Heat Sterilization Effects on Polymeric, FDM-Optimized Orthopedic Cutting Guide for Surgical Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
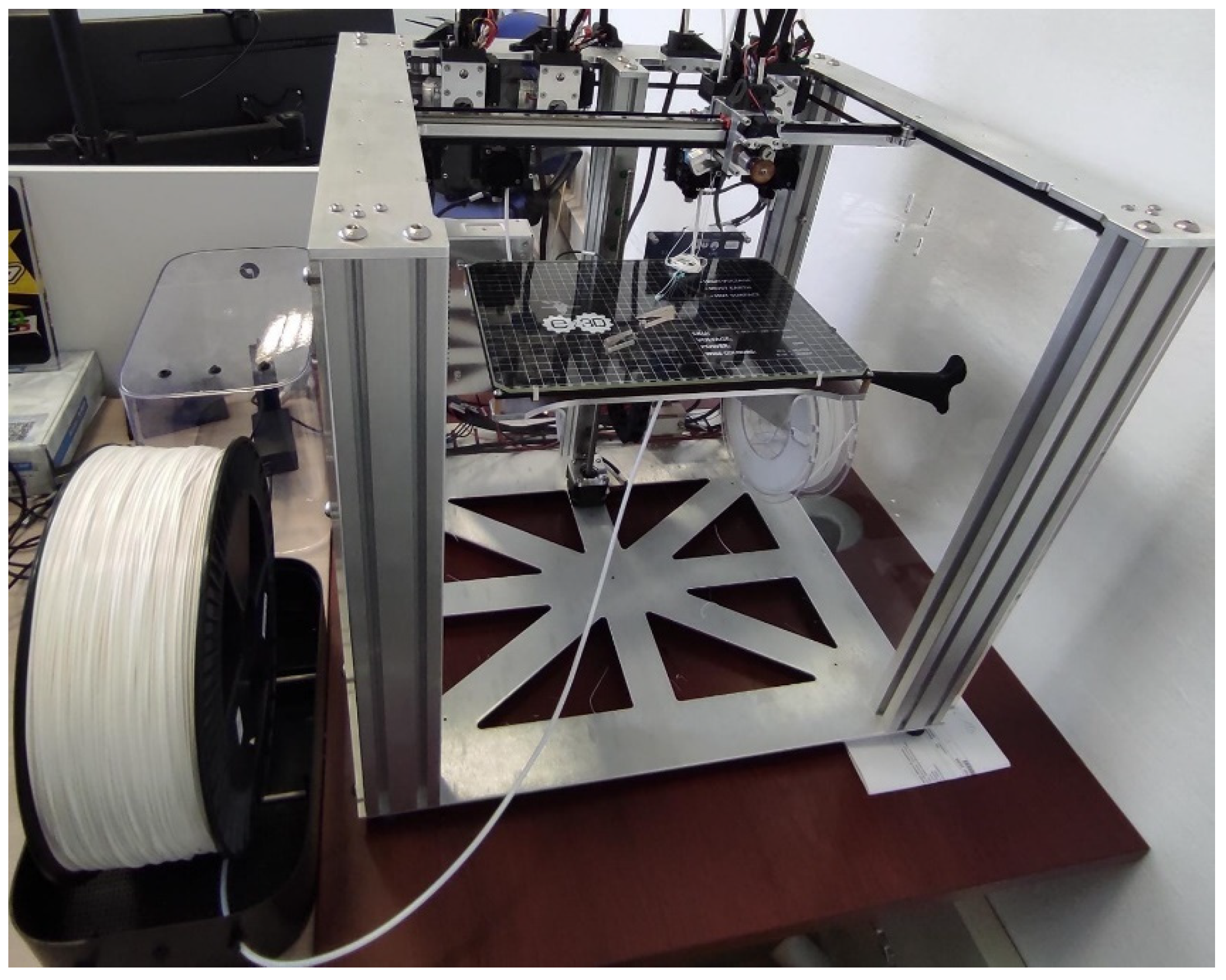
2.2. Methodology
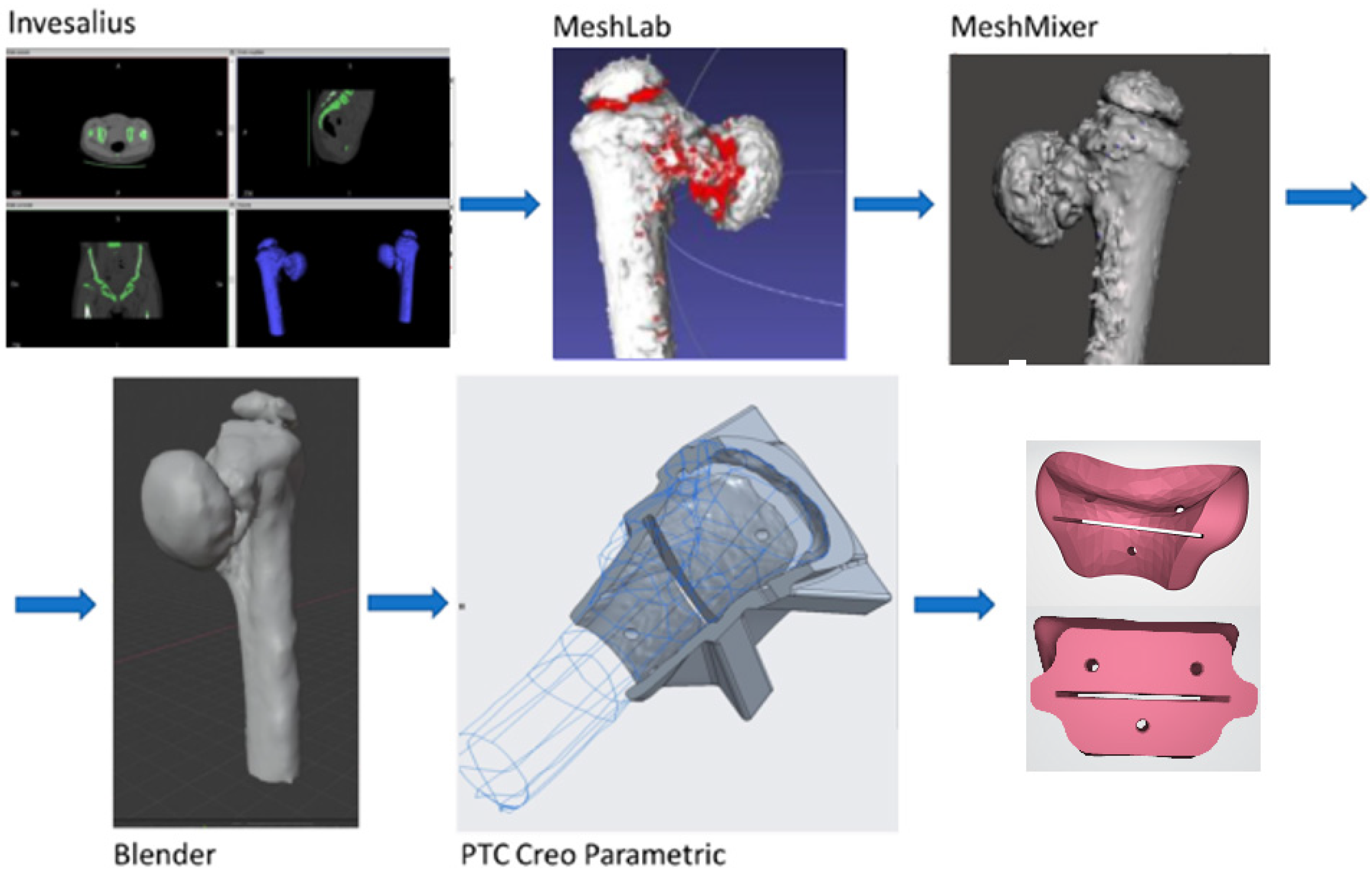
2.2.1. From TAC Image to the Drawing Board, to the 3D Printer
2.2.2. FDM Process Parameters
- Extrusion multiplier: This is a parameter that determines the quantity of material to add to the standard value of the slicing software and is important since it allows the reduction in defects such as the deposition of material between lines.
- Extrusion width: as a property indicating the distance between centers of two continuous filaments, this parameter allows the online width to be controlled, but it is impossible to have this value for a single line.
