFC-MS/MS)
2.4. Handling Properties
2.5. Differential Scanning Calorimetry
2.6. Mechanical Testing
2.7. Statistical Analysis
3. Results
3.1. 1H NMR Analysis
3.2. SFC-MS/MS Analysis
3.3. Handling Properties
3.4. DSC Analysis
3.5. Mechanical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charnley, J. The Classic: The bonding of Protheses to bone by Cement. J. Bone Jt. Surg. 1964, 46, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Haboush, E.J. A new operation for arthroplasty of the hip based on biomechanics, photoelasticity, fast setting dental acrylic, and other considerations. Bull. Hosp. Jt. Dis. 1953, 14, 242–277. [Google Scholar]
- Galibert, P.; Deramond, H.; Rosat, P.; Le Gars, D. Deramond, Note préliminaire sur le traitement des angiomes vertébraux par vertébroplastie acrylique percutanée. Neurochirurgie 1987, 33, 166–168. [Google Scholar] [PubMed]
- Watts, N.B. Osteoporotic vertebral fractures. Neurosurg. Focus. 2001, 10, 1–3. [Google Scholar] [CrossRef]
- Anselmetti, G.C.; Corrao, G.; della Monica, P.; Tartaglia, V.; Manca, A.; Eminefendic, H.; Russo, F.; Tosetti, I.; Regge, D. Pain Relief Following Percutaneous Vertebroplasty: Results of a Series of 283 Consecutive Patients Treated in a Single. Cardiovasc. Intervent. Radiol. 2007, 30, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.M.; Barr, J.D.; Belkoff, S.M.; Barr, M.S.; Jensen, M.E. Percutaneous Vertebroplasty: A Developing Standard of Care for Vertebral Compression Fractures. Am. J. Neuroradiol. 2001, 22, 373–381. [Google Scholar]
- Barr, J.D.; Barr, M.S.; Lemley, T.J.; Mccann, R.M. Percutaneous Vertebroplasty for Pain Relief and Spinal Stabilization. Spine 2000, 25, 923–928. [Google Scholar] [CrossRef]
- Belkoff, S.M.; Molloy, S. Temperature Measurement During Polymerization of Polymethylmethacrylate Cement Used for Vertebroplasty. Spine 2003, 28, 1555–1559. [Google Scholar] [CrossRef]
- Jefferiss, C.D.; Lee, A.J.; Ling, R.S. Thermal aspects of self-curing polymethylmethacrylate. J. Bone Joint Surg. Br. 1975, 57, 511–518. [Google Scholar] [CrossRef]
- Mjöberg, B.; Pettersson, H.; Rosenqvist, R.; Rydholm, A. Bone cement, thermal injury and the radiolucent zone. Acta Orthop. Scand. 1984, 55, 597–600. [Google Scholar] [CrossRef]
- Leeson, M.C.; Lippitt, S.B. Thermal Aspects of the Use of Polymethacrylate in Large Methaphyseal Defects in Bone: A clinical review and Laboratory study. Clin. Orthop. Relat. Res. 1993, 295, 239–245. [Google Scholar]
- Dahl, O.E.; Garvik, L.J.; Lyberg, T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro. Acta Orthop. 1994, 65, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.F.; Chappard, D.; Lesourd, M.; Monthéard, J.P.; Baslé, M.F. Free radicals and side products released during methylmethacrylate polymerization are cytotoxic for osteoblastic cells. J. Biomed. Mater. Res. 1998, 40, 124–131. [Google Scholar] [CrossRef]
