Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and 3D Printing of EW-Alg 3D Constructs for Mechanical Characterization
2.2. Rheological Characterization
2.3. Swelling and Degradation Behavior
2.4. Mechanical Strength
2.5. Cell Culture
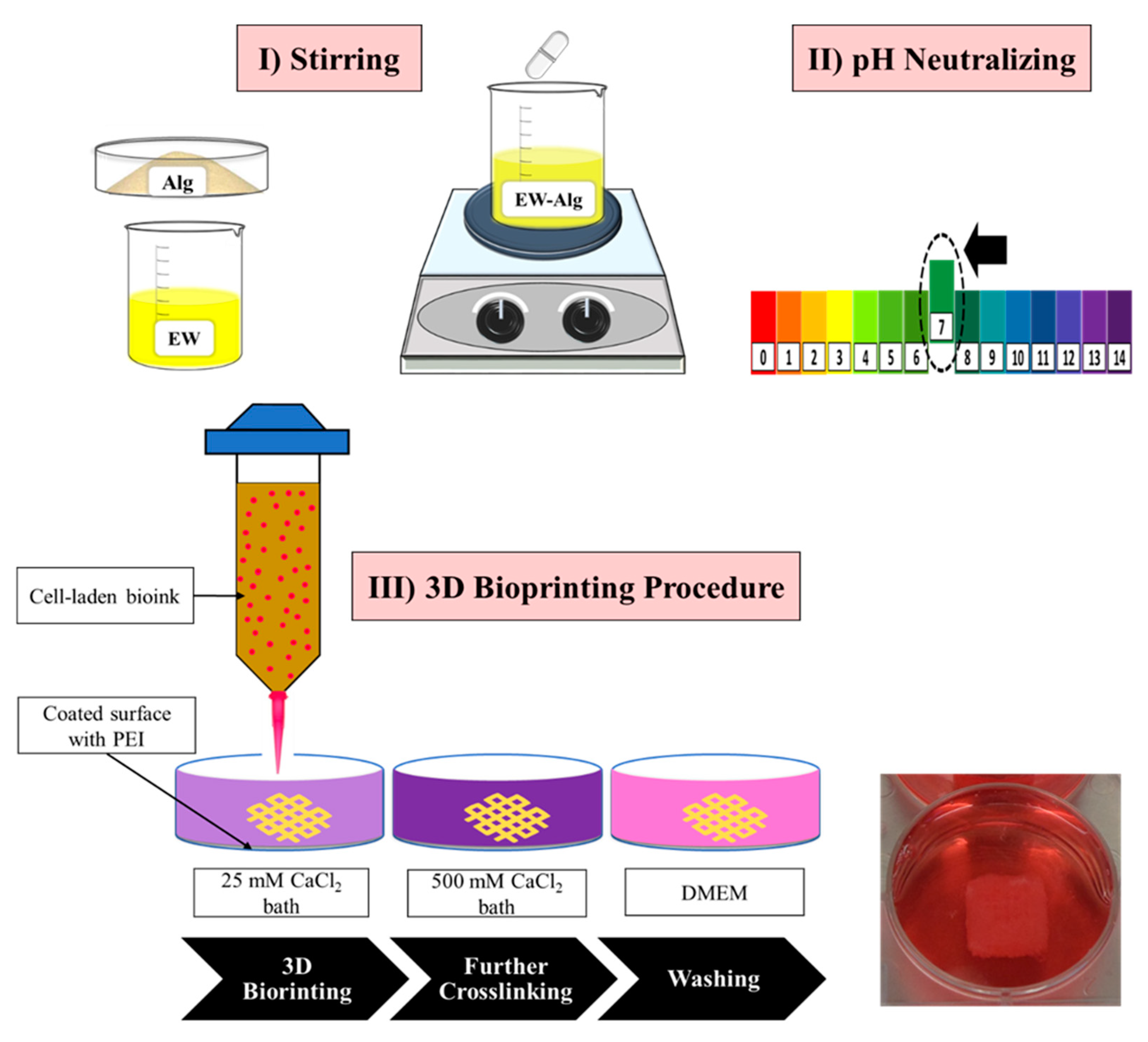
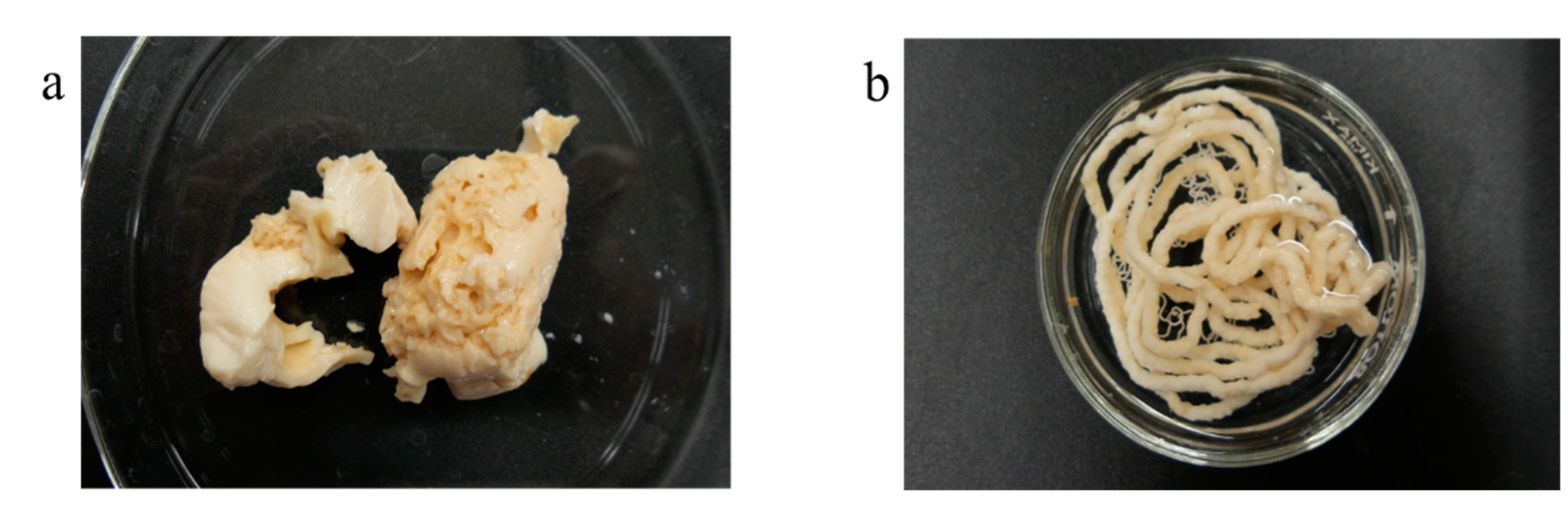
2.6. Bioprinting the Cell-Laden Patches
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
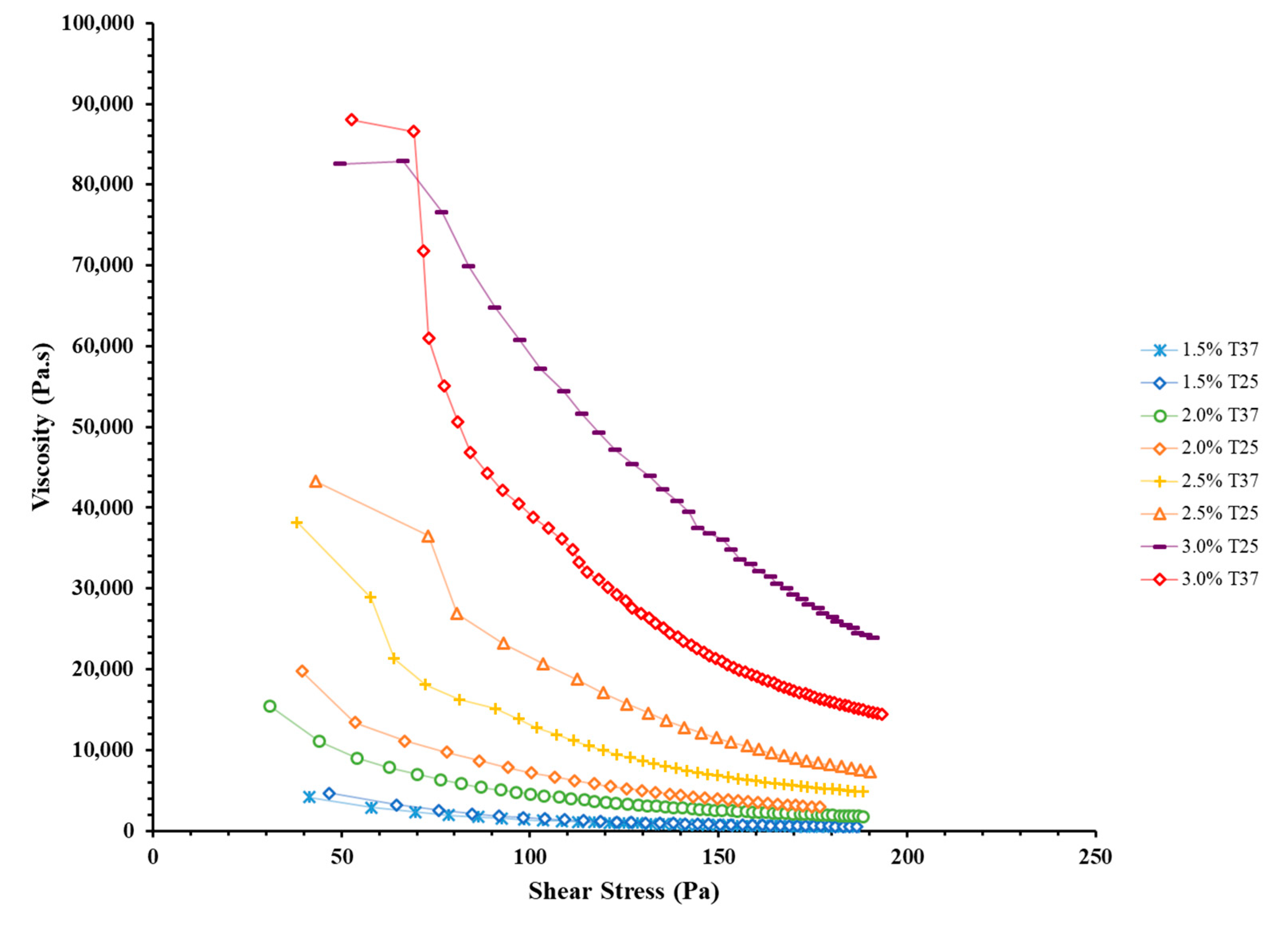
3.1. Rheological Characterization
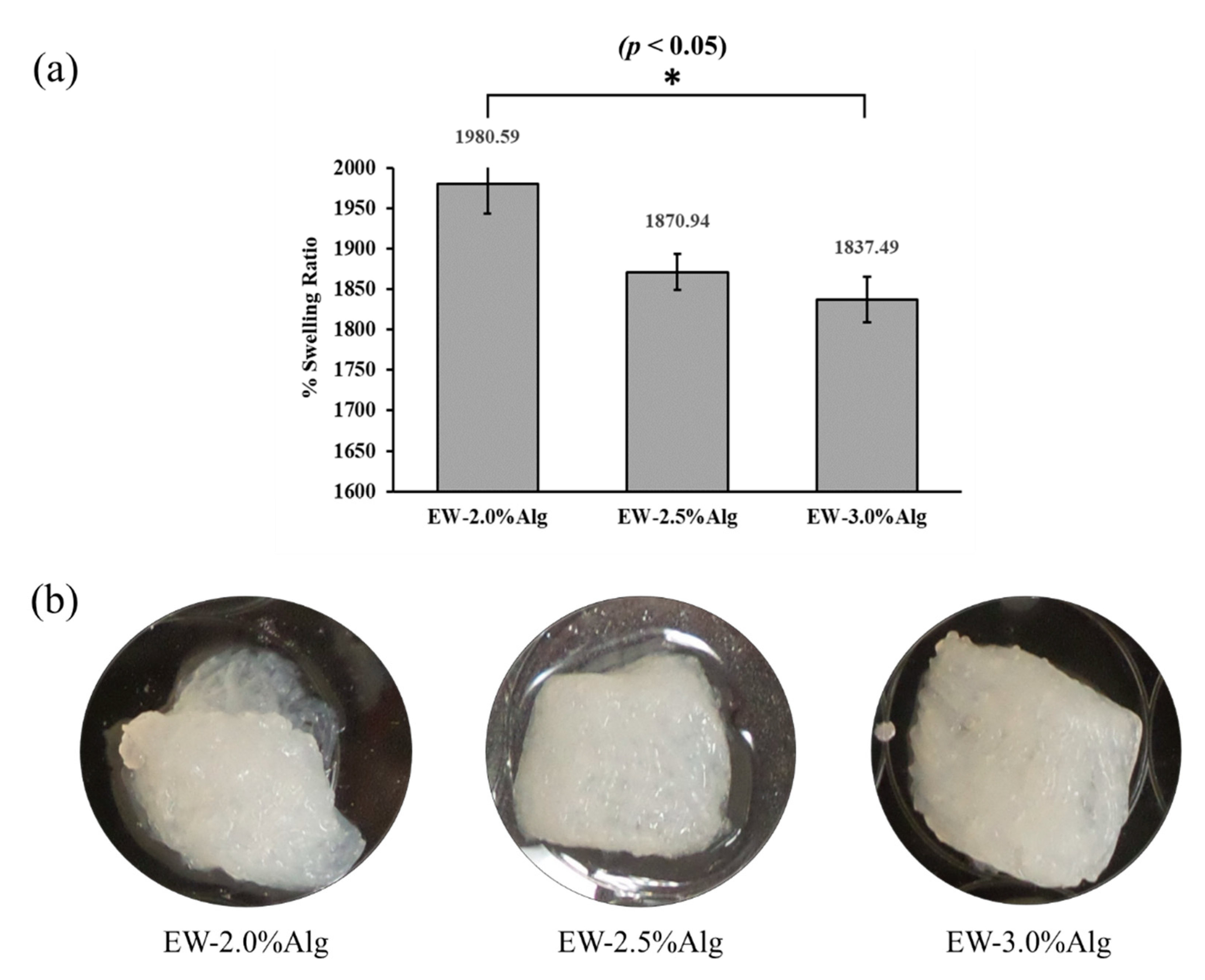
3.2. Water Uptake Behavior and Biodegradability
3.3. Mechanical Strength
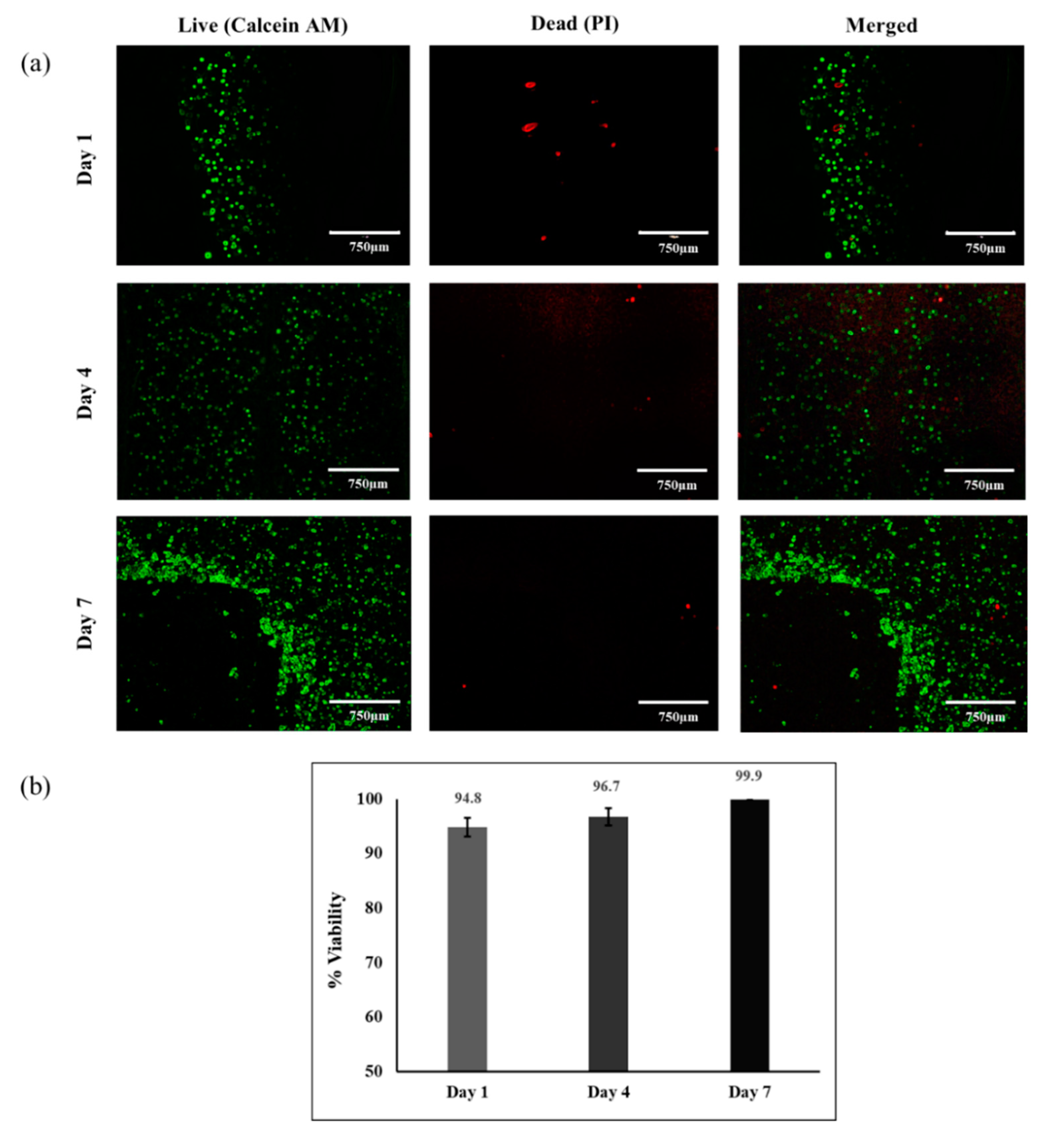
3.4. Cell Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
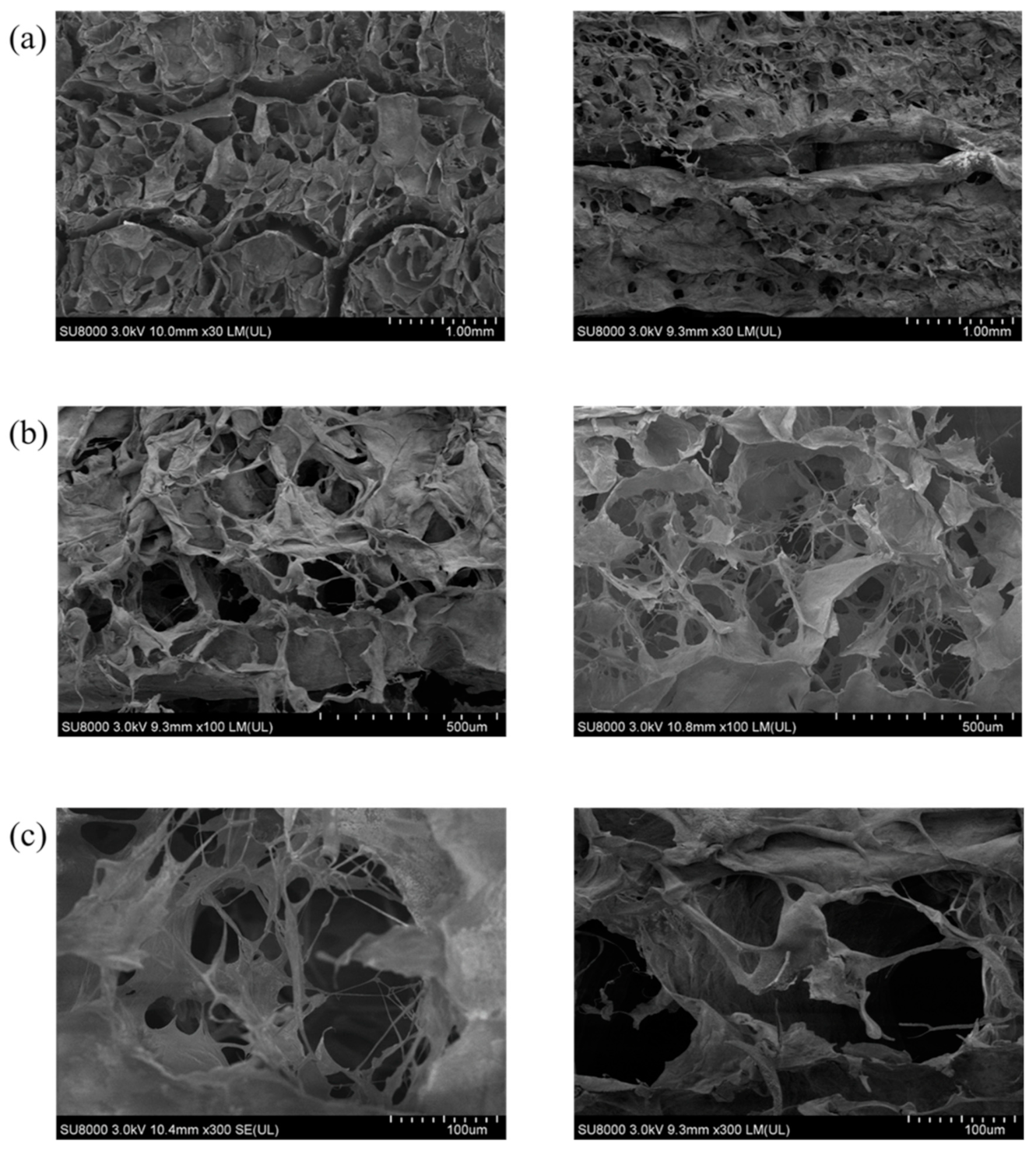
Appendix B

References
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Chen, X. 3D biofabrication of vascular networks for tissue regeneration : A report on recent advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.D. Extrusion Bioprinting of Scaffolds for Tissue Engineering Applications; Springer: Cham, Switzerland, 2019; pp. 16–30. [Google Scholar]
- Zhang, Y. Three-dimensional-printing for microfluidics or the other way around? Int. J. Bioprinting 2019, 5, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, A.; Chen, X. Bioprinting for combating infectious diseases. Bioprinting 2020, 20, e00104. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in extrusion bioprinting. Biofabrication 2021, 13, 33001. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhao, H.; Lewinski, N. Key parameters and applications of extrusion-based bioprinting. Bioprinting 2021, 23, e00156. [Google Scholar] [CrossRef]
- Nizeet, I.; Olivos, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.G.; Kacena, M.A.; Wagner, D.R. Bioprinting Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer : Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef]
- He, L.; Chen, X. Cardiomyocyte Induction and Regeneration for Myocardial Infarction Treatment: Cell Sources and Administration Strategies. Adv. Healthc. Mater. 2020, 9, 2001175. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Kelly, M.; Chen, X. Bioprinting and in vitro characterization of alginate dialdehyde–gelatin hydrogel bio-ink. Bio-Des. Manuf. 2020, 3, 48–59. [Google Scholar] [CrossRef]
- Ning, L.; Yang, B.; Mohabatpour, F.; Betancourt, N.; Sarker, M.D.; Papagerakis, P.; Chen, X. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Rybka, J.D. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting 2020, 18, e00070. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhadi, S.; Rajabi-Zeleti, S.; Mohammadi, P.; Gaudiello, E.; Bonakdar, S.; Solati-Hashjin, M.; Marsano, A.; Aghdami, N.; Scherberich, A.; Baharvand, H.; et al. Facile fabrication of egg white macroporous sponges for tissue regeneration. Adv. Healthc. Mater. 2015, 4, 2281–2290. [Google Scholar] [CrossRef]
- Aiyelabegan, H.T.; Zaidi, S.S.Z.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.K.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 853–861. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-based hydrogels for regenerative engineering and cell transplantation. Biotechnol. Bioeng. 2019, 116, 3457–3468. [Google Scholar] [CrossRef]
- Li, P.S.; Lee, I.L.; Yu, W.L.; Sun, J.S.; Jane, W.N.; Shen, H.H. A novel albumin-based tissue scaffold for autogenic tissue engineering applications. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Nseir, N.; Regev, O.; Kaully, T.; Blumenthal, J.; Levenberg, S.; Zussman, E. Biodegradable scaffold fabricated of electrospun albumin fibers: Mechanical and biological characterization. Tissue Eng. Part C Methods 2013, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, H.; Wu, J.; Li, S.H.; Weisel, R.D.; Sung, H.W.; Li, J.; Li, R.K. Preservation of conductive propagation after surgical repair of cardiac defects with a bio-engineered conductive patch. J. Hear. Lung Transplant. 