- Outline overlap: This parameter increases infill quality. A value of 30% showed the best outcome.
2.2.3. Heat Treatment before Sterilization Test
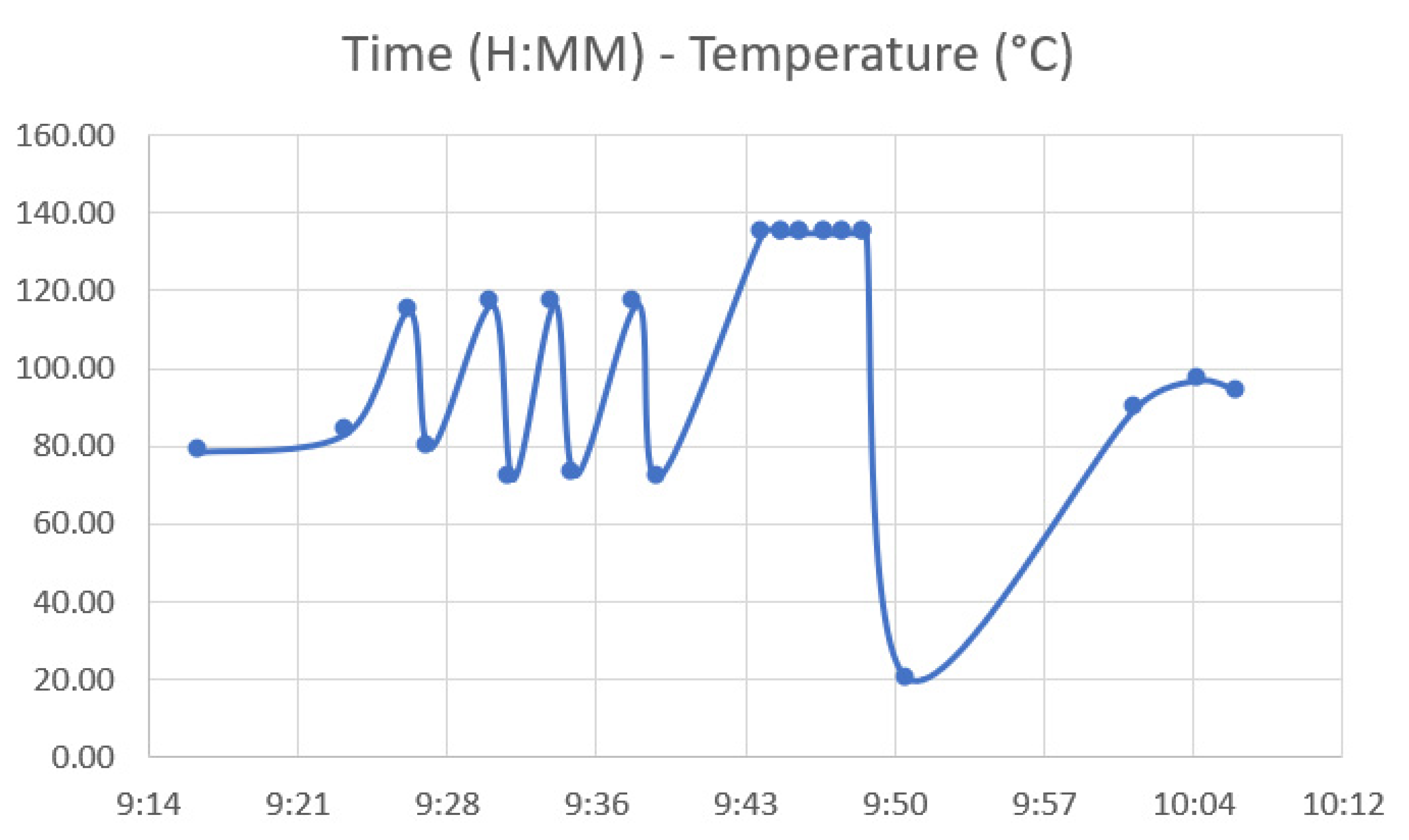
2.2.4. Autoclave Temperature Analysis
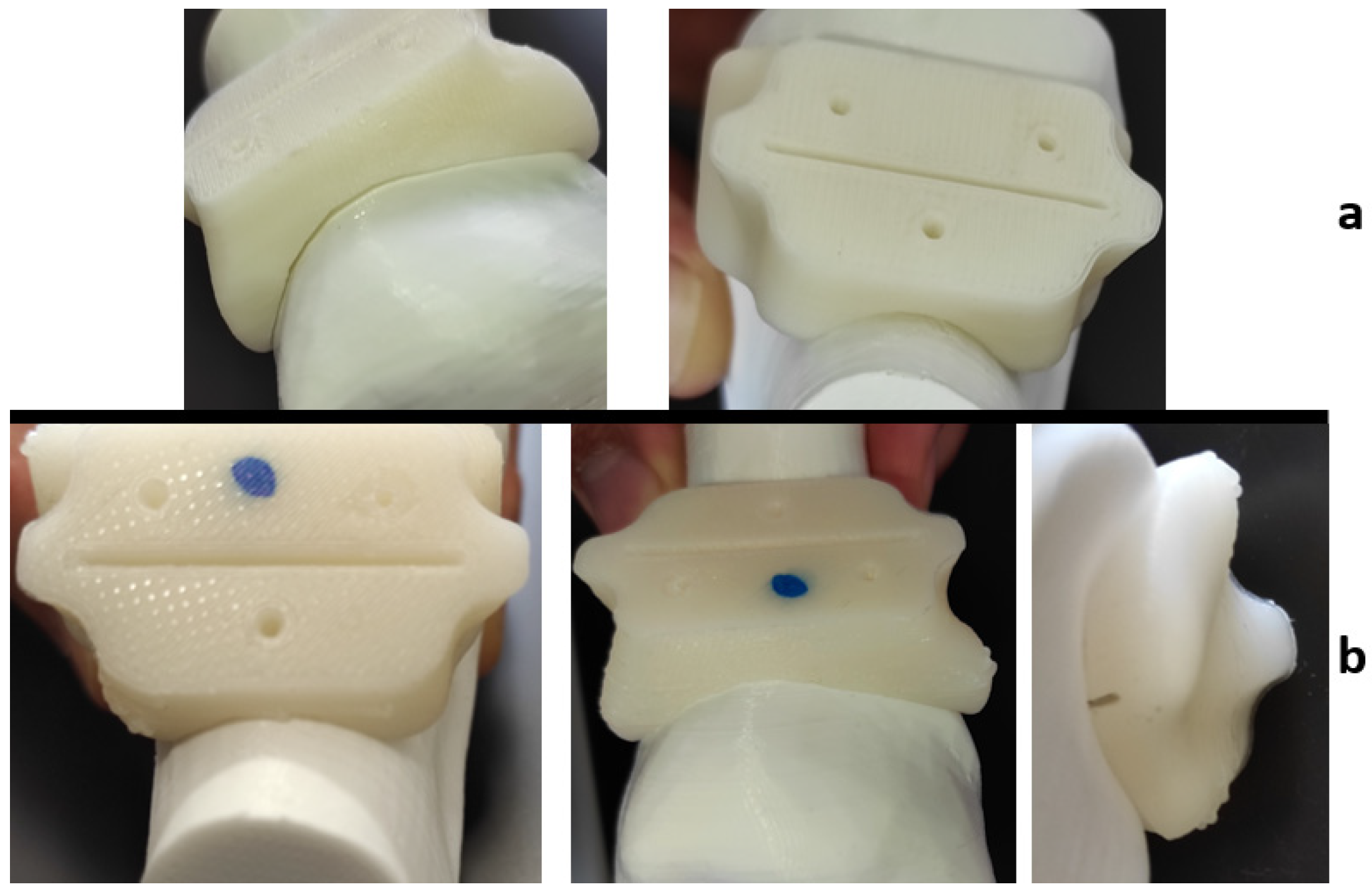
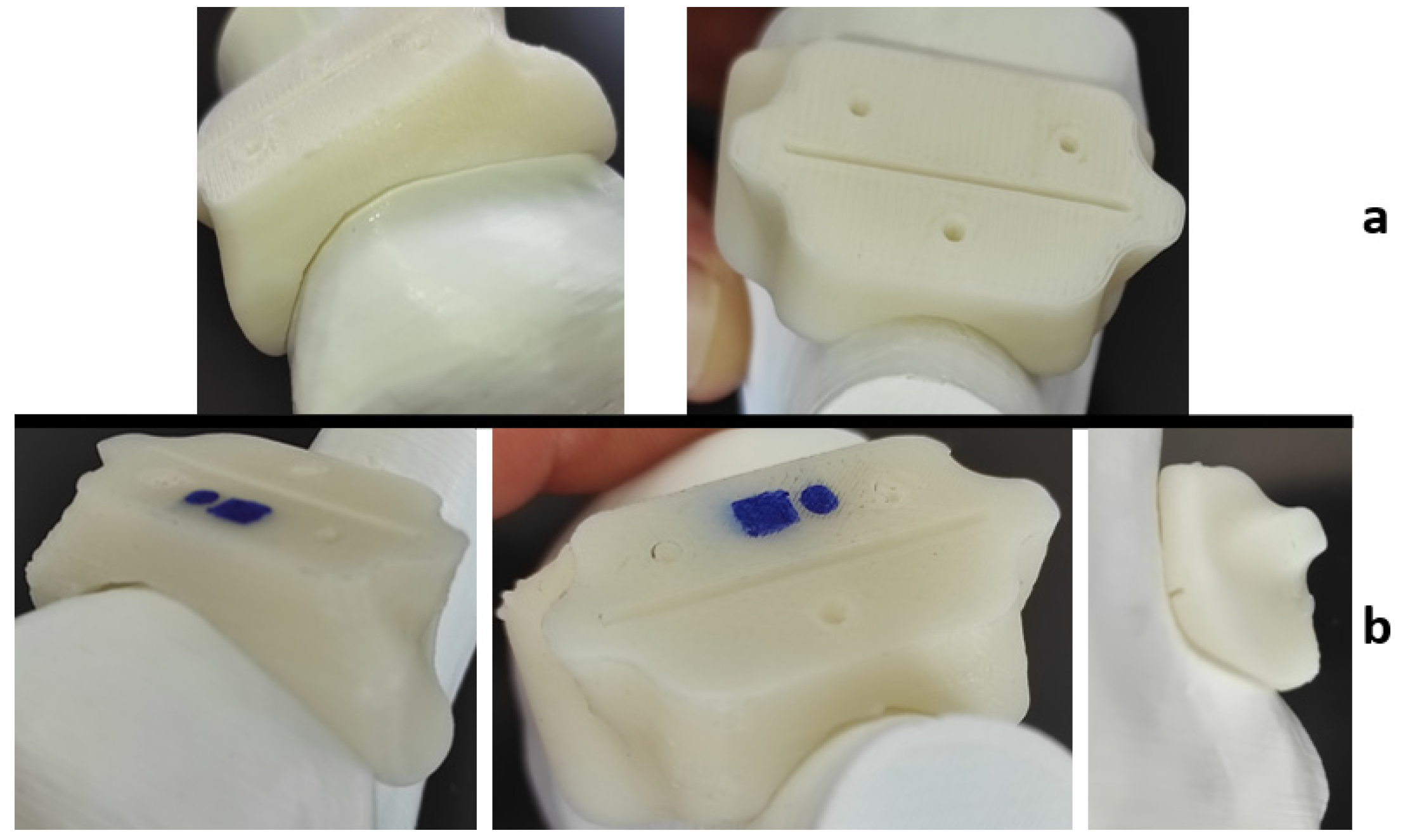
2.3. Dimensional Assessment after Sterilization Procedure
3. Results
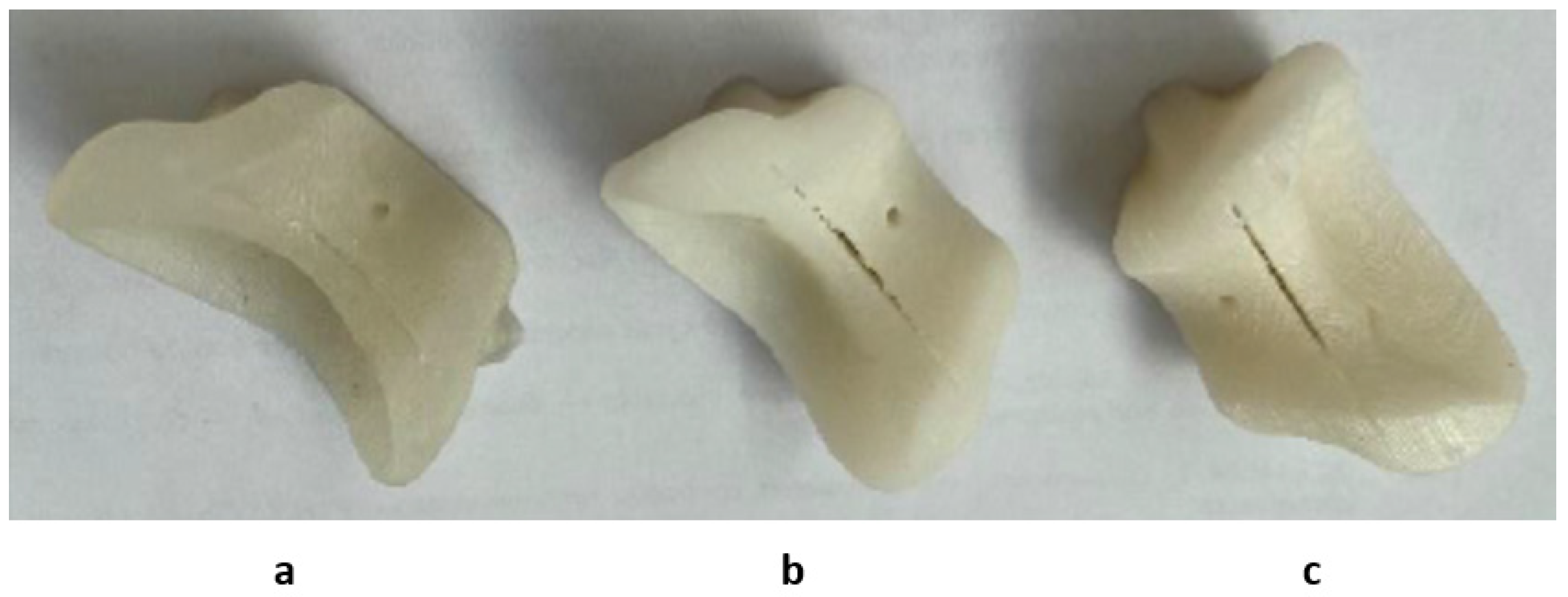
3.1. Quality Assessment of the Product after Sterilization
3.2. Autoclave Temperature Analysis Results—Sterilization Test
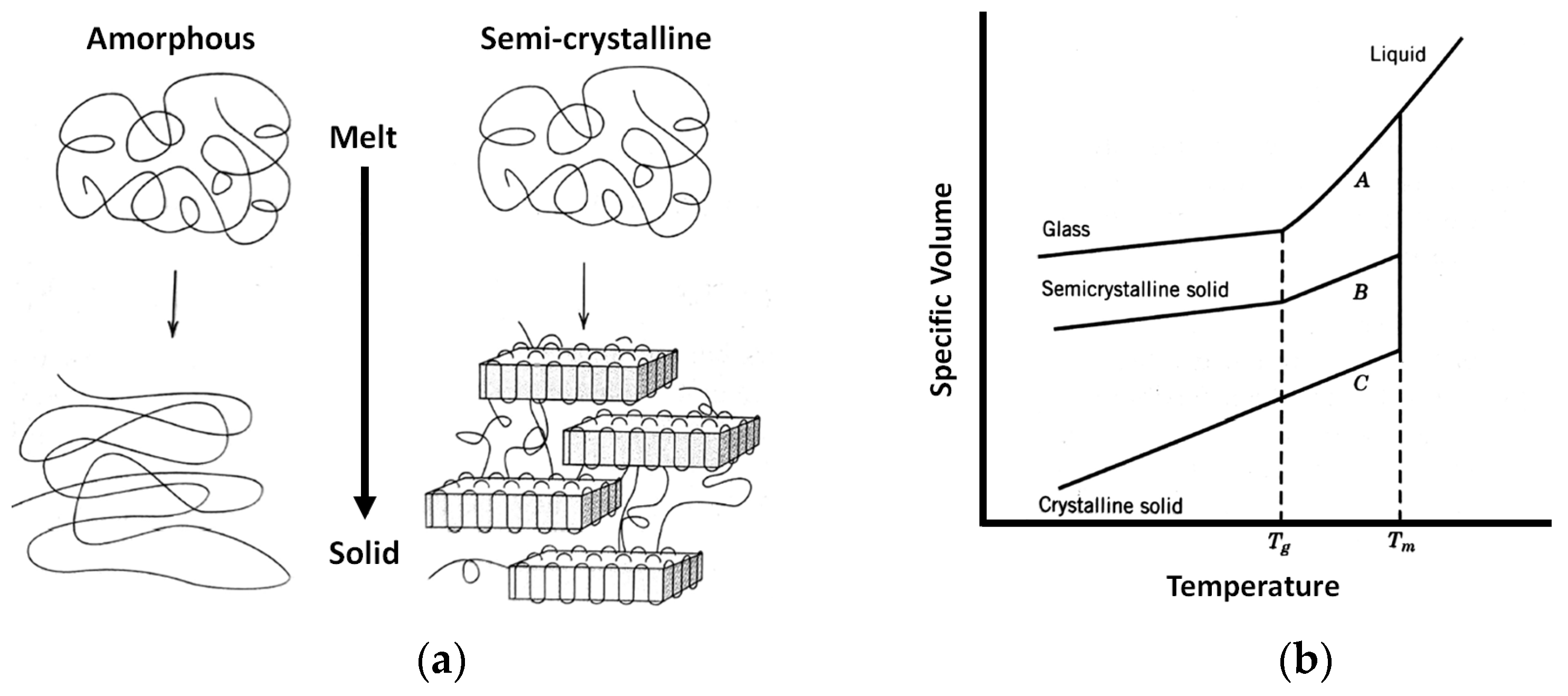
3.3. Material Shrinkage and Molecular Semi-Crystallization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakof, K.S.A.; Zabudin, N.F.; Mat Sahat, I.; Mohd Adib, M.A.H. Development of 3D Printed Heart Model for Medical Training. In Lecture Notes in Mechanical Engineering; Hassan, M.H.A., Ed.; Springer: Singapore, 2018; pp. 109–116. ISBN 9783319666969. [Google Scholar] [CrossRef]
- Osti, F.; Santi, G.M.; Neri, M.; Liverani, A.; Frizziero, L.; Stilli, S.; Maredi, E.; Zarantonello, P.; Gallone, G.; Stallone, S.; et al. CT Conversion Workflow for Intraoperative Usage of Bony Models: From DICOM Data to 3D Printed Models. Appl. Sci. 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Waran, V.; Narayanan, V.; Karuppiah, R.; Pancharatnam, D.; Chandran, H.; Raman, R.; Rahman, Z.A.A.; Owen, S.L.F.; Aziz, T.Z. Injecting realism in surgical training—initial simulation experience with custom 3D models. J. Surg. Educ. 2014, 71, 193–197. [Google Scholar] [CrossRef]
- Frizziero, L.; Liverani, A.; Donnici, G.; Osti, F.; Neri, M.; Maredi, E.; Trisolino, G.; Stilli, S. New Methodology for Diagnosis of Orthopedic Diseases through Additive Manufacturing Models. Symmetry 2019, 11, 542. [Google Scholar] [CrossRef] [Green Version]
- Caligiana, P.; Liverani, A.; Ceruti, A.; Santi, G.M.; Donnici, G.; Osti, F. An Interactive Real-Time Cutting Technique for 3D Models in Mixed Reality. Technologies 2020, 8, 23. [Google Scholar] [CrossRef]
- McGurk, M.; Amis, A.A.; Potamianos, P.; Goodger, N.M. Rapid prototyping techniques for anatomical modelling in medicine. Ann. R. Coll. Surg. Engl. 1997, 79, 169. [Google Scholar]
- Waran, V.; Menon, R.; Pancharatnam, D.; Rathinam, A.K.; Balakrishnan, Y.K.; Tung, T.S.; Raman, R.; Prepageran, N.; Chandran, H.; Rahman, Z.A.A. The creation and verification of cranial models using three-dimensional rapid prototyping technology in field of transnasal sphenoid endoscopy. Am. J. Rhinol. Allergy 2012, 26, e132–e136. [Google Scholar] [CrossRef]
- Waran, V.; Devaraj, P.; Hari Chandran, T. Validation of 3D anatomical accuracy of cranial models created by Rapid Prototyping Techniques using a neuronavigation station (Medtronic S7®). J. Clin. Neurosci. 2012, 19, 574–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandler, A.G. Touch Surgery: A Twenty-First Century Platform for Surgical Training. J. Digit. Imaging 2018, 31, 585–590. [Google Scholar] [CrossRef]
- Michalski, A.; Stopa, M.; Miśkowiak, B. Use of multimedia technology in the doctor- patient relationship for obtaining patient informed consent. Med. Sci. Monit. 2016, 22, 3994–3999. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Tanaka, K.; Dang, A.; Dang, A. Identifying a commercially-available 3D printing process that minimizes model distortion after annealing and autoclaving and the effect of steam sterilization on mechanical strength. 3D Print. Med. 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, E.; Alhelwani, A.; Van de Casteele, E.; Politis, C.; Jacobs, R. Evaluation of Dimensional Changes of 3D Printed Models After Sterilization: A Pilot Study. Open Dent. J. 2018, 12, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Deloitte. The Future of Health Care: Potential, Impacts and Models of 3D Printing in the Health Care Sector; Deloitte Italy S.p.A.: Milan, Italy, 2018. [Google Scholar]