- Mousavi, P.; Roth, S.; Finkelstein, J.; Cheung, G.; Whyne, C. Volumetric quantification of cement leakage following percutaneous vertebroplasty in metastatic and osteoporotic vertebrae. J. Neurosurg. 2003, 99, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, K.D. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: A case report. J. Bone Joint Surg. Am. 2001, 83, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, P.; Chen, Y.; Khalil, R.; Plakseychuk, A.; Cho, S.K.; Tillman, B.; Kumta, P.N.; Chun, Y.J. A review of PMMA bone cement and intra-cardiac embolism. Materials 2016, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Padovani, B.; Kasriel, O.; Brunner, P.; Peretti-viton, P. Pulmonary Embolism Caused by Acrylic Cement: A Rare Complication of Percutaneous Vertebroplasty. Am. J. Neuroradiol. 1999, 20, 375–377. [Google Scholar]
- Wang, L.; Yang, H.; Shi, Y.; Jiang, W.; Chen, L. Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: A systematic review. Orthop. Surg. 2012, 4, 182–189. [Google Scholar] [CrossRef]
- Chen, W.J.; Kao, Y.H.; Yang, S.C.; Yu, S.W.; Tu, Y.K.; Chung, K.C. Impact of cement leakage into disks on the development of Adjacent vertebral compression fractures. Clin. Spine Surg. 2010, 23, 35–39. [Google Scholar] [CrossRef]
- Luo, J.; Annesley-Williams, D.J.; Adams, M.A.; Dolan, P. How are adjacent spinal levels affected by vertebral fracture and by vertebroplasty? A biomechanical study on cadaveric spines. Spine J. 2017, 17, 863–874. [Google Scholar] [CrossRef]
- Sun, Y.; Mu, M.; Teng, H.; Yuan, W.; Luo, C. Risk of post-vertebroplasty fracture in adjacent vertebral bodies appears correlated with the morphologic extent of bone cement. J. Chin. Med. Assoc. 2011, 74, 357–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuijse, M.J.; Putter, H.; van Erkel, A.R.; Dijkstra, P.D.S. New Vertebral Fractures after Percutaneous Vertebroplasty for Painful Osteoporotic Vertebral Compression Fractures: A Clustered Analysis and the Relevance of Intradiskal Cement Leakage. Radiology 2013, 266, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-A.; Lin, C.-L.; Chang, M.-C.; Liu, C.-L.; Chen, T.-H.; Lai, S.-C. Subsequent Vertebral Fracture After Vertebroplasty. Spine 2012, 37, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Uppin, A.A.; Hirsch, J.A.; Centenera, L.V.; Pfiefer, B.A.; Pazianos, A.G. Occurrence of New Vertebral Body Fracture after Percutaneous Vertebroplasty in Patients with Osteoporosis. Radiology 2003, 226, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Grados, F.; Depriester, C.; Cayrolle, G.; Hardy, N.; Deramond, H.; Fardellone, P. Long-term observations of vertebral oeteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology 2000, 39, 1410–1414. [Google Scholar] [CrossRef] [Green Version]