2018, 37, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Horváthy, D.B.; Schandl, K.; Schwarz, C.M.; Renner, K.; Hornyák, I.; Szabó, B.T.; Niculescu-Morzsa, E.; Nehrer, S.; Dobó-Nagy, C.; Doros, A.; et al. Serum albumin-coated bone allograft (BoneAlbumin) results in faster bone formation and mechanically stronger bone in aging rats. J. Tissue Eng. Regen. Med. 2019, 13, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.; Shapira, A.; Regev, O.; Nseir, N.; Zussman, E.; Dvir, T. Albumin fiber scaffolds for engineering functional cardiac tissues. Biotechnol. Bioeng. 2014, 111, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.-H.; Torchiana, D.F. BioGlue: Albumin/glutaraldehyde sealant in cardiac surgery. J. Card. Surg. 2003, 18, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Gundry, S.R.; Black, K.; Izutani, H. Sutureless coronary artery bypass with biologic glued anastomoses: Preliminary in vivo and in vitro results. J. Thorac. Cardiovasc. Surg. 2000, 120, 473–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.M.; Sarbu Boeti, P.M.; Efrimescu, I.C. Collagen-based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar] [PubMed]
- Kaipparettu, B.A.; Kuiatse, I.; Chan, B.T.Y.; Kaipparettu, M.B.; Lee, A.V.; Oesterreich, S. Novel egg white-based 3-D cell culture system. Biotechniques 2008, 45, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Balaji, P.; Murugadas, A.; Ramkumar, A.; Thirumurugan, R.; Shanmugaapriya, S.; Akbarsha, M.A. Characterization of Hen’s Egg White to Use It as a Novel Platform to Culture Three-Dimensional Multicellular Tumor Spheroids. ACS Omega 2020, 5, 19760–19770. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, T.; Chen, X.; Fang, K.; Hou, M.; Gu, N. The effects of porosity and stiffness of genipin cross-linked egg white simulating aged extracellular matrix on proliferation and aggregation of ovarian cancer cells. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 649–660. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Chang, Q.; Darabi, M.A.; Liu, Y.; He, Y.; Zhong, W.; Mequanin, K.; Li, B.; Lu, F.; Xing, M.M.Q. Hydrogels from natural egg white with extraordinary stretchability, direct-writing 3D printability and self-healing for fabrication of electronic sensors and actuators. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 24626–24640. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of neutralization on the proliferation and cytoskeletal organization of chondrocytes on chitosan-based matrices. Int. J. Carbohydr. Chem. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Rajaram, A.; Schreyer, D.J.; Chen, D.X.B. Use of the polycation polyethyleneimine to improve the physical properties of alginate-hyaluronic acid hydrogel during fabrication of tissue repair scaffolds. J. Biomater. Sci. Polym. Ed. 2015, 26, 433–445. [Google Scholar] [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and cell viability in bioprinting alginate dialdehyde-gelatin scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Li, L. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. Int. J. Bioprinting 2016, 2, 54–66. [Google Scholar] [CrossRef]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef]
- Izadifar, M.; Kelly, M.E.; Chen, X. Engineering angiogenesis for myocardial infarction repair: Recent developments, challenges, and future directions. Cardiovasc. Eng. Technol. 2014, 5, 281–307. [Google Scholar] [CrossRef]
- Perets, A.; Baruch, Y.; Weisbuch, F.; Shoshany, G.; Neufeld, G.; Cohen, S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. Part A 2003, 65, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.S.; Crowley, E.; Vanderby, R. The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009, 17, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Ghosh, S. Carriers for the tunable release of therapeutics: Etymological classification and examples. Expert Opin. Drug Deliv. 2016, 13, 1729–1741. [Google Scholar] [CrossRef]