- Ferretti, P.; Leon, C.; Sali, M.; Frizziero, L.; Donnici, G.; Liverani, A. Application of TPU—Sourced 3D Printed FDM Organs for Improving the Realism in Surgical Planning and Training. In Proceedings of the International Conference on Industrial Engineering and Operations Management, Singapore, 8 March 2021. [Google Scholar]
- Witowski, J.S.; Coles-Black, J.; Zuzak, T.Z.; Pędziwiatr, M.; Chuen, J.; Major, P.; Budzyński, A. 3D Printing in Liver Surgery: A Systematic Review. Telemed. J. e-Health Off. J. Am. Telemed. Assoc. 2017, 23, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Sampogna, G.; Pugliese, R.; Elli, M.; Vanzulli, A.; Forgione, A. Routine clinical application of virtual reality in abdominal surgery. Minim. Invasive Ther. Allied Technol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Brunso, J.; Franco, M.; Constantinescu, T.; Barbier, L.; Santamaría, J.A.; Alvarez, J. Custom-Machined Miniplates and Bone-Supported Guides for Orthognathic Surgery: A New Surgical Procedure. J. Oral Maxillofac. Surg. 2016, 74, 1061.e1–1061.e12. [Google Scholar] [CrossRef]
- Jacobi, M.; Wahl, P.; Bouaicha, S.; Jakob, R.P.; Gautier, E. Distal femoral varus osteotomy: Problems associated with the lateral open-wedge technique. Arch. Orthop. Trauma Surg. 2011, 131, 725–728. [Google Scholar] [CrossRef]
- Pape, D.; Rupp, S. Preoperative Planning for High Tibial Osteotomies. Oper. Tech. Orthop. 2007, 17, 2–11. [Google Scholar] [CrossRef]
- Imhoff, F.B.; Schnell, J.; Magaña, A.; Diermeier, T.; Scheiderer, B.; Braun, S.; Imhoff, A.B.; Arciero, R.A.; Beitzel, K. Single cut distal femoral osteotomy for correction of femoral torsion and valgus malformity in patellofemoral malalignment—Proof of application of new trigonometrical calculations and 3D-printed cutting guides. BMC Musculoskelet. Disord. 2018, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Bianchi, A.; Schiariti, G.; Badiali, G.; Marchetti, C. Computer-Aided Design and Computer-Aided Manufacturing Cutting Guides and Customized Titanium Plates Are Useful in Upper Maxilla Waferless Repositioning. J. Oral Maxillofac. Surg. 2015, 73, 701–707. [Google Scholar] [CrossRef]
- Nam, D.; Williams, B.; Hirsh, J.; Johnson, S.R.; Nunley, R.M.; Barrack, R.L. Planned Bone Resections Using an MRI-Based Custom Cutting Guide System Versus 3-Dimensional, Weight-Bearing Images in Total Knee Arthroplasty. J. Arthroplast. 2015, 30, 567–572. [Google Scholar] [CrossRef]
- Arnal-Burró, J.; Pérez-Mañanes, R.; Gallo-del-Valle, E.; Igualada-Blazquez, C.; Cuervas-Mons, M.; Vaquero-Martín, J. Three dimensional-printed patient-specific cutting guides for femoral varization osteotomy: Do it yourself. Knee 2017, 24, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xing, W.; Wu, Z.; Huang, H.; Huang, W. A combination of three-dimensional printing and computer-assisted virtual surgical procedure for preoperative planning of acetabular fracture reduction. Injury 2016, 47, 2223–2227. [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; Laptoiu, D.; Marinescu, R.; Botezatu, I. Design and 3D printing customized guides for orthopaedic surgery–lessons learned. Rapid Prototyp. J. 2018, 25, 901–913. [Google Scholar] [CrossRef]
- Vlasceanu, D.; Baciu, F.; Popescu, D.; Hadar, A.; Marinescu, R. Development and 3D Printing of an ABS Ergonomic Handle for Medical Use. Mater. Plast. 2018, 55, 630. [Google Scholar] [CrossRef]
- Rankin, T.M.; Giovinco, N.A.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Three-dimensional printing surgical instruments: Are we there yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, M.; Aroom, K.R.; Hawes, H.G.; Gill, B.S.; Love, J. 3D printed surgical instruments: The design and fabrication process. World J. Surg. 2017, 41, 314–319. [Google Scholar] [CrossRef]
