Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nano Hydroxyapatite
2.2. Preparation and Characterization of n-HAp, Sr-n-HAp, Zn-n-HAp and Sr-Zn-n-HAp
2.3. Fabrication of Sr/Zn-n-HAp Incorporated PLGA Composite Scaffolds
2.4. Characterization of Composite Scaffolds
2.5. Mechanical Testing of the Scaffolds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of nHAp, Sr-nHAp, Zn-nHAp, Sr/Zn-nHAp
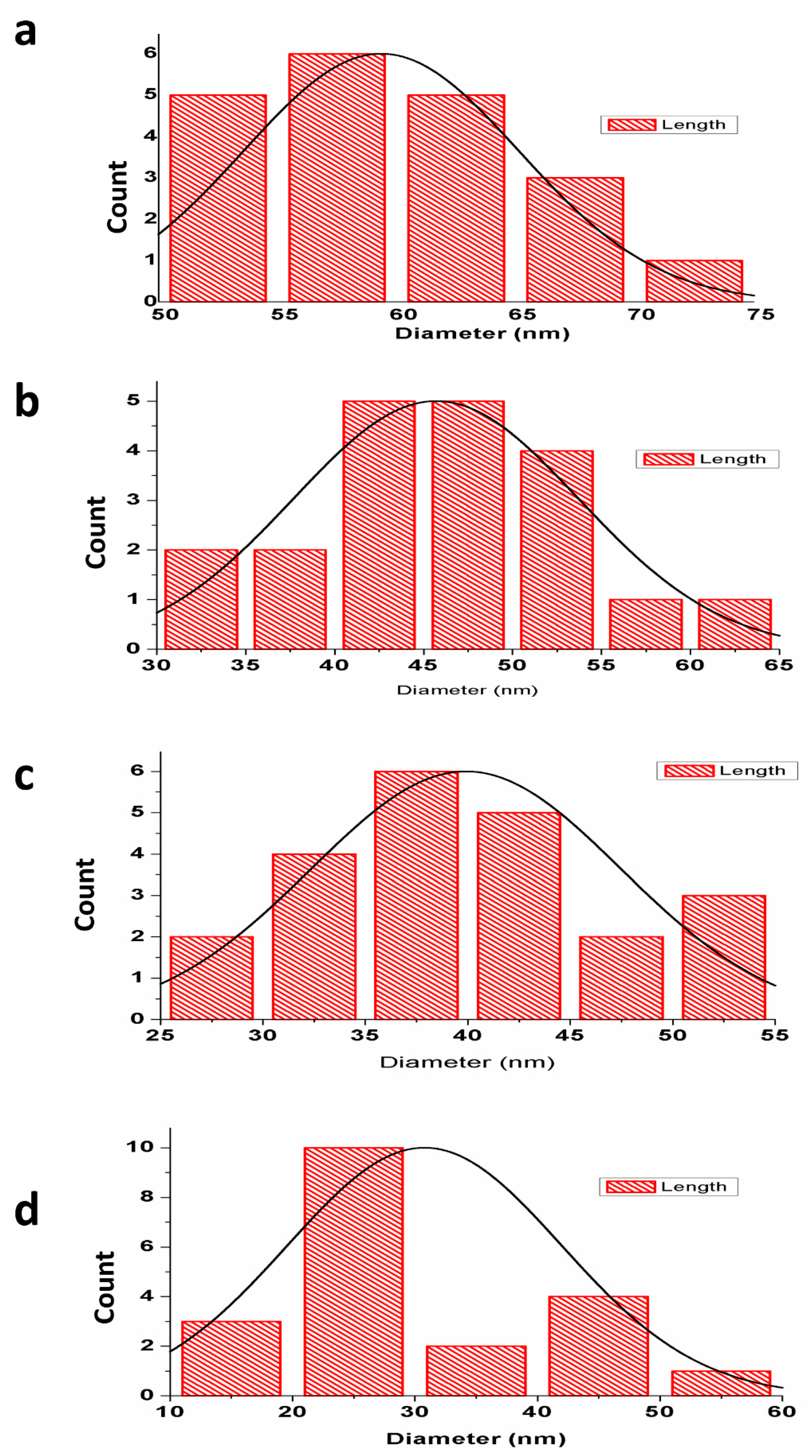
3.1.1. Particle Size Analysis
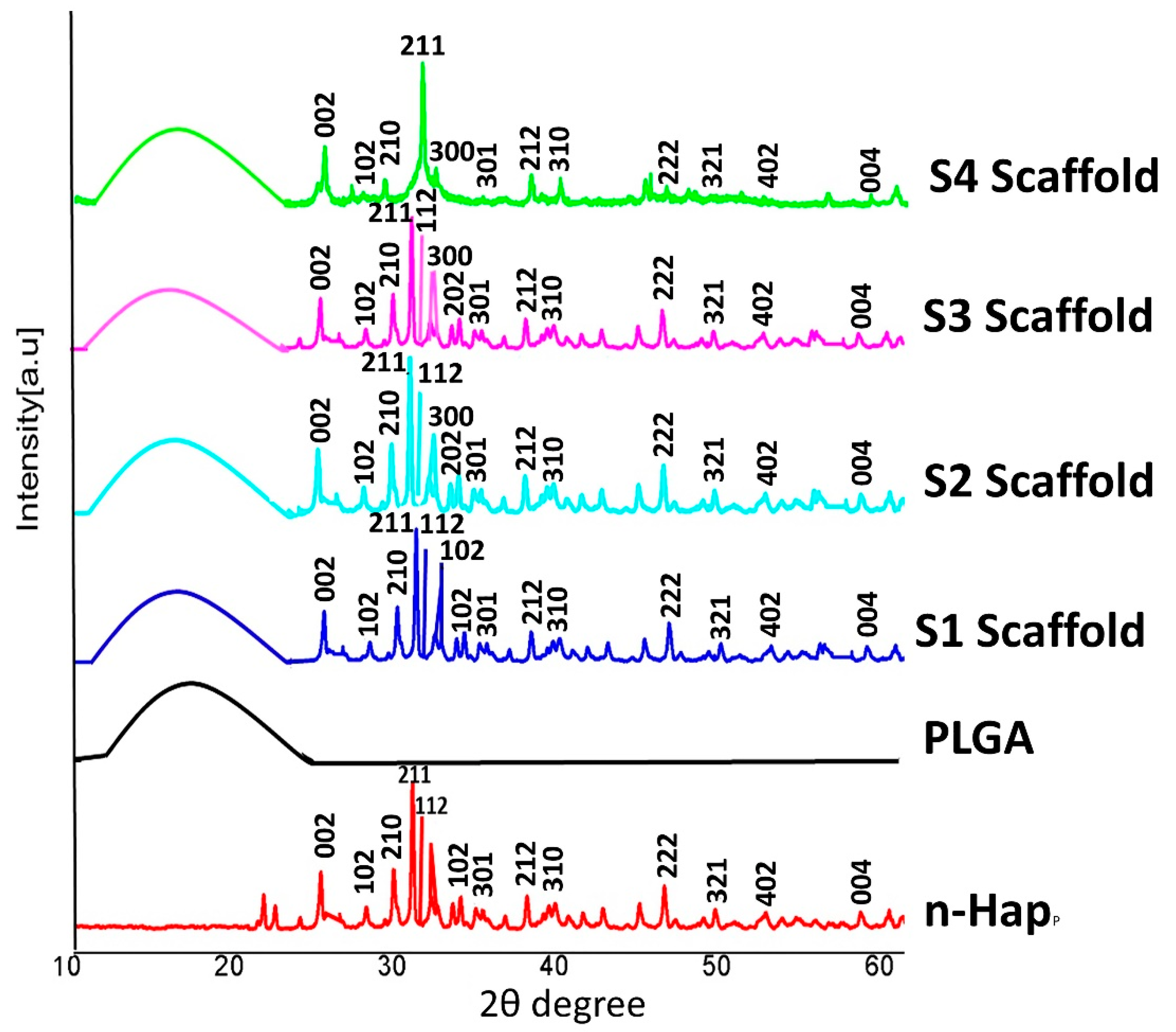
3.1.2. X-ray Diffraction Analysis
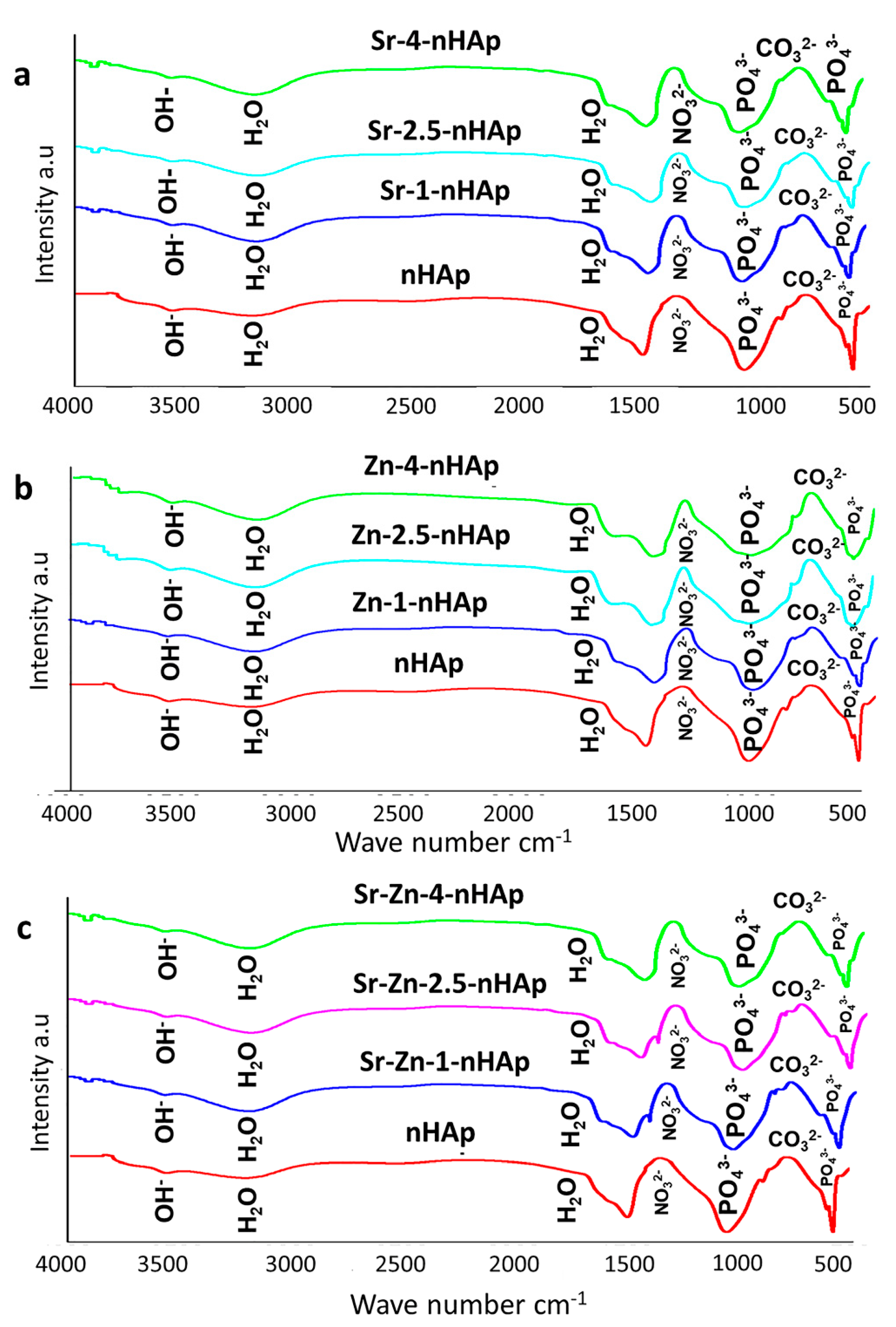
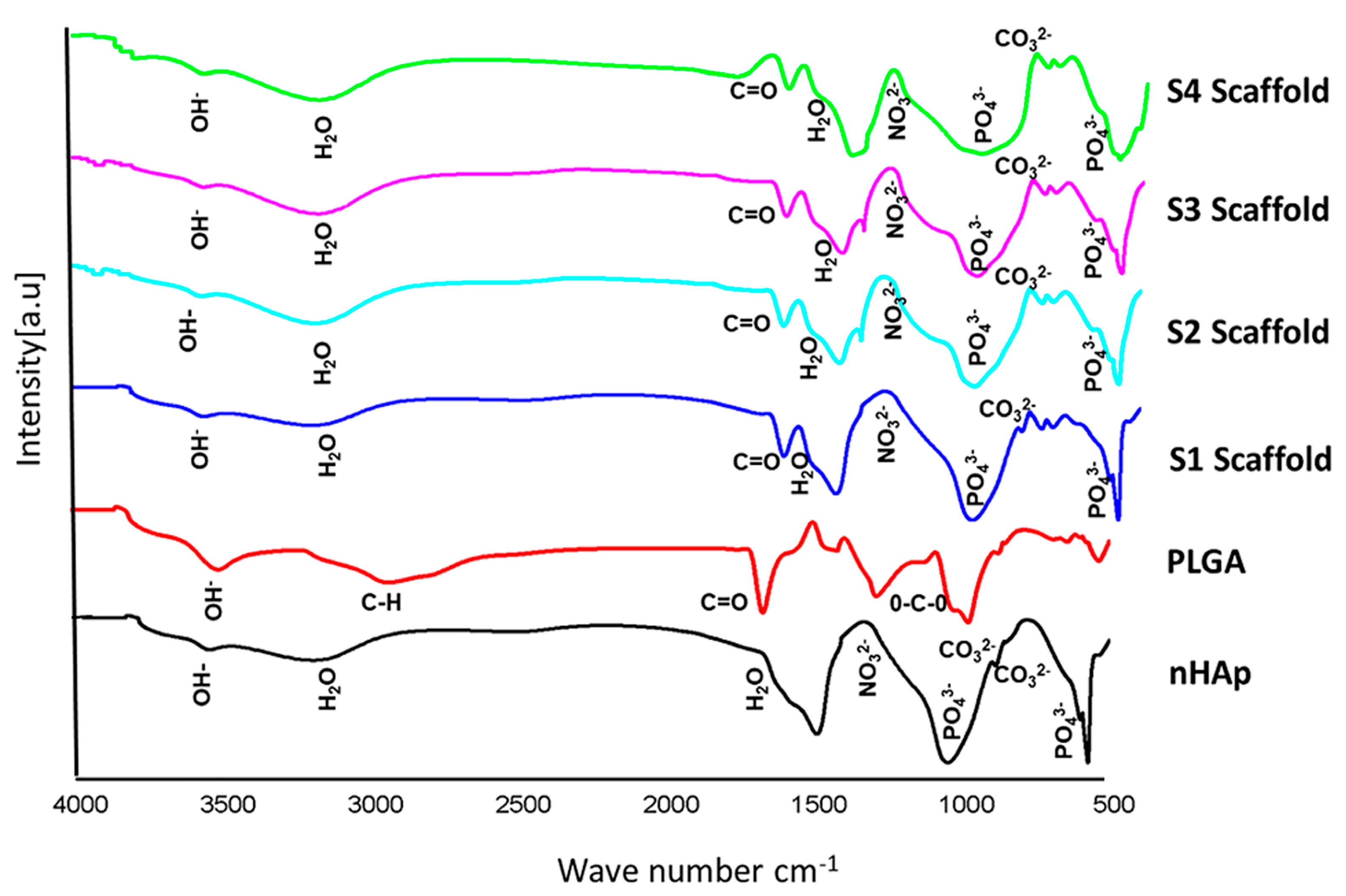
3.1.3. Fourier Transform Infrared Spectroscopy Analysis
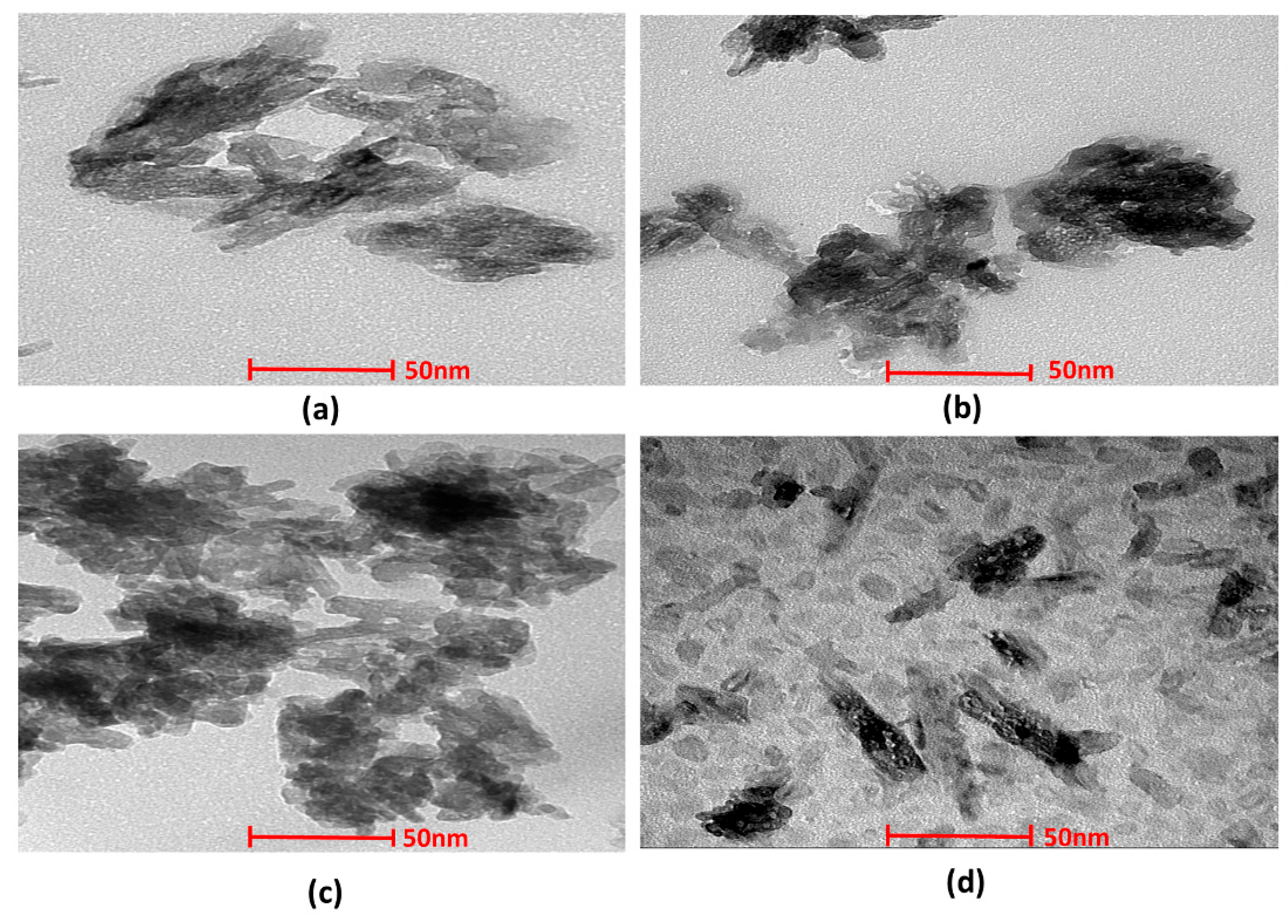
3.1.4. Transmission Electron Microscopy
3.2. Characterization of Scaffolds after PLGA Incorporation
3.2.1. X-ray Diffraction Analysis of Scaffolds
3.2.2. FTIR Spectra of Scaffolds
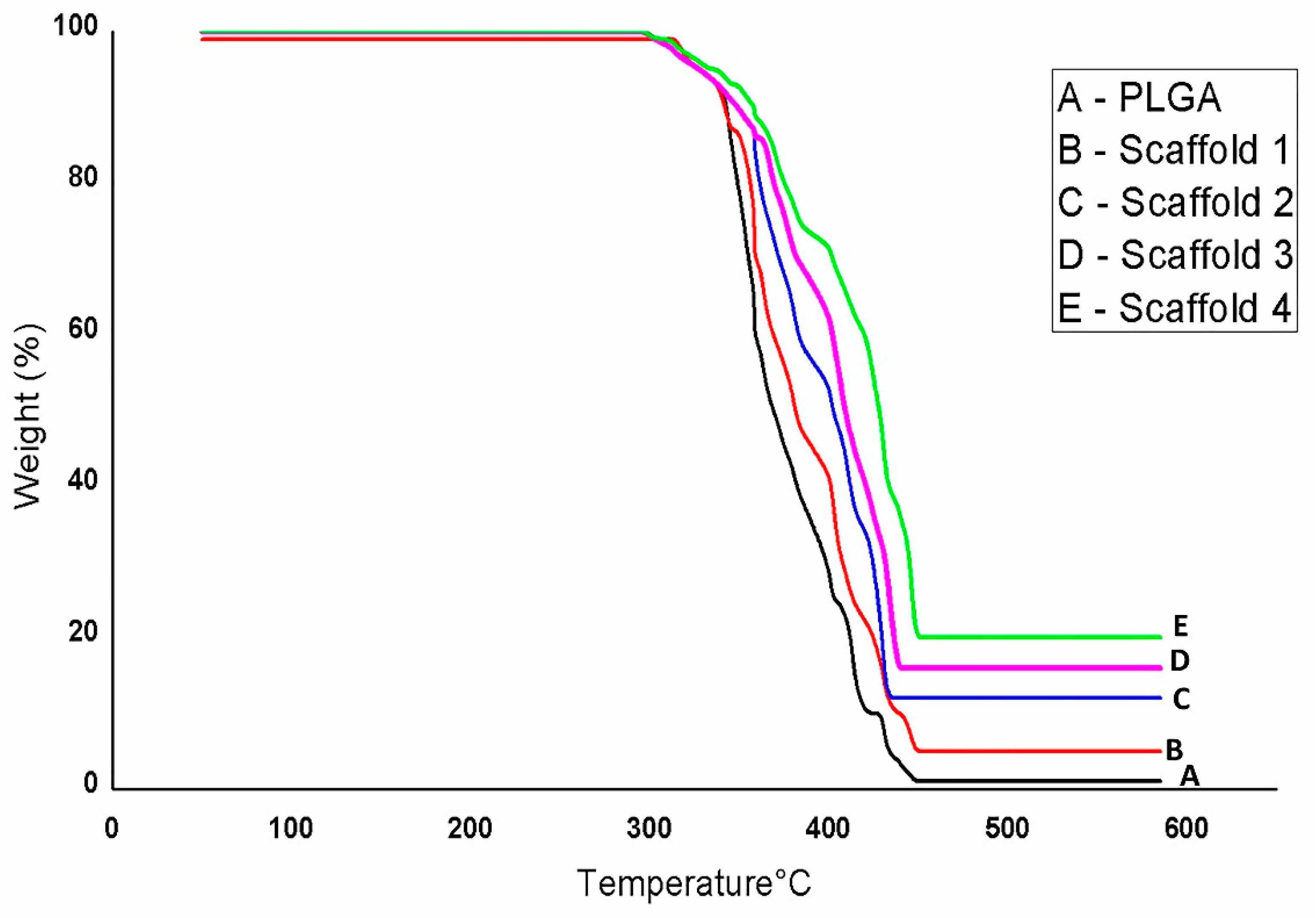
3.2.3. TGA Analysis of Scaffolds
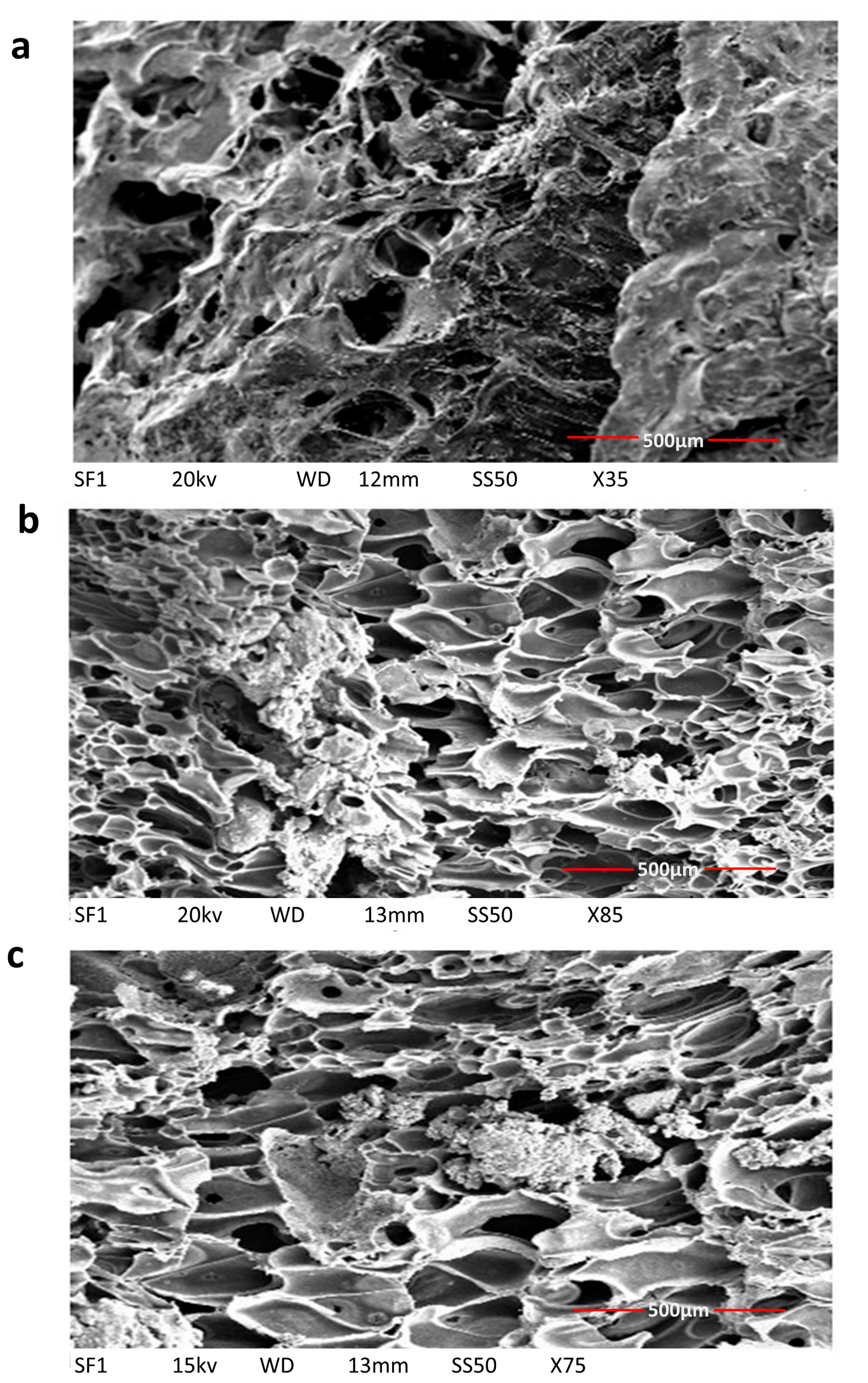
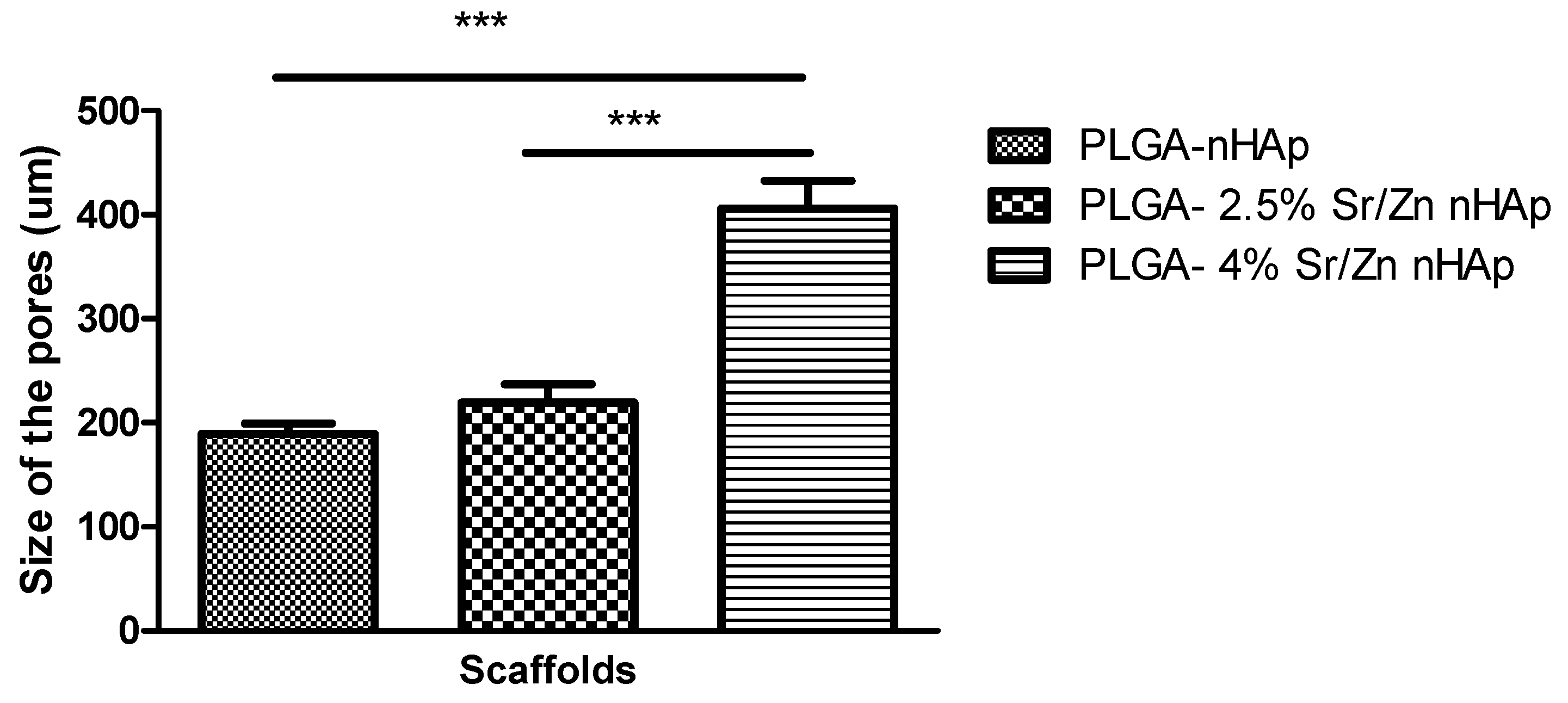
3.2.4. Scanning Electron Microscopy of Scaffolds
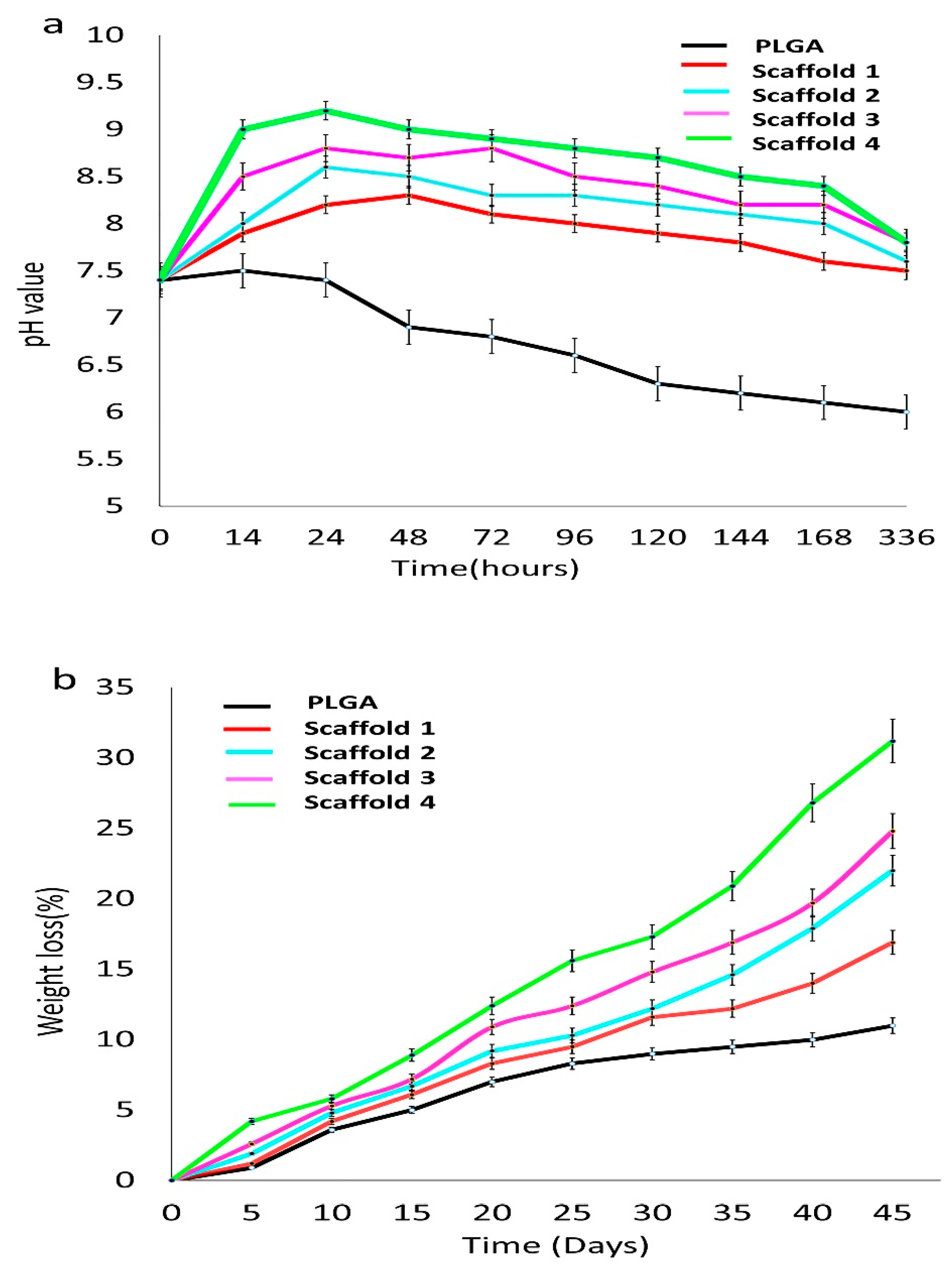
3.2.5. Bioactivity of Composite Scaffolds
PH Measurement
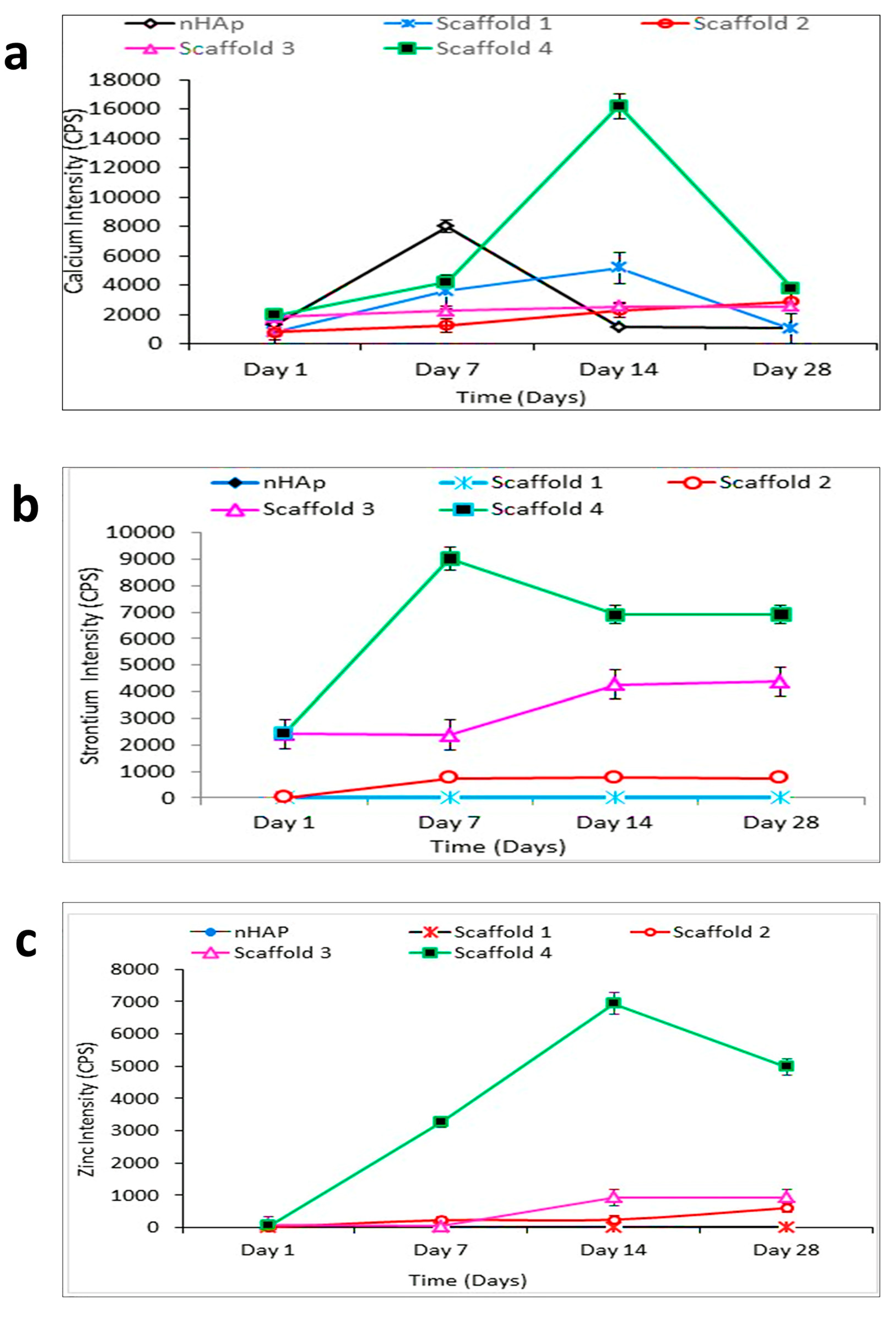
Inductive Coupled Plasma-Mass Spectrometry (ICP-MS)
3.2.6. Characterization of Scaffolds after Immersion in SBF
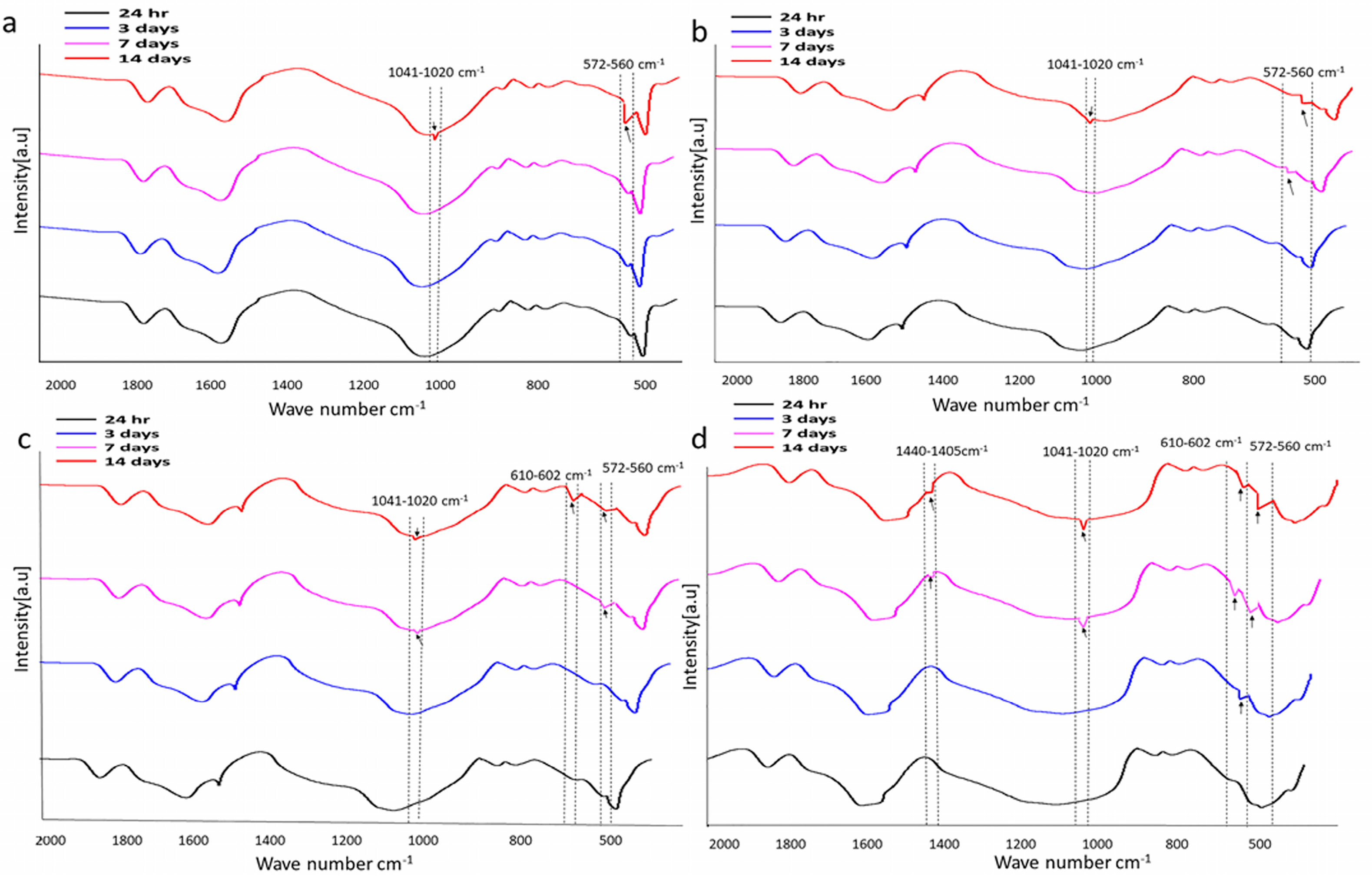
Fourier Transform Infrared Spectroscopy Analysis
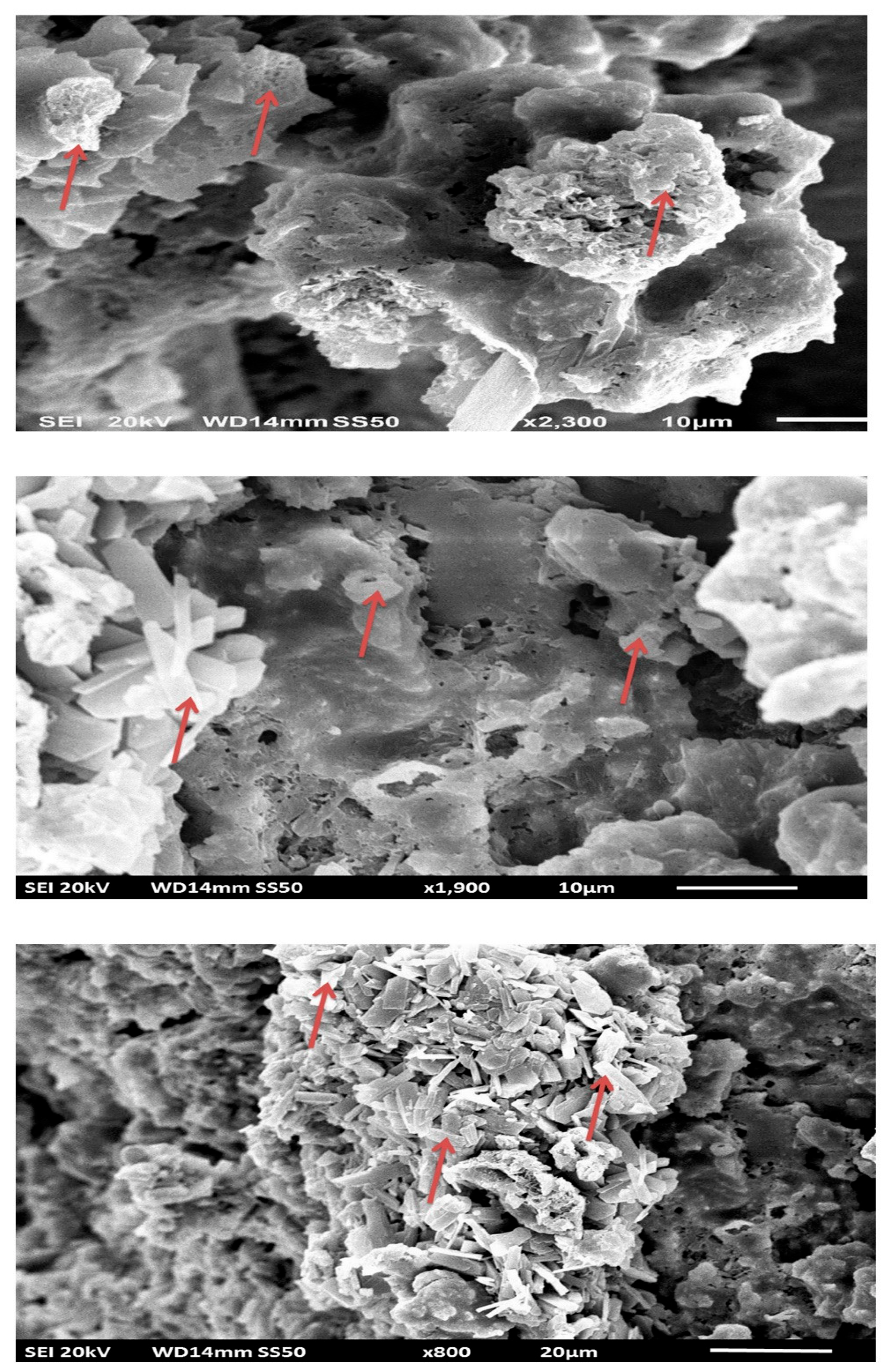
Scanning and Transmission Electron Microscopy
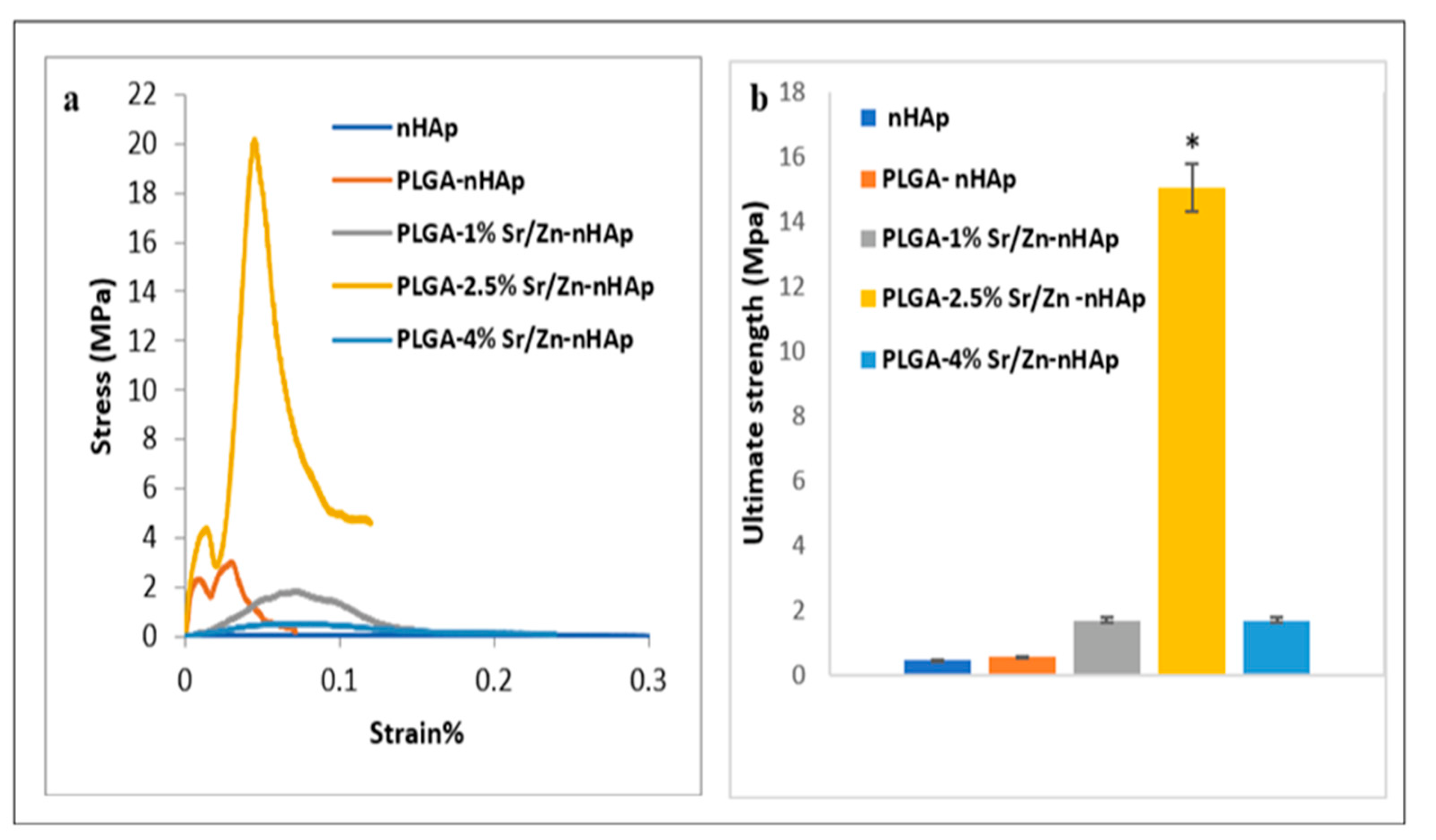
3.3. Mechanical Properties of the Scaffolds
4. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichte, P.; Pape, H.C.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for bone healing: Concepts, materials and evidence. Injury 2011, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Fang, W.; Chen, J.; Chen, Y. Poly(lactic-co-glycolic acid)-bioactive glass composites as nanoporous scaffolds for bone tissue engineering: In vitro and in vivo studies. Exp. Ther. Med. 2019, 18, 4874–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.G.; Hwang, J.H.; Cho, Y.S.; Lee, K.S.; Jeong, H.J.; Park, S.H.; Park, Y.; Cho, Y.S.; Lee, B.K. Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 2019, 98, 949–959. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Abinaya, B.; Prasith, T.P.; Ashwin, B.; Viji Chandran, S.; Selvamurugan, N. Chitosan in Surface Modification for Bone Tissue Engineering Applications. Biotechnol. J. 2019, 14, 1900171. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef]
- Young, M.F. Bone matrix proteins: Their function, regulation, and relationship to osteoporosis. Osteoporos. Int. 2003, 14, 35–42. [Google Scholar] [CrossRef]
- Family, R.; Solati-Hashjin, M.; Nik, S.N.; Nemati, A. Surface modification for titanium implants by hydroxyapatite nanocomposite. Casp. J. Intern. Med. 2012, 3, 460–465. [Google Scholar]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Korolkov, I.; Rusakov, V.; Nikolaevich, L.; Fesenko, O.; Budnyk, O.; Yakimchuk, D.; Shumskaya, A.; et al. FeCo nanotubes: Possible tool for targeted delivery of drugs and proteins. Appl. Nanosci. 2019, 9, 1091–1099. [Google Scholar] [CrossRef]
- Lodi, M.B.; Fanti, A.; Vargiu, A.; Bozzi, M.; Mazzarella, G. A Multiphysics Model for Bone Repair Using Magnetic Scaffolds for Targeted Drug Delivery. IEEE J. Multiscale Multiphysics Comput. Tech. 2021, 6, 201–213. [Google Scholar] [CrossRef]
- Fan, D.; Wang, Q.; Zhu, T.; Wang, H.; Liu, B.; Wang, Y.; Liu, Z.; Liu, X.; Fan, D.; Wang, X. Recent Advances of Magnetic Nanomaterials in Bone Tissue Repair. Front. Chem. 2020, 8, 745. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Best, S.M. Calcium phosphate scaffolds for bone repair. JOM 2011, 63, 83–92. [Google Scholar] [CrossRef]
- dos Santos, T.M.B.K.; Merlini, C.; Aragones, Á.; Fredel, M.C. Manufacturing and characterization of plates for fracture fixation of bone with biocomposites of poly (lactic acid-co-glycolic acid) (PLGA) with calcium phosphates bioceramics. Mater. Sci. Eng. C 2019, 103, 109728. [Google Scholar] [CrossRef]