- Agrawal, C.M.; McKinney, J.S.; Lanctot, D.; Athanasiou, K.A. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials 2000, 21, 2443–2452. [Google Scholar] [CrossRef]
- Momtahan, N.; Poornejad, N.; Struk, J.A.; Castleton, A.A.; Herrod, B.J.; Vance, B.R.; Eatough, J.P.; Roeder, B.L.; Reynolds, P.R.; Cook, A.D. Automation of pressure control improves whole porcine heart decellularization. Tissue Eng. Part C Methods 2015, 21, 1148–1161. [Google Scholar] [CrossRef]
- Escobar, P.; Wittles, S.; Asfour, S.; Latta, L. Mechanical characteristics of muscle, skin and fat-elastic moduli for finite element modeling of limbs. In Proceedings of the ORS (Orthopaedic Research Society) 2014 Annual Meeting, New Orleans, LA, USA, 15–18 March 2014. Poster No. 1173. [Google Scholar]
- Mansy, H.A.; Grahe, J.R.; Sandler, R.H. Elastic properties of synthetic materials for soft tissue modeling. Phys. Med. Biol. 2008, 53, 2115–2130. [Google Scholar] [CrossRef]
- Datta, S.; Barua, R.; Das, J. Importance of alginate bioink for 3D bioprinting in tissue engineering and regenerative medicine. In Alginates: Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2019. [Google Scholar]
- Naghieh, S.; Sarker, M.; Izadifar, M.; Chen, X. Dispensing-based bioprinting of hybrid scaffolds with vessel-like channels for tissue engineering applications—A brief review. J. Mech. Behav. Biomed. Mater. 