- Angela, W.Y.; Khan, M. On-demand three-dimensional printing of surgical supplies in conflict zones. J. Trauma Acute Care Surg. 2015, 78, 201–203. [Google Scholar]
- de Melo Costa, D.; de Oliveira Lopes, L.K.; Vickery, K.; Watanabe, E.; de Oliveira Leão, L.S.N.; de Paula, M.C.; Melo, D.S.; Hu, H.; Deva, A.K.; Tipple, A.F.V. Reprocessing safety issues associated with complex-design orthopaedic loaned surgical instruments and implants. Injury 2018, 49, 2005–2012. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection and sterilization in health care facilities: What clinicians need to know. Clin. Infect. Dis. 2004, 39, 702–709. [Google Scholar] [CrossRef] [Green Version]
- Aguado-Maestro, I.; De Frutos-Serna, M.; González-Nava, A.; Merino-De Santos, A.B.; García-Alonso, M. Are the common sterilization methods completely effective for our in-house 3D printed biomodels and surgical guides? Injury 2020, 52, 1341–1345. [Google Scholar] [CrossRef]
- Marinescu, R.; Antoniac, V.I.; Stoia, D.I.; Lăptoiu, D.C. Clavicle anatomical osteosynthesis plate breakage-failure analysis report based on patient morphological parameters. Rom. J. Morphol Embryol 2017, 58, 593–598. [Google Scholar]
- Brown, S.A.; Merritt, K.; Woods, T.O.; McNamee, S.G.; Hitchins, V.M. Effects of different disinfection and sterilization methods on tensile strength of materials used for single-use devices. Biomed. Instrum. Technol. 2002, 36, 23–27. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.-L.; Kim, C.H.; Lee, S.H.; Kim, J.K.; Lee, S.J.; Park, J.-C. Effects of low temperature hydrogen peroxide gas on sterilization and cytocompatibility of porous poly (d, l-lactic-co-glycolic acid) scaffolds. Surf. Coatings Technol. 2008, 202, 5762–5767. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.-H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 34108. [Google Scholar] [CrossRef]
- Sosnowski, E.P.; Morrison, J. Sterilization of Medical 3d Printed Plastics: Is H2O2 Vapour Suitable? CMBES Proc. 2017, 40. [Google Scholar]
- Perez, M.; Block, M.; Espalin, D.; Winker, R.; Hoppe, T.; Medina, F.; Wicker, R. Sterilization of FDM-manufactured parts. In Proceedings of the 23rd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Dallas, TX, USA, 6–8 August 2012; pp. 285–296. [Google Scholar]
- Rogers, W.J. Steam and dry heat sterilization of biomaterials and medical devices. In Sterilisation of Biomaterials and Medical Devices; Woodhead Publishing: Cambridge, UK, 2012; pp. 20–55. [Google Scholar]
- Rogers, W.J. 7—Sterilisation techniques for polymers. In Woodhead Publishing Series in Biomaterials; Lerouge, S., Simmons, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 151–211. ISBN 978-1-84569-932-1. [Google Scholar]
- Modjarrad, K.; Ebnesajjad, S. Handbook of Polymer Applications in Medicine and Medical Devices; William Andrew Publishing: Norwich, NY, USA, 2014. [Google Scholar]
- Mardis, N.J. Emerging Technology and Applications of 3D Printing in the Medical Field. Mo. Med. 2018, 115, 368–373. [Google Scholar]
- Singh, R.; Singh, N.; Amendola, A.; Fraternali, F. On the wear properties of Nylon6-SiC-Al2O3 based fused deposition modelling feed stock filament. Compos. Part. B Eng. 2017, 119, 125–131. [Google Scholar] [CrossRef]
- Hou, Z.; Tian, X.; Zhang, J.; Li, D. 3D printed continuous fibre reinforced composite corrugated structure. Compos. Struct. 2018, 184, 1005–1010. [Google Scholar] [CrossRef]