- Holub, O.; López, A.; Borse, V.; Engqvist, H.; Kapur, N.; Hall, R.M.; Persson, C. Biomechanics of low-modulus and standard acrylic bone cements in simulated vertebroplasty: A human ex vivo study. J. Biomech. 2015, 48, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Baroud, G.; Nemes, J.; Heini, P.; Steffen, T. Load shift of the intervertebral disc after a vertebroplasty: A finite-element study. Eur. Spine J. 2003, 12, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Baroud, G.; Bohner, M. Biomechanical impact of vertebroplasty. Postoperative biomechanics of vertebroplasty. Jt. Bone Spine 2006, 73, 144–150. [Google Scholar] [CrossRef]
- Kim, J.M.; Shin, D.A.; Byun, D.H.; Kim, H.S.; Kim, S.; Kim, H.I. Effect of bone cement volume and stiffness on occurrences of adjacent vertebral fractures after vertebroplasty. J. Korean Neurosurg. Soc. 2012, 52, 435–440. [Google Scholar] [CrossRef]
- Liebschner, M.A.; Rosenberg, W.S.; Keaveny, T.M. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine 2001, 26, 1547–1554. [Google Scholar] [CrossRef]
- Belkoff, S.M.; Mathis, J.M.; Jasper, L.E.; Deramond, H. The biomechanics of vertebroplasty: The effect of cement volume on mechanical behavior. Spine 2001, 26, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Polikeit, A.; Nolte, L.P.; Ferguson, S.J. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit: Finite-element analysis. Spine 2003, 28, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Kinzl, M.; Benneker, L.M.; Boger, A.; Zysset, P.K.; Pahr, D.H. The effect of standard and low-modulus cement augmentation on the stiffness, strength, and endplate pressure distribution in vertebroplasty. Eur. Spine J. 2012, 21, 920–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boger, A.; Bohner, M.; Heini, P.; Verrier, S.; Schneider, E. Properties of an Injectable Low Modulus PMMA Bone Cement for Osteoporotic Bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Boger, A.; Bisig, A.; Bohner, M.; Heini, P.; Schneider, E. Variation of the mechanical properties of PMMA to suit osteoporotic cancellous bone. J. Biomater. Sci. Polym. Ed. 2008, 19, 1125–1142. [Google Scholar] [CrossRef]
- Puska, M.; Yli-urpo, A.; Vallittu, P.; Airola, K.; Puska, M.; Yli-urpo, A.; Vallittu, P.; Airola, K. Synthesis and Characterization of Polyamide of Trans-4-hydroxy-L-proline used as Porogen Filler in Acrylic Bone Cement. J. Biomater. Appl. 2005, 19, 287–301. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Puska, M.A.; Kokkari, A.K.; Timo, O.N. Mechanical properties of oligomer-modified acrylic bone cement. Biomaterials 2003, 24, 417–425. [Google Scholar]