- Safiaghdam, H.; Nokhbatolfoghahaei, H.; Khojasteh, A. Therapeutic metallic ions in bone tissue engineering: A systematic review of the literature. Iran. J. Pharm. Res. 2019, 18, 101–118. [Google Scholar] [CrossRef]
- Robinson, L.; Salma-Ancane, K.; Stipniece, L.; Meenan, B.J.; Boyd, A.R. The deposition of strontium and zinc Co-substituted hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2017, 28, 51. [Google Scholar] [CrossRef]
- Drouet, C.; Carayon, M.-T.; Combes, C.; Rey, C. Surface enrichment of biomimetic apatites with biologically-active ions Mg2+ and Sr2+: A preamble to the activation of bone repair materials. Mater. Sci. Eng. C 2008, 28, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Aoki, H.; Okayama, S.; Akao, M. Effect of Strontium Content on the Mechanical Properties of Bone and Sintered Hydroxyapatite; Bonfield, W., Hastings, G.W., Tanner, K.E.B.T.-B., Eds.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 87–90. ISBN 9780750602693. [Google Scholar]
- Qamar, A.; Zia, R.; Raiz, M. Tuning the Mechanical and Dielectric Properties of Zinc Incorporated Hydroxyapatite. Curr. Nanosci. 2020, 16, 982–993. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Yang, X.; Xi, Z.; Zhao, L.; Cen, L.; Lu, E.; Yang, Y. Novel Fabricating Process for Porous Polyglycolic Acid Scaffolds by Melt-Foaming Using Supercritical Carbon Dioxide. ACS Biomater. Sci. Eng. 2018, 4, 694–706. [Google Scholar] [CrossRef]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.N.; Bahir, M.M.; Mene, R.U.; Mahabole, M.P.; Khairnar, R.S. Study of nanobiomaterial hydroxyapatite in simulated body fluid: Formation and growth of apatite. Mater. Sci. Eng. B 2010, 168, 224–230. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-S.; Kumta, P.N. Sol–gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Gates-Rector, S.D.; Blanton, T.N. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Mou, Z.-L.; Zhao, L.-J.; Zhang, Q.-A.; Zhang, J.; Zhang, Z.-Q. Preparation of porous PLGA/HA/collagen scaffolds with supercritical CO2 and application in osteoblast cell culture. J. Supercrit. Fluids 2011, 58, 398–406. [Google Scholar] [CrossRef]