- Tian, X.; Liu, T.; Yang, C.; Wang, Q.; Li, D. Interface and performance of 3D printed continuous carbon fiber reinforced PLA composites. Compos. Part. A Appl. Sci. Manuf. 2016, 88, 198–205. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Liu, S. Rapid prototyping of continuous carbon fiber reinforced polylactic acid composites by 3D printing. J. Mater. Process. Technol. 2016, 238, 218–225. [Google Scholar] [CrossRef]
- Hao, W.; Liu, Y.; Zhou, H.; Chen, H.; Fang, D. Preparation and characterization of 3D printed continuous carbon fiber reinforced thermosetting composites. Polym. Test. 2018, 65, 29–34. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Saghlatoon, H.; Honari, M.M.; Mirzavand, R.; Montemagno, C.; Mousavi, P. Investigation on electrical and mechanical properties of 3D printed nylon 6 for RF/microwave electronics applications. Addit. Manuf. 2018, 21, 69–75. [Google Scholar] [CrossRef]
- Marascio, M.G.M.; Antons, J.; Pioletti, D.P.; Bourban, P. 3D printing of polymers with hierarchical continuous porosity. Adv. Mater. Technol. 2017, 2, 1700145. [Google Scholar] [CrossRef]
- Jackson, R.J.; Patrick, P.S.; Page, K.; Powell, M.J.; Lythgoe, M.F.; Miodownik, M.A.; Parkin, I.P.; Carmalt, C.J.; Kalber, T.L.; Bear, J.C. Chemically treated 3D printed polymer scaffolds for biomineral formation. ACS Omega 2018, 3, 4342–4351. [Google Scholar] [CrossRef] [PubMed]
- Justo, J.; Távara, L.; García-Guzmán, L.; París, F. Characterization of 3D printed long fibre reinforced composites. Compos. Struct. 2018, 185, 537–548. [Google Scholar] [CrossRef]
- Spackman, C.C.; Frank, C.R.; Picha, K.C.; Samuel, J. 3D printing of fiber-reinforced soft composites: Process study and material characterization. J. Manuf. Process. 2016, 23, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Melenka, G.W.; Cheung, B.K.O.; Schofield, J.S.; Dawson, M.R.; Carey, J.P. Evaluation and prediction of the tensile properties of continuous fiber-reinforced 3D printed structures. Compos. Struct. 2016, 153, 866–875. [Google Scholar] [CrossRef]
- Das, S.; Hollister, S.J.; Flanagan, C.; Adewunmi, A.; Bark, K.; Chen, C.; Ramaswamy, K.; Rose, D.; Widjaja, E. Freeform fabrication of Nylon-6 tissue engineering scaffolds. Rapid Prototyp. J. 2003, 9, 43–49. [Google Scholar] [CrossRef]
- Boparai, K.S.; Singh, R.; Fabbrocino, F.; Fraternali, F. Thermal characterization of recycled polymer for additive manufacturing applications. Compos. Part. B Eng. 2016, 106, 42–47. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar]
- DeJong, L.T.C.E.S.; DeBerardino, L.T.C.T.M.; Brooks, D.E.; Judson, K. In vivo comparison of a metal versus a biodegradable suture anchor. Arthrosc. J. Arthrosc. Relat. Surg. 2004, 20, 511–516. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erne, P.; Schier, M.; Resink, T.J. The road to bioabsorbable stents: Reaching clinical reality? Cardiovasc. Intervent. Radiol. 2006, 29, 11–16. [Google Scholar] [CrossRef]
- Eyring, H. Viscosity, plasticity, and diffusion as examples of absolute reaction rates. J. Chem. Phys. 1936, 4, 283–291. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyauchi, S. Poly (L-lactide): VI Effects of crystallinity on enzymatic hydrolysis of poly (L-lactide) without free amorphous region. Polym. Degrad. Stab. 2001, 71, 415–424. [Google Scholar] [CrossRef]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lembeck, B.; Wülker, N. Severe cartilage damage by broken poly–L–lactic acid (PLLA) interference screw after ACL reconstruction. Knee Surgery Sport. Traumatol. Arthrosc. 2005, 13, 283–286. [Google Scholar] [CrossRef]
- Da Silva Soares, J.F. Constitutive Modeling for Biodegradable Polymers for Application in Endovascular Stents. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2008. [Google Scholar]
- Ceresana, Bioplastics—Study: Market., Analysis, Trends. Available online: https://www.ceresana.com/en/market-studies/plastics/bioplastics/market-study-bioplastics.html (accessed on 26 April 2021).