- Boger, A.; Wheeler, K.; Montali, A.; Gruskin, E. NMP-modified PMMA bone cement with adapted mechanical and hardening properties for the use in cancellous bone augmentation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 760–766. [Google Scholar] [CrossRef]
- Lam, W.M.; Pan, H.B.; Fong, M.K.; Cheung, W.S.; Wong, K.L.; Li, Z.Y.; Luk, K.D.K.; Chan, W.K.; Wong, C.T.; Yang, C.; et al. In Vitro characterization of low modulus linoleic acid coated strontium- substituted hydroxyapatite containing PMMA bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 96, 76–83. [Google Scholar] [CrossRef]
- Persson, C.; Lopez, A.; Fathali, H.; Hoess, A.; Rojas, R.; Ott, M.K.; Hilborn, J.; Engqvist, H. The effect of oligo(trimethylene carbonate) addition on the stiffness of acrylic bone cement. Biomatter 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Mejía, A.; Herrera-Kao, W.; Duarte-Aranda, S.; Loría-Bastarrachea, M.I.; Canché-Escamilla, G.; Moscoso-Sánchez, F.J.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Synthesis and characterization of core-shell nanoparticles and their influence on the mechanical behavior of acrylic bone cements. Mater. Sci. Eng. C 2013, 33, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Weightman, B.; Freeman, M.A.; Revell, P.A.; Braden, M.; Albrektsson, B.E.; Carlson, L.V. The mechanical properties of cement and loosening of the femoral component of hip replacements. J. Bone Joint Surg. Br. 1987, 69, 558–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, E.J.; Behiri, J.C.; Bonfield, W. Flexural and fatigue properties of a bone cement based upon polyethylmethacrylate and hydroxyapatite. J. Mater. Sci. Mater. Med. 1995, 6, 799–803. [Google Scholar] [CrossRef]
- López, A.; Mestres, G.; Ott, M.K.; Engqvist, H.; Ferguson, S.J.; Persson, C.; Helgason, B. Compressive mechanical properties and cytocompatibility of bone-compliant, linoleic acid-modified bone cement in a bovine model. J. Mech. Behav. Biomed. Mater. 2014, 32, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, A.; Hoess, A.; Thersleff, T.; Ott, M.; Engqvist, H.; Persson, C. Low-modulus PMMA bone cement modified with castor oil. Biomed. Mater. Eng. 2011, 21, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Persson, C.; Robert, E.; Carlsson, E.; Robo, C.; Lopez, A.; Godoy-Gallardo, M.; Ginebra, M.-P.; Engqvist, H. The effect of unsaturated fatty acid and triglyceride oil addition on the mechanical and antibacterial properties of acrylic bone cements. J. Biomater. Appl. 2015, 30, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, E.; Mestres, G.; Treerattrakoon, K.; López, A.; Ott, M.K.; Larsson, S.; Persson, C. In Vitro and In Vivo Response to Low-Modulus PMMA-Based Bone Cement. BioMed Res. Int. 2015, 2015, 594284. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G.; Mladsi, S. Effect of sterilization method on properties of Palacos R acrylic bone cement. Biomaterials 1998, 19, 117–124. [Google Scholar] [CrossRef]