- Stanković, A.; Sezen, M.; Milenković, M.; Kaišarević, S.; Andrić, N.; Stevanović, M. PLGA/Nano-ZnO Composite Particles for Use in Biomedical Aplications: Preparation, Characterization, and Antimicrobial Activity. J. Nanomater. 2016, 2016, 9425289. [Google Scholar] [CrossRef]
- Li, S.; Song, C.; Yang, S.; Yu, W.; Zhang, W.; Zhang, G.; Xi, Z.; Lu, E. Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019, 94, 253–267. [Google Scholar] [CrossRef]
- Hatton, J.; Davis, G.R.; Mourad, A.-H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. J. Funct. Biomater. 2019, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, C.S.; Iconaru, S.L.; Chifiriuc, M.C.; Costescu, A.; Le Coustumer, P.; Predoi, D. Synthesis and Antimicrobial Activity of Silver-Doped Hydroxyapatite Nanoparticles. BioMed Res. Int. 2013, 2013, 916218. [Google Scholar] [CrossRef] [Green Version]
- Garbo, C.; Locs, J.; D’Este, M.; Demazeau, G.; Mocanu, A.; Roman, C.; Horovitz, O.; Tomoaia-Cotisel, M. Advanced Mg, Zn, Sr, Si Multi-Substituted Hydroxyapatites for Bone Regeneration. Int. J. Nanomed. 2020, 15, 1037–1058. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Xia, L.; Gan, J.; Zhang, Z.; Chen, H.; Jiang, X.; Chang, J. Tailoring the Nanostructured Surfaces of Hydroxyapatite Bioceramics to Promote Protein Adsorption, Osteoblast Growth, and Osteogenic Differentiation. ACS Appl. Mater. Interfaces 2013, 5, 8008–8017. [Google Scholar] [CrossRef]
- Luo, Y.; Lode, A.; Wu, C.; Chang, J.; Gelinsky, M. Alginate/Nanohydroxyapatite Scaffolds with Designed Core/Shell Structures Fabricated by 3D Plotting and in Situ Mineralization for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6541–6549. [Google Scholar] [CrossRef]
- Boyd, A.R.; Rutledge, L.; Randolph, L.D.; Meenan, B.J. Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater. Sci. Eng. C 2015, 46, 290–300. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and structural analysis of zinc-substituted hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Miyaji, F.; Kono, Y.; Suyama, Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater. Res. Bull. 2005, 40, 209–220. [Google Scholar] [CrossRef]