- Valerga, A.P.; Batista, M.; Salguero, J.; Girot, F. Influence of PLA filament conditions on characteristics of FDM parts. Materials 2018, 11, 1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Gramlich, W.M.; Gardner, D.J. Improving the impact strength of Poly(lactic acid) (PLA) in fused layer modeling (FLM). Polymer 2017, 114, 242–248. [Google Scholar] [CrossRef]
- Tran, P.; Ngo, T.D.; Ghazlan, A.; Hui, D. Bimaterial 3D printing and numerical analysis of bio-inspired composite structures under in-plane and transverse loadings. Compos. Part. B Eng. 2017, 108, 210–223. [Google Scholar] [CrossRef]
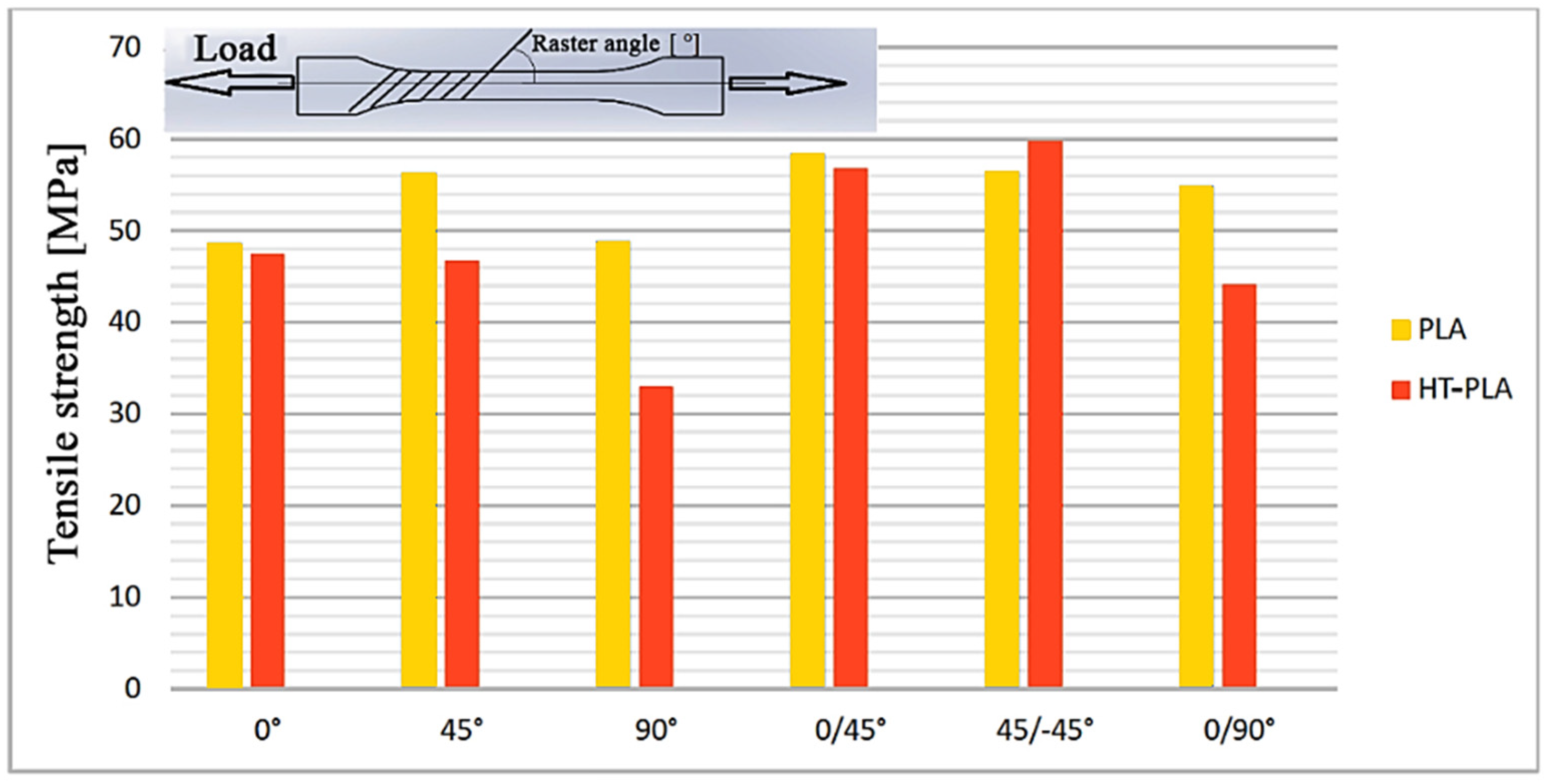
- Hanon, M.M.; Marczis, R.; Zsidai, L. Influence of the 3D Printing Process Settings on Tensile Strength of PLA and HT-PLA. Period. Polytech. Mech. Eng. 2021, 65, 38–46. [Google Scholar] [CrossRef]
- Ferretti, P.; Leon-Cardenas, C.; Santi, G.M.; Sali, M.; Ciotti, E.; Frizziero, L.; Donnici, G.; Liverani, A. Relationship between FDM 3D Printing Parameters Study: Parameter Optimization for Lower Defects. Polymers 2021, 13, 2190. [Google Scholar] [CrossRef] [PubMed]
- Jakub Kočí How to Improve Your 3D Prints with Annealing. Available online: https://blog.prusaprinters.org/how-to-improve-your-3d-prints-with-annealing_31088/ (accessed on 7 January 2020).
- Espalin, D.; Arcaute, K.; Rodriguez, D.; Medina, F.; Posner, M.; Wicker, R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar] [CrossRef]
- ADA Council on Scientific Affairs; ADA Council on Dental Practice. Infection control recommendations for the dental office and the dental laboratory. J. Am. Dent. Assoc. 1996, 127, 672–680. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Recommended infection-control practices for dentistry. Morb. Mortal. Wkly. Rep. Recom-Mendation Rep. 1993, 41, 1–12. [Google Scholar]
- Hupp, J.R.; Tucker, M.R.; Ellis, E. Contemporary Oral and Maxillofacial Surgery-E-book; Elsevier Health Sciences: London, UK, 2013; ISBN 0323226876. [Google Scholar]
- Dentsply Sirona SIMPLANT Procedure Manual from Scan, to Plan. Available online: https://assets.dentsplysirona.com/dentsply/pim/manufacturer/Implants/Guided_surgery/32670466-USX-1702%20Simplant%20Procedure%20Manual_LR.pdf (accessed on 14 December 2020).