- Graham, J.; Pruitt, L.; Ries, M.; Gundiah, N. Fracture and fatigue properties of acrylic bone cement: The effects of mixing method, sterilization treatment, and molecular weight. J. Arthroplasty 2000, 15, 1028–1035. [Google Scholar] [CrossRef]
- ISO 5833. Implants for Surgery—Acrylic Resin Cements; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Baroud, G.; Bohner, M.; Heini, P.; Steffen, T. Injection biomechanics of bone cements used in vertebroplasty. Biomed. Mater. Eng. 2004, 14, 487–504. [Google Scholar]
- Krebs, J.; Ferguson, S.J.; Bohner, M.; Baroud, G.; Steffen, T.; Heini, P.F. Clinical Measurements of Cement Injection Pressure During Vertebroplasty. Spine 2005, 30, E118–E122. [Google Scholar] [CrossRef] [PubMed]
- Baroud, G.; Vant, C.; Giannitsios, D.; Bohner, M.; Steffen, T. Effect of vertebral shell on injection pressure and intravertebral pressure in vertebroplasty. Spine 2005, 30, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Gasser, B.; Baroud, G.; Heini, P. Theoretical and experimental model to describe the injection of a polymethylmethacrylate cement into a porous structure. Biomaterials 2003, 24, 2721–2730. [Google Scholar] [CrossRef]
- Steeves, M.; Mogaard, C.S.J.; Byrne, S. How pilot-hole size affects bone-screw pullout strength in human cadaveric cancellous bone. Can. J. Surg. 2005, 48, 207–212. [Google Scholar]
- ASTM F543-07, Standard Specification and Test Methods for Metallic Medical Bone Screws. 2007, pp. 1–20. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/F543-07.htm (accessed on 15 January 2021).
- Pujari-Palmer, M.; Robo, C.; Persson, C.; Procter, P.; Engqvist, H. Influence of cement compressive strength and porosity on augmentation performance in a model of orthopedic screw pull-out. J. Mech. Behav. Biomed. Mater. 2018, 77, 624–633. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.; Tsiafoulis, C.; Gerothanassis, I. High Resolution NMR Spectroscopy as a Structural and Analytical Tool for Unsaturated Lipids in Solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Fidler, N.; Sauerwald, T.U.; Koletzko, B.; Demmelmair, H. Effects of human milk pasteurization and sterilization on available fat content and fatty acid composition. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 317–322. [Google Scholar] [CrossRef]