- Lowry, N.; Han, Y.; Meenan, B.J.; Boyd, A.R. Strontium and zinc co-substituted nanophase hydroxyapatite. Ceram. Int. 2017, 43, 12070–12078. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Stoch, A.; Jastrzębski, W.; Brożek, A.; Stoch, J.; Szaraniec, J.; Trybalska, B.; Kmita, G. FTIR absorption–reflection study of biomimetic growth of phosphates on titanium implants. J. Mol. Struct. 2000, 555, 375–382. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Deniaud, A.; Chevallet, M.; Michaud-Soret, I.; Buton, N.; Prodan, A.M. Textural, Structural and Biological Evaluation of Hydroxyapatite Doped with Zinc at Low Concentrations. Materials 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Sun, T.-W.; Qi, C.; Ding, Z.; Zhao, H.; Zhao, S.; Shi, Z.; Zhu, Y.-J.; Chen, D.; He, Y. Evaluation of zinc-doped mesoporous hydroxyapatite microspheres for the construction of a novel biomimetic scaffold optimized for bone augmentation. Int. J. Nanomed. 2017, 12, 2293–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Nedelec, J.-M.; Renaudin, G. On the effect of temperature on the insertion of zinc into hydroxyapatite. Acta Biomater. 2012, 8, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.L.; Deniaud, A.; Michaud-Soret, I.; Guégan, R.; Motelica-Heino, M.; Predoi, D. Structural and Biological Assessment of Zinc Doped Hydroxyapatite Nanoparticles. J. Nanomater. 2016, 2016, 1062878. [Google Scholar] [CrossRef] [Green Version]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Surendiran, M.; Kavitha, L. Development of strontium and magnesium substituted porous hydroxyapatite/poly(3,4-ethylenedioxythiophene) coating on surgical grade stainless steel and its bioactivity on osteoblast cells. Colloids Surf. B Biointerfaces 2014, 114, 234–240. [Google Scholar] [CrossRef]