- Nobel Biocare. Treatment Workflow for the Partially Edentulous Patient. Available online: https://www.nobelbiocare.com/en-int/tempshell (accessed on 15 December 2020).
- Doornmalen, J.; van Wezel, R.; Onsea, T.; Oussoren, H.; Henrotin, K.; Kopinga, K. Steam sterilisation according to EN 285:2015. Zentralsterilisation Cent. Serv. 2020, 28, 51–54. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; Wiley: New York, NY, USA, 2018; Volume 9. [Google Scholar]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Biosurfaces: A Materials Science and Engineering Perspective; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 334. ISBN 9781118950623. [Google Scholar]
- Zhang, S.; Horrocks, A.R.; Hull, R.; Kandola, B.K. Flammability, degradation and structural characterization of fibre-forming polypropylene containing nanoclay–flame retardant combinations. Polym. Degrad. Stab. 2006, 91, 719–725. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, D.; Zhao, J.; Wu, Y.; Zheng, B. 3D Printout Models vs. 3D-Rendered Images: Which Is Better for Preoperative Planning? J. Surg. Educ. 2016, 73, 518–523. [Google Scholar] [CrossRef]
- Rebong, R.E.; Stewart, K.T.; Utreja, A.; Ghoneima, A.A. Accuracy of three-dimensional dental resin models created by fused deposition modeling, stereolithography, and Polyjet prototype technologies: A comparative study. Angle Orthod. 2018, 88, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]





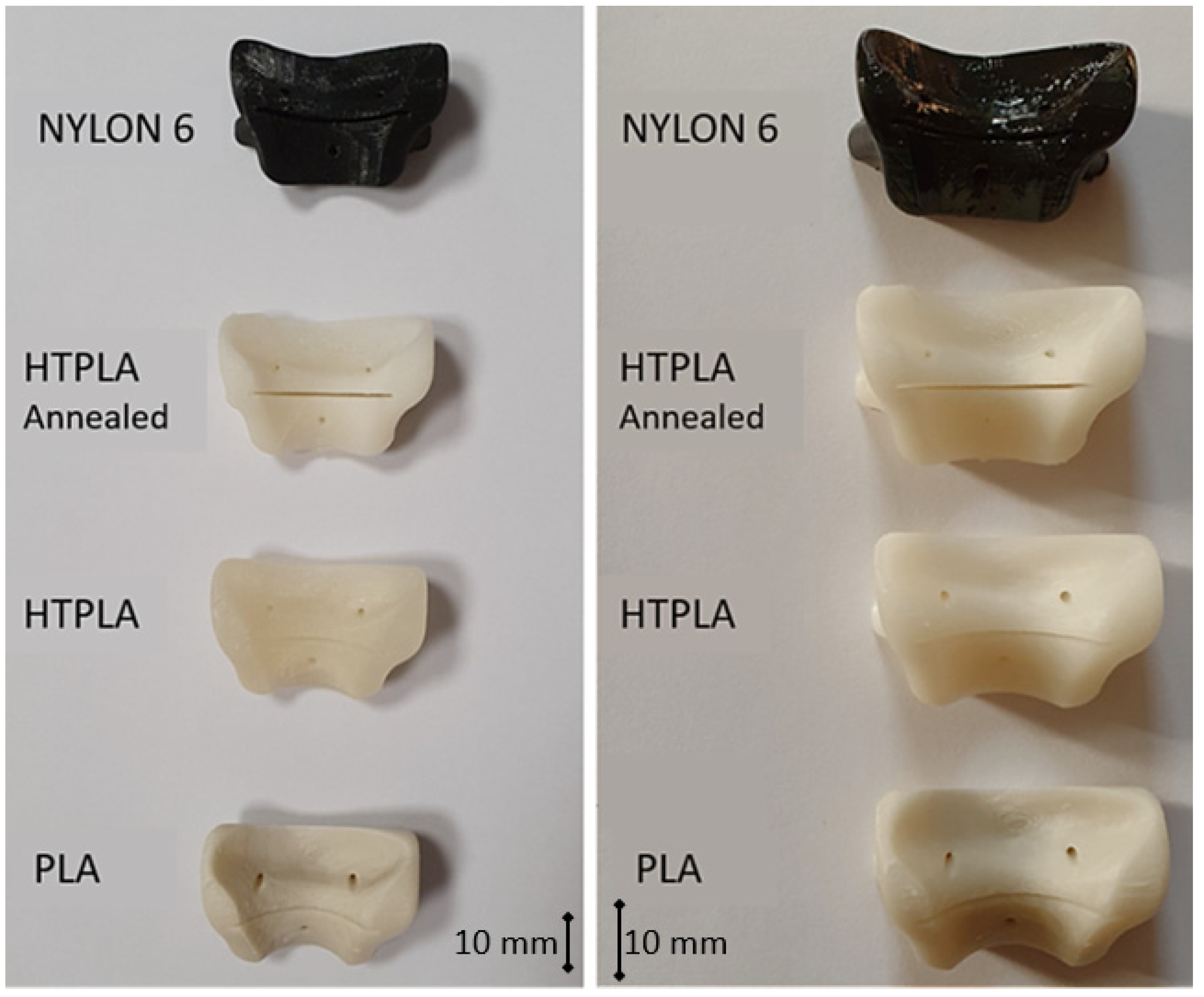
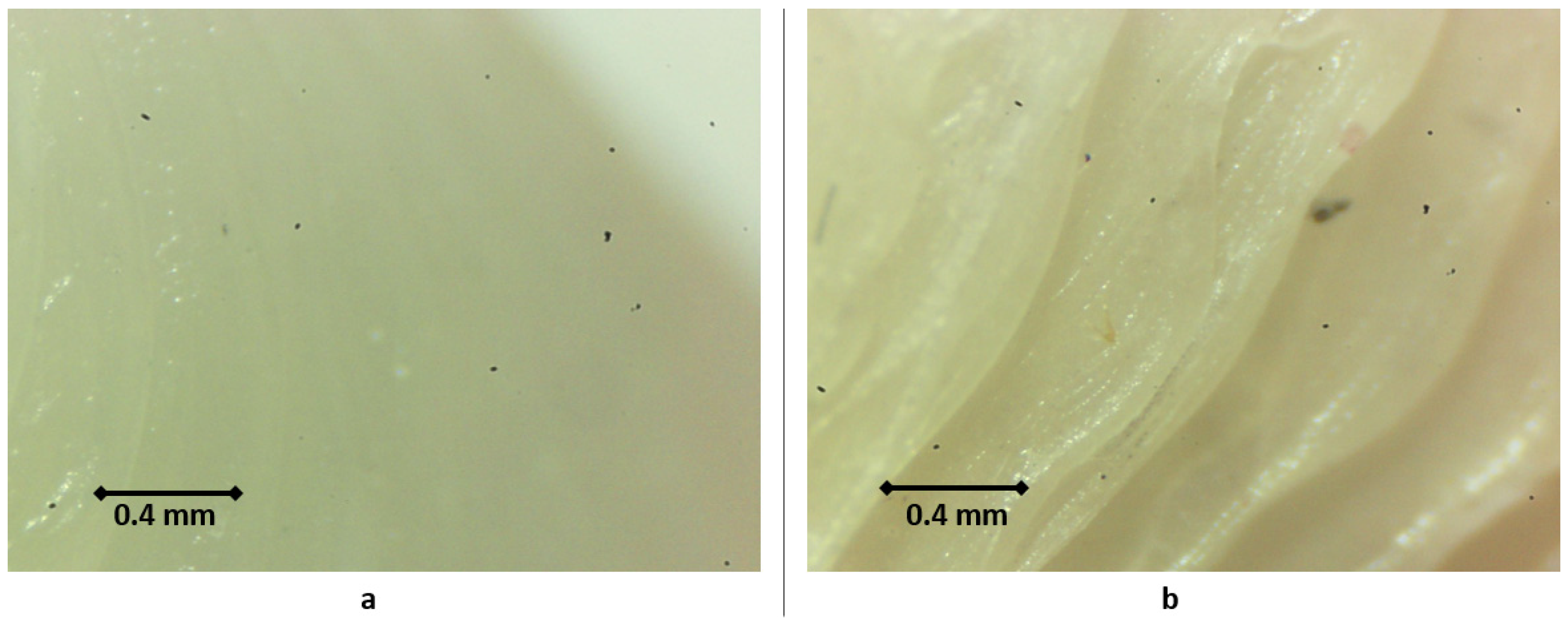
| Type | Item | PLA | HTPLA | NYLON d = dry; m = moist | Based on |
|---|---|---|---|---|---|
| Filament | Diameter | 1.75 mm | 1.75 mm | 1.75 mm | - |
| Specific Gravity | 1.24 (g/cc) | 1.25 (g/cc) | 1.12 (g/cc) | (ASTM D1505) | |
| Supplier | FiloAlfa® | Fabbrix® | Polymaker® | - | |
| Part Number | 1PLA10002 | FHT020075 | 200805107 | - | |
| Mechanical | Tensile Strength | 47.8 Mpa | 40.8 Mpa | 66.2 Mpa (d), | ISO 527, (ASTM D638) |
| 36.4 Mpa (m) | |||||
| Tensile Modulus | 2436 Mpa | 2190 Mpa | 2223 Mpa (d), | ISO 527, (ASTM D638) | |
| 1053 Mpa (m) | |||||
| Yield Strength | - | 90.9 Mpa | 97 ± 1.1 Mpa (d), 41.6 ± 11.6 Mpa (m) | ISO 178, (ASTM D790) | |
| Elongation at Break | 4.59% | 5% | - | DIN EN ISO 6892-1 | |
| Hardness-Vickers | 83 Shore D | - | - | DIN EN ISO 6507-1 | |
| Impact Strength (v-notched Izod) | 17.91 kJ/m2 | 25.31 kJ/m2 | 9.6 ± 1.4 KJ/m2 (d) | ISO 180 (d), ISO 179 (m), ASTM D256 (t) | |
| 17.2 ± 1.4 KJ/m2 (m) | |||||
| Thermal | Melting Point | 145–160 °C | 165–180 °C | 180 °C | (ASTM D3418) |
| Glass Transition (Tg) | 60 °C | 55–60 °C | - | (ASTM D3418) | |
| Fusion Temperature (Tm) | 145–160 °C | 165–180 °C | 190 °C | (ASTM D3418) |
| Printing Technology | FDM (Fused Deposition Modeling) |
|---|---|
| Printing volume | 300 mm × 200 mm × 290 mm |
| Layer resolution | 0.05 mm |
| Positioning accuracy | 5 μm |
| Extruder quantity | 4 |
| Supported materials | Polymer filaments up to 300 °C |
| Ambient operating temperature | 5–40 °C |
| Operational extruder temperature | max 300 °C |
| Operational bed temperature | max 120 °C |
| Software input formats | G Code (.txt) |
| Connectivity | Wifi—Network Managed |
| Weight | 20 kg |
| Parameter/Material | Extrusion | Extrusion | Layer (mm) | Infill | T (°C) Nozzle | T (°C) Bed | Printing Speed (mm/s) |
|---|---|---|---|---|---|---|---|
| Multiplier | Width | ||||||
| (%) | (mm) | ||||||