- Nawar, W.W. Thermal degradation of lipids. J. Agric. Food Chem. 1969, 17, 18–21. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Saldaña, M.D.A.; Torres, J.A.; Kennelly, J.J. Effect of pressure-assisted thermal sterilization on conjugated linoleic acid (CLA) content in CLA-enriched milk, Innov. Food Sci. Emergy Technol. 2012, 16, 291–297. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Monitoring by H-nuclear magnetic resonance of the changes in the composition of virgin linseed oil heated at frying temperature. Comparison with the evolution of other edible oils. Food Control 2012, 28, 59–68. [Google Scholar] [CrossRef]
- Xu, J.; Ho, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical properties and cytocompatibility improvement of vertebroplasty PMMA bone cements by incorporating mineralized collagen. Materials 2015, 8, 2616–2634. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; Santos, L.O.D.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Schork, F.J. Hybrid Miniemulsion Polymerization of Acrylate/Oil and Acrylate/Fatty Acid Systems. Macromol. React. Eng. 2008, 2, 265–276. [Google Scholar] [CrossRef]
- Demchuk, Z.; Shevchuk, O.; Tarnavchyk, I.; Kirianchuk, V.; Lorenson, M.; Kohut, A.; Voronov, S.; Voronov, A. Free-radical copolymerization behavior of plant-oil-based vinyl monomers and their feasibility in latex synthesis. ACS Omega 2016, 1, 1374–1382. [Google Scholar] [CrossRef]
- Tanio, N.; Nakanishi, T. Physical aging and refractive index of poly(methyl methacrylate) glass. Polym. J. 2006, 38, 814–818. [Google Scholar] [CrossRef] [Green Version]
- Banse, X.; Sims, T.J.; Bailey, A.J. Mechanical properties of adult vertebral cancellous bone: Correlation with collagen intermolecular cross-links. J. Bone Miner. Res. 2002, 17, 1621–1628. [Google Scholar] [CrossRef]
- Öhman-Mägi, C.; Holub, O.; Hall, R.M.; Persson, C. Density and mechanical properties of vertebral trabecular bone—A review. 2021, unpublished work. [Google Scholar]
- Kopperdahl, D.L.; Keaveny, T.M. Yield strain behavior of trabecular bone. J. Biomech. 1998, 31, 601–608. [Google Scholar] [CrossRef]
- Wilke, H.J.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 1999, 24, 755–762. [Google Scholar] [CrossRef]
- Lewis, G. Properties of Acrylic Bone Cement: State of the Art Review. J. Biomed. Mater. Res. Part A 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Stadelmann, V.A.; Bretton, E.; Terrier, A.; Procter, P.; Pioletti, D.P. Calcium phosphate cement augmentation of cancellous bone screws can compensate for the absence of cortical fixation. J. Biomech. 2010, 43, 2869–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Ajaxon, I.; Ginebra, M.P.; Engqvist, H.; Persson, C. Compressive, diametral tensile and biaxial flexural strength of cutting-edge calcium phosphate cements. J. Mech. Behav. Biomed. Mater. 