- Ehret, C.; Aid-Launais, R.; Sagardoy, T.; Siadous, R.; Bareille, R.; Rey, S.; Pechev, S.; Etienne, L.; Kalisky, J.; de Mones, E.; et al. Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLoS ONE 2017, 12, e0184663. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite Nanoparticles: A Review of Preparation Methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74–80. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C 2016, 60, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Paragkumar, N.T.; Edith, D.; Six, J.-L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Ignjatović, N.; Uskoković, V.; Ajduković, Z.; Uskoković, D. Multifunctional hydroxyapatite and poly(d,l-lactide-co-glycolide) nanoparticles for the local delivery of cholecalciferol. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene Oxide Hybridized nHAC/PLGA Scaffolds Facilitate the Proliferation of MC3T3-E1 Cells. Nanoscale Res. Lett. 2018, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101A, 1080–1094. [Google Scholar] [CrossRef]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The effect of temperature on the properties of hydroxyapatite precipitated from calcium hydroxide and phosphoric acid. Thermochim. Acta 2001, 374, 13–22. [Google Scholar] [CrossRef]
- Skwarek, E. Thermal analysis of hydroxyapatite with adsorbed oxalic acid. J. Therm. Anal. Calorim. 2015, 122, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Jose, G.; Shalumon, K.T.; Liao, H.-T.; Kuo, C.-Y.; Chen, J.-P. Preparation and Characterization of Surface Heat Sintered Nanohydroxyapatite and Nanowhitlockite Embedded Poly (Lactic-co-glycolic Acid) Microsphere Bone Graft Scaffolds: In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2020, 21, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B. Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Widiyanti, P. Composition Variation on Bone Graft Synthesis Based on Hydroxyapatite and Alginate. J. Biomim. Biomater. Biomed. Eng. 2016, 29, 14–21. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Labbaf, S. Hardystonite-diopside nanocomposite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2017, 202, 95–103. [Google Scholar] [CrossRef]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef] [Green Version]