| PLA | 1.03 | 0.42 | 0.15 | 100% | 200 | 65 | 50 |
| HTPLA | 0.97 | 0.42 | 0.15 | 100% | 220 | 70 | 50 |
| NYLON 6 | 0.93 | 0.42 | 0.15 | 100% | 250 | 40 | 50 |
| Temperature (C) | Pressure (Bar) | Time (Exposure) (min) |
|---|---|---|
| 112 | 0.5 | 30 |
| 121 | 1 | 15 |
| 134 | 2 | 10 |
| Step | Condition | Temperature (°C) | Pressure (kPa) | Time (min) |
|---|---|---|---|---|
| Conditioning | - | 79.6 | 93.4 | 0:00 |
| (5 Steps) | empty + vapor steps | 78.40→116.50 | varies on each cycle 91.2 (min), 182 (max) | 0–21.00 |
| Heating | - | 71.9 | 28.6 | 22 |
| 134.7 | 312 | 27 | ||
| Sterilization | - | 134.8 | 311.4 | 28 |
| 134.8 | 311.9 | 32 | ||
| Wash | - | 134.8 | 311.8 | 32 |
| 25 | ||||
| Addition | - | 20 | - | 34 |
| Drying | - | 89.8 | 86.7 | 55 |
| 25 | ||||
| Drying | - | 96.8 | 24.8 | 58 |
| Ventilation | - | 94 | 17.4 | 60 |
| End | - | 89.2 | 98.6 | 61 |
| D1 (mm) | CREO | Not Sterilized | PLA | HTPLA | HTPLA-Ann | Nylon 6 |
| Media | 20.00 | 19.90 | 19.46 | 19.44 | 19.03 | 20.4 |
| Absolute deviation (mm) | - | 0.105 | 0.54 | 0.56 | 0.97 | 0.4 |
| Deviation % | - | 0.53 | 2.7 | 2.8 | 4.85 | −2.02 |
| D2 (mm) | CREO | Not Sterilized | PLA | HTPLA | HTPLA-Ann | Nylon 6 |
| Media | 39.21 | 39.05 | 38.76 | 38.41 | 38.82 | 38.59 |
| Absolute deviation (mm) | - | 0.16 | 0.45 | 0.8 | 0.37 | 0.62 |
| Deviation % | - | 0.41 | 1.15 | 2.05 | 0.99 | 1.57 |
| D3 (mm) | CREO | Not Sterilized | PLA | HTPLA | HTPLA-Ann | Nylon 6 |
| Media | 40.35 | 40.28 | 40.56 | 40.03 | 39.95 | 38.99 |
| Absolute deviation (mm) | - | 0.07 | 0.21 | 0.32 | 0.4 | 1.36 |
| Deviation % | - | 0.17 | −0.52 | 0.79 | 0.98 | 3.37 |
| D4 (mm) | CREO | Not Sterilized | PLA | HTPLA | HTPLA-Ann | Nylon 6 |
| Media | 24.55 | 24.42 | 24.3 | 23.93 | 24.44 | 23.9 |
| Absolute deviation (mm) | - | 0.13 | 0.25 | 0.62 | 0.11 | 0.65 |
| Deviation % | - | 0.53 | 1.03 | 2.53 | 0.43 | 2.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frizziero, L.; Santi, G.M.; Leon-Cardenas, C.; Ferretti, P.; Sali, M.; Gianese, F.; Crescentini, N.; Donnici, G.; Liverani, A.; Trisolino, G.; et al. Heat Sterilization Effects on Polymeric, FDM-Optimized Orthopedic Cutting Guide for Surgical Procedures. J. Funct. Biomater. 2021, 12, 63. https://doi.org/10.3390/jfb12040063
Frizziero L, Santi GM, Leon-Cardenas C, Ferretti P, Sali M, Gianese F, Crescentini N, Donnici G, Liverani A, Trisolino G, et al. Heat Sterilization Effects on Polymeric, FDM-Optimized Orthopedic Cutting Guide for Surgical Procedures. Journal of Functional Biomaterials. 2021; 12(4):63. https://doi.org/10.3390/jfb12040063
Chicago/Turabian StyleFrizziero, Leonardo, Gian Maria Santi, Christian Leon-Cardenas, Patrich Ferretti, Merve Sali, Francesco Gianese, Nicola Crescentini, Giampiero Donnici, Alfredo Liverani, Giovanni Trisolino, and et al. 2021. "Heat Sterilization Effects on Polymeric, FDM-Optimized Orthopedic Cutting Guide for Surgical Procedures" Journal of Functional Biomaterials 12, no. 4: 63. https://doi.org/10.3390/jfb12040063
APA StyleFrizziero, L., Santi, G. M., Leon-Cardenas, C., Ferretti, P., Sali, M., Gianese, F., Crescentini, N., Donnici, G., Liverani, A., Trisolino, G., Zarantonello, P., Stallone, S., & Di Gennaro, G. L. (2021). Heat Sterilization Effects on Polymeric, FDM-Optimized Orthopedic Cutting Guide for Surgical Procedures. Journal of Functional Biomaterials, 12(4), 63. https://doi.org/10.3390/jfb12040063