2016, 60, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, T.; Nuutinen, J.P.; Koistinen, A.P.; Kröger, H.; Lappalainen, R. Biomechanical evaluation of bone screw fixation with a novel bone cement. Biomed. Eng. Online 2015, 14, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzier, J.S.; Evans, A.J.; Cahill, D.W. Increased pedicle screw pullout strength with vertebroplasty augmentation in osteoporotic spines. J. Neurosurg. 2002, 96, 309–312. [Google Scholar] [CrossRef]
- Cook, S.D.; Salkeld, S.L.; Stanley, T.; Faciane, A.; Miller, S.D. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J. 2004, 4, 402–408. [Google Scholar] [CrossRef] [PubMed]
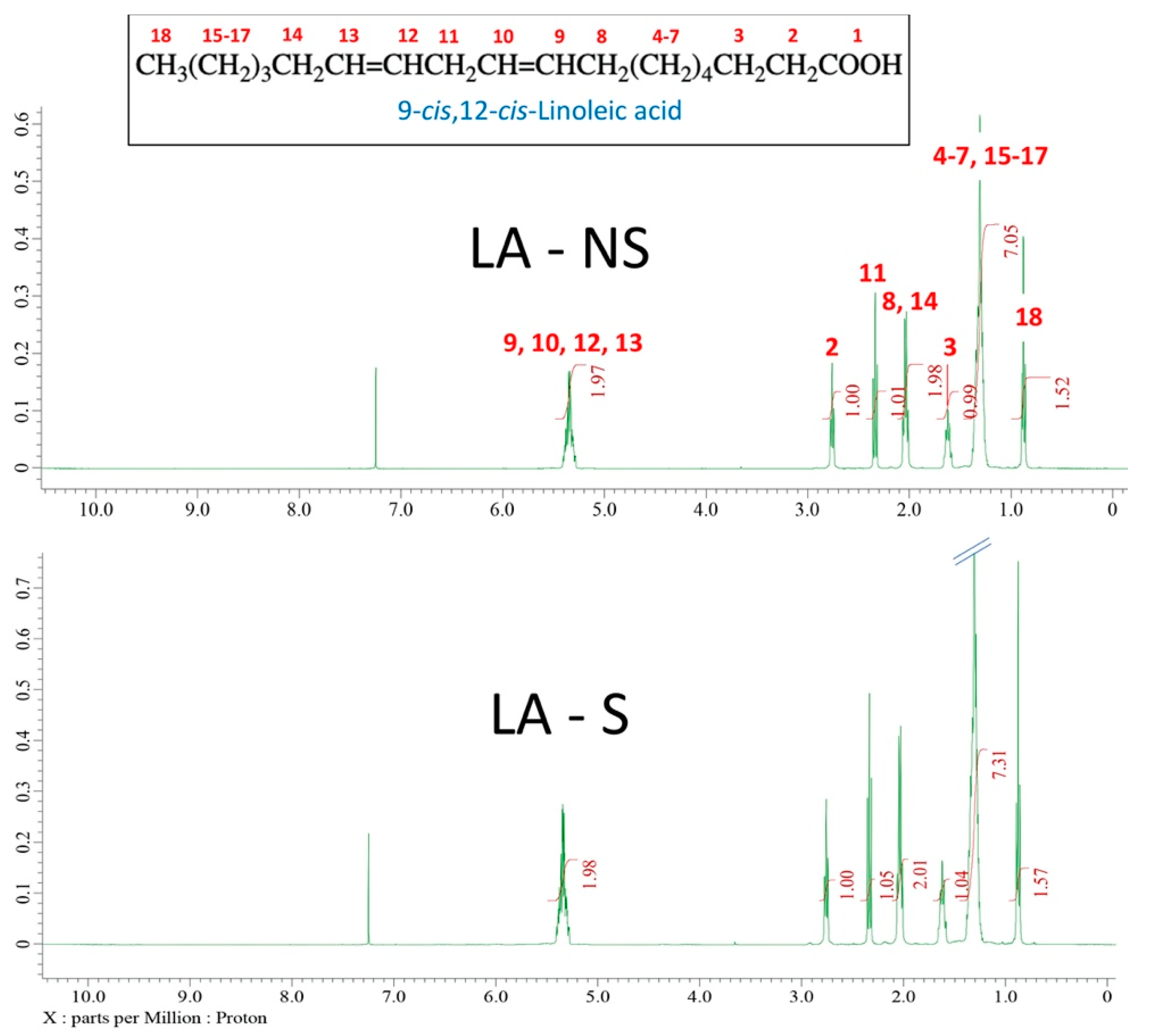



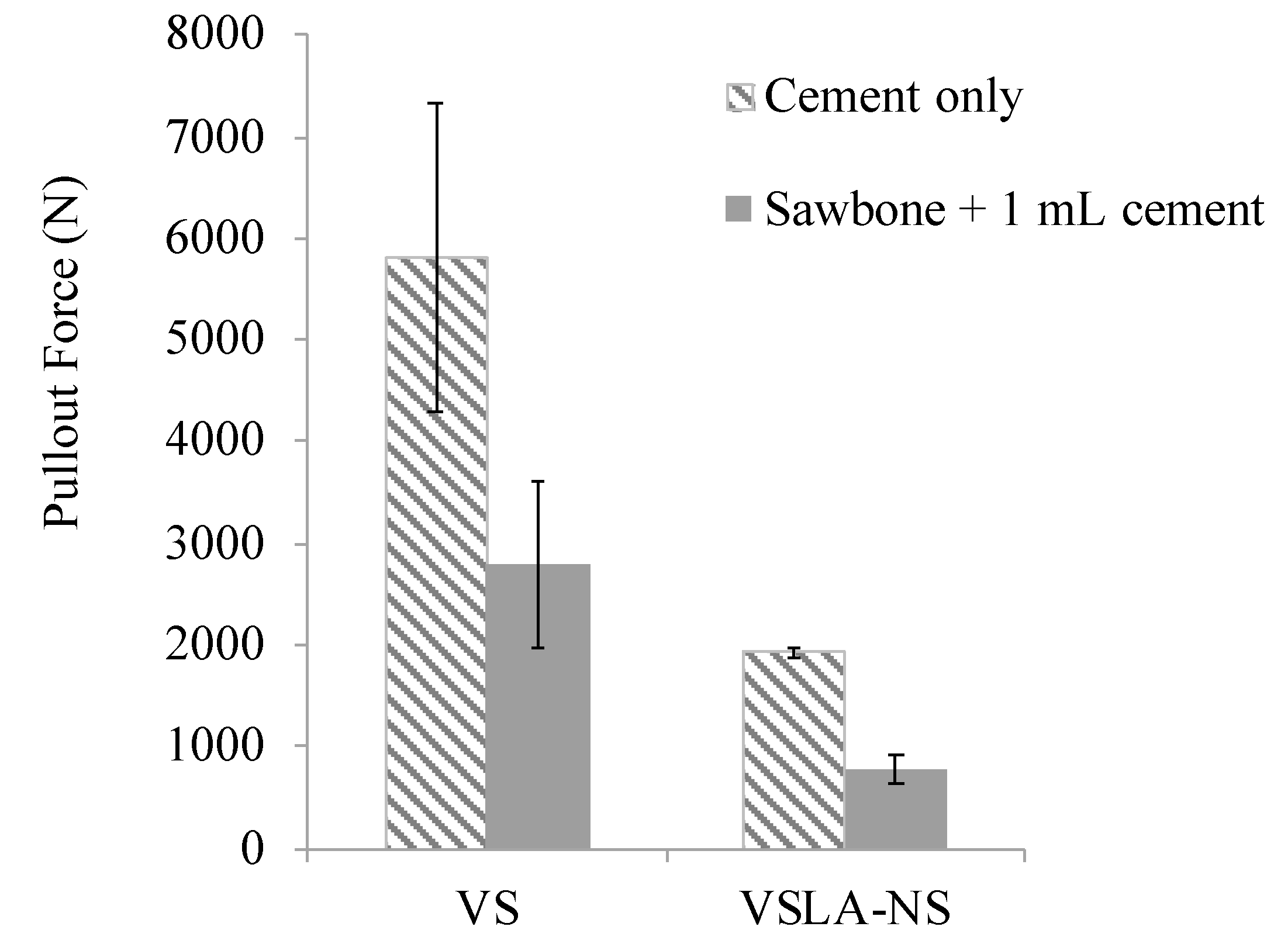
| Cement | tsetting [Min] | tdoughing [Min] | Tmax [°C] | Tg [°C] | |
|---|---|---|---|---|---|
| 24 h | 2 weeks | ||||
| VS | 22.1 (±1.0) | 9.1 (±0.4) | 66.8 (±2.7) | 102.8 (±1.3) | 114.0 (±2.5) |
| VSLA-NS | 24.7 (±1.0) | 12.3 (±0.3) | 28.2 (±0.4) | 74.7 (±4.8) | 87.8 (±2.5) |
| VSLA-S | 20.8 (±1.0) | 9.9 (±0.1) | 31.1 (±1.1) | 78.0 (±3.3) | 78.0 (±0.6) |
| VS | VS-LA (NS) | VS-LA (S) | ||||
|---|---|---|---|---|---|---|
| Time Point | CS (±SD) | E (±SD) | CS (±SD) | E (±SD) | CS (±SD) | E (±SD) |
| 24 h | 100.7 (±3.1) | 2140.4 (±128.8) | 28.3 (±5.1) | 494.7 (±51.8) | 20.5 (±7.1) | 559.6 (±60.3) |
| 2 weeks | 96.3 (±5.2) | 2075.2 (±114.3) | 30.5 (±0.8) | 803.3 (±65.8) | 31.8 (±0.7) | 927.1 (±60.4) |
| 4 weeks | 91.5 (±16.5) | 2070.0 (±103.1) | 36.5 (±0.6) | 947.8 (±64.4) | 38.3 (±0.7) | 1028.3 (±89.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robo, C.; Wenner, D.; Ubhayasekera, S.J.K.A.; Hilborn, J.; Öhman-Mägi, C.; Persson, C. Functional Properties of Low-Modulus PMMA Bone Cements Containing Linoleic Acid. J. Funct. Biomater. 2021, 12, 5. https://doi.org/10.3390/jfb12010005
Robo C, Wenner D, Ubhayasekera SJKA, Hilborn J, Öhman-Mägi C, Persson C. Functional Properties of Low-Modulus PMMA Bone Cements Containing Linoleic Acid. Journal of Functional Biomaterials. 2021; 12(1):5. https://doi.org/10.3390/jfb12010005
Chicago/Turabian StyleRobo, Céline, David Wenner, S. J. Kumari A. Ubhayasekera, Jöns Hilborn, Caroline Öhman-Mägi, and Cecilia Persson. 2021. "Functional Properties of Low-Modulus PMMA Bone Cements Containing Linoleic Acid" Journal of Functional Biomaterials 12, no. 1: 5. https://doi.org/10.3390/jfb12010005
APA StyleRobo, C., Wenner, D., Ubhayasekera, S. J. K. A., Hilborn, J., Öhman-Mägi, C., & Persson, C. (2021). Functional Properties of Low-Modulus PMMA Bone Cements Containing Linoleic Acid. Journal of Functional Biomaterials, 12(1), 5. https://doi.org/10.3390/jfb12010005