- Chakkalakal, D.A.; Mashoof, A.A.; Novak, J.; Strates, B.S.; McGuire, M.H. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J. Biomed. Mater. Res. 1994, 28, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, W.; Wen, C.; Pan, H.; Wang, T.; Darvell, B.W.; Lu, W.W.; Huang, W. Bone regeneration: Importance of local pH—strontium-doped borosilicate scaffold. J. Mater. Chem. 2012, 22, 8662–8670. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Zhou, X.; Yang, D.; Shi, Z.; Hao, Y. Effect of strontium-containing on the properties of Mg-doped wollastonite bioceramic scaffolds. Biomed. Eng. Online 2019, 18, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, T.R. Extracellular pH Regulates Bone Cell Function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Zhou, Q.; Gao, C.; Shen, J. In vitro and in vivo degradability and cytocompatibility of poly(l-lactic acid) scaffold fabricated by a gelatin particle leaching method. Acta Biomater. 2007, 3, 531–540. [Google Scholar] [CrossRef]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, P.; Ge, X.; Li, P.; Lv, C.; Wang, M.; Wang, K.; Fang, L.; Lu, X. Experimental and simulation studies of strontium/zinc-codoped hydroxyapatite porous scaffolds with excellent osteoinductivity and antibacterial activity. Appl. Surf. Sci. 2018, 462, 118–126. [Google Scholar] [CrossRef]
- Hadagalli, K.; Panda, A.K.; Mandal, S.; Basu, B. Faster Biomineralization and Tailored Mechanical Properties of Marine-Resource-Derived Hydroxyapatite Scaffolds with Tunable Interconnected Porous Architecture. ACS Appl. Bio Mater. 2019, 2, 2171–2184. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O. Synthesis and characterization of Zn-Doped hydroxyapatite: Scaffold application, antibacterial and bioactivity studies. Heliyon 2019, 5, e01716. [Google Scholar] [CrossRef] [Green Version]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Chrzanowski, W. Bioactive nanocomposite PLDL/nano-hydroxyapatite electrospun membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.O.; Kim, H.S.; Stahl, T.; Nukavarapu, S.P. Self-neutralizing PLGA/magnesium composites as novel biomaterials for tissue engineering. Biomed. Mater. 2018, 13, 35013. [Google Scholar] [CrossRef]