2018, 78, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, J.S.; Kim, J.Y.; Cho, D.W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.G.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P.G. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delkash, Y.; Gouin, M.; Rimbeault, T.; Mohabatpour, F.; Papagerakis, P.; Maw, S.; Chen, X. Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications. J. Funct. Biomater. 2021, 12, 45. https://doi.org/10.3390/jfb12030045
Delkash Y, Gouin M, Rimbeault T, Mohabatpour F, Papagerakis P, Maw S, Chen X. Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications. Journal of Functional Biomaterials. 2021; 12(3):45. https://doi.org/10.3390/jfb12030045
Chicago/Turabian StyleDelkash, Yasaman, Maxence Gouin, Tanguy Rimbeault, Fatemeh Mohabatpour, Petros Papagerakis, Sean Maw, and Xiongbiao Chen. 2021. "Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications" Journal of Functional Biomaterials 12, no. 3: 45. https://doi.org/10.3390/jfb12030045
APA StyleDelkash, Y., Gouin, M., Rimbeault, T., Mohabatpour, F., Papagerakis, P., Maw, S., & Chen, X. (2021). Bioprinting and In Vitro Characterization of an Eggwhite-Based Cell-Laden Patch for Endothelialized Tissue Engineering Applications. Journal of Functional Biomaterials, 12(3), 45. https://doi.org/10.3390/jfb12030045






