Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application
Abstract
:1. Introduction
2. Materials and Methods
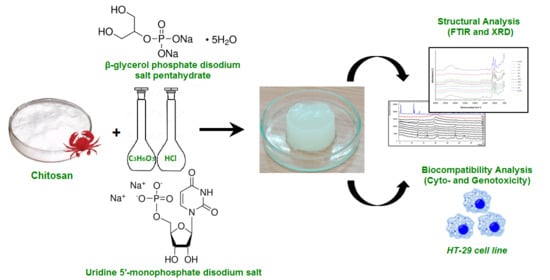
2.1. Materials of Hydrogels
2.2. Preparation of Solutions and Hydrogels Manufacture
2.3. In Vitro Conditioning
2.4. Physico-Chemical Studies
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. X-ray Diffraction
2.5. Biological Studies
2.5.1. Cell Culture
2.5.2. Preparation of CH Solutions for Cytotoxicity and Genotoxicity Studies
2.5.3. Cytotoxicity Analysis
2.5.4. Genotoxicity Assessment
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Spectra
3.2. Crystallinity—XRD Diffractograms
3.3. Analysis of the Cytotoxicity of Chitosan Hydrogels
3.4. Evaluation of Genotoxicity of Chitosan Hydrogels
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castells-Sala, C.; Alemany-Ribes, M.; Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Aloy-Reverté, C.; Caballero-Camino, J.; Márquez-Gil, A.; Semino, C.E. Current applications of tissue engineering in biomedicine. J. Biochips Tissue Chips 2013, S2, 1. [Google Scholar]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kruk, A.; Gadomska-Gajadhur, A.; Sebai, A.; Ruśkowski, P. Rusztowania komórkowe w inżynierii tkankowej. Wyroby Medyczne 2017, 4, 31–35. [Google Scholar]
- Błażewicz, M. Materiały dla inżynierii tkankowej. Inżynieria Biomateriałów 2001, 4, 32–35. [Google Scholar]
- Deluzio, T.G.; Seifu, D.G.; Mequanint, K. 3D scaffolds in tissue engineering and regenerative medicine: Beyond structural templates? Pharm. Bioprocess. 2013, 1, 267–281. [Google Scholar] [CrossRef]
- Kumar, R.; Griffin, M.; Butler, P. A Review of Current Regenerative Medicine Strategies that Utilize Nanotechnology to Treat Cartilage Damage. Open Orthop. J. 2016, 10, 862–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grolik, M. Inżynieria tkankowa—Nowe narzędzie w rekonstrukcji tkanek. Zeszyty Naukowe Towarzystwa Doktorantów Uniwersytetu Jagiellońskiego. Nauki Ścisłe 2011, 3, 33–41. [Google Scholar]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Ahn, S.-K.; Kasi, R.M.; Kim, S.-C.; Sharma, N.; Zhou, Y. Stimuli-responsive polymer gels. Soft Matter 2008, 4, 1151–1157. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A. Hydrogel: Classification, Properties, Preparation and Technical Features. Asian J. Biomater. Res. 2016, 2, 163–170. [Google Scholar]
- Dutta, S.D.; Patel, D.K.; Lim, K.-T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019, 13, 1–19. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Anamica; Pande, P.P. Polymer Hydrogels and Their Applications. Int. J. Mater. Sci. 2017, 12, 11–14. [Google Scholar]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric Hydrogels: Characterization and Biomedical Applications. Des. Monomers Polym. 2009, 12, 197–220. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Grassi, G.; Farra, R.; Caliceti, P.; Guarnieri, G.; Salmaso, S.; Carenza, M.; Grassi, M. Temperature-Sensitive Hydrogels. Am. J. Drug Deliv. 2005, 3, 239–251. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Suwantong, O. The potential use of thermosensitive chitosan/silk sericin hydrogels loaded with longan seed extract for bone tissue engineering. RSC Adv. 2018, 8, 40219–40231. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-T.; Cheng, Y.-Z.; Qin, M.; Wang, Y.-H.; Yu, H.-L.; Wang, A.-L.; Zhang, W.-F. Thermosensitive Chitosan Hydrogels Containing Polymeric Microspheres for Vaginal Drug Delivery. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.; Qi, T.; Wei, X.; Qu, Y.; Wu, Q.; Luo, F.; Qian, Z. Thermosensitive Polymeric Hydrogels as Drug Delivery Systems. Curr. Med. Chem. 2012, 20, 79–94. [Google Scholar] [CrossRef]
- Pokhrel, S.; Yadav, P.N.; Adhikari, R. Applications of Chitin and Chitosan in Industry and Medical Science: A Review. Nepal J. Sci. Technol. 2016, 16, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Pöppelmann, B.; Pappelbaum, K.; Moerschbacher, B.M.; Schneider, S.W. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials 2010, 31, 8556–8563. [Google Scholar] [CrossRef]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef]
- Minami, S.; Suzuki, H.; Okamoto, Y.; Fujinaga, T.; Shigemasa, Y. Chitin and chitosan activate complement via the alternative pathway. Carbohydr. Polym. 1998, 36, 151–155. [Google Scholar] [CrossRef]
- Dobolyi, A.; Juhász, G.; Kovacs, Z.; Kardos, J. Uridine function in the central nervous system. Curr. Top. Med. Chem. 2011, 11, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Negrão, L.; Almeida, P.; Alcino, S.; Duro, H.; Libório, T.; Silva, U.M.; Figueira, R.; Gonçalves, S.; Parra, L.N. Effect of the combination of uridine nucleotides, folic acid and vitamin B12 on the clinical expression of peripheral neuropathies. Pain Manag. 2014, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, S.J.; Chun, H.J.; Kim, C.-H. Thermosensitive Hydrogels for Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 8, 117–123. [Google Scholar]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Dessì, M.; Borzacchiello, A.; Mohamed, T.H.A.; Abdel-Fattah, W.I.; Ambrosio, L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2984–2993. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Su, W.-T.; Chou, W.-L.; Chou, C.-M. Osteoblastic differentiation of stem cells from human exfoliated deciduous teeth induced by thermosensitive hydrogels with strontium phosphate. Mater. Sci. Eng. C 2015, 52, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, S.N.; Zakharova, A.N.; Drenichev, M.S.; Ershov, A.V.; Kasatkina, M.A.; Vladimirov, L.V.; Novikov, V.V.; Kildeeva, N.R. Crosslinking of Chitosan with Dialdehyde Derivatives of Nucleosides and Nucleotides. Mechanism and Comparison with Glutaraldehyde. Nucleosides Nucleotides Nucleic Acids 2016, 35, 114–129. [Google Scholar] [CrossRef]
- Pieklarz, K.; Tylman, M.; Modrzejewska, Z. Preparation and characterization of a new generation of chitosan hydrogels containing pyrimidine ribonucleotides. Prog. Chem. Appl. Chitin Deriv. 2020, XXV, 192–200. [Google Scholar] [CrossRef]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and Characterization of Lyophilized Chitosan-Based Hydrogels Cross-Linked with Benzaldehyde for Controlled Drug Release. J. Chem. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Novel poss reinforced chitosan composite membranes for guided bone tissue regeneration. J. Mater. Sci. Mater. Med. 2017, 29, 1. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Hund, R.-D.; Aibibu, D.; Horakova, J.; Cherif, C. Pure Chitosan and Chitsoan/Chitosan Lactate Blended Nanofibres made by Single Step Electrospinning. Autex Res. J. 2013, 13, 128–133. [Google Scholar] [CrossRef]
- Bajer, D.; Kaczmarek, H. Study of the Influence OV UV Radiation on Biodegradable Blends Based on Chitosan and Starch. Prog. Chem. Appl. Chitin Deriv. 2010, XV, 17–24. [Google Scholar]
- Tylman, M.; Pieklarz, K.; Owczarz, P.; Maniukiewicz, W.; Modrzejewska, Z. Structure of chitosan thermosensitive gels containing graphene oxide. J. Mol. Struct. 2018, 1161, 530–535. [Google Scholar] [CrossRef]
- Carmona, P.; Molina, M.; Escobar, R. Conformation-sensitive infrared bands of uridine-5′-monophosphate. J. Mol. Struct. 1991, 243, 297–306. [Google Scholar] [CrossRef]
- Muntean, C.M.; Bratu, I.; Tripon, C.; Nalpantidis, K.; Purcaru, M.A.P.; Deckert, V. Molecular Relaxation Processes in Nucleic Acids Components as Probed with Raman Spectroscopy. Rev. Chim. 2017, 68, 2471–2475. [Google Scholar] [CrossRef]
- Singh, M.S.; Homendra, N.; Lonibala, R.K. Coordinating properties of uridine 5′-monophosphate with selected Ln3+ ions in ionic micellar media. BioMetals 2012, 25, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz Antonino, R.; Lia Fook, B.; De Oliveira Lima, V.; De Farias Rached, R.; Lima, E.; Da Silva Lima, R.; Peniche Covas, C.; Lia Fook, M. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; Barros, F.D.M.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Eskandani, M. Comet Assay: A Method to Evaluate Genotoxicity of Nano-Drug Delivery System. BioImpacts 2011, 1, 87–97. [Google Scholar] [PubMed]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. J. Funct. Biomater. 2021, 12, 37. https://doi.org/10.3390/jfb12020037
Pieklarz K, Galita G, Tylman M, Maniukiewicz W, Kucharska E, Majsterek I, Modrzejewska Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. Journal of Functional Biomaterials. 2021; 12(2):37. https://doi.org/10.3390/jfb12020037
Chicago/Turabian StylePieklarz, Katarzyna, Grzegorz Galita, Michał Tylman, Waldemar Maniukiewicz, Ewa Kucharska, Ireneusz Majsterek, and Zofia Modrzejewska. 2021. "Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application" Journal of Functional Biomaterials 12, no. 2: 37. https://doi.org/10.3390/jfb12020037
APA StylePieklarz, K., Galita, G., Tylman, M., Maniukiewicz, W., Kucharska, E., Majsterek, I., & Modrzejewska, Z. (2021). Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. Journal of Functional Biomaterials, 12(2), 37. https://doi.org/10.3390/jfb12020037