- Lindahl, C.; Engqvist, H.; Xia, W. Effect of strontium ions on the early formation of biomimetic apatite on single crystalline rutile. Appl. Surf. Sci. 2013, 266, 199–204. [Google Scholar] [CrossRef]
- Christoffersen, J.; Christoffersen, M.R.; Kolthoff, N.; Bärenholdt, O. Effects of strontium ions on growth and dissolution of hydroxyapatite and on bone mineral detection. Bone 1997, 20, 47–54. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Persson, C.; Thomsen, P.; Lausmaa, J.; Engqvist, H. Changes of Surface Composition and Morphology after Incorporation of Ions into Biomimetic Apatite Coating. J. Biomater. Nanobiotechnol. 2010, 1, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.J.; Sun, X.D.; Cui, F.Z.; Wei, Y.T.; Cheng, Z.J.; Kong, X.D. Preparation and Characterization of Antheraea pernyi Silk Fibroin Based Nanohydroxyapatite Composites. J. Bioact. Compat. Polym. 2007, 22, 465–474. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhu, S.; Luo, E.; Feng, G.; Chen, Q.; Hu, J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 418, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Jayasuriya, A.C.; Kohn, D.H. Effect of ionic activity products on the structure and composition of mineral self assembled on three-dimensional poly(lactide-co-glycolide) scaffolds. J. Biomed. Mater. Res. A 2007, 83, 1076–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, C.; Yang, B.; Peng, S.; Li, Z. Development of composite porous scaffolds based on poly(lactide-co- glycolide)/nano-hydroxyapatite via selective laser sintering. Int. J. Adv. Manuf. Technol. 2013, 69, 51–57. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Mechanical properties of dispersed ceramic nanoparticles in polymer composites for orthopedic applications. Int. J. Nanomed. 2010, 5, 299–313. [Google Scholar] [CrossRef] [Green Version]


| Sample | Sr (mol %) | Zn (mol %) | nHAp (mol %) |
|---|---|---|---|
| Sr-1-nHAp | 1.0 | 0.0 | 1.0 |
| Sr-2.5-nHAp | 2.5 | 0.0 | 1.0 |
| Sr-4-nHAp | 4.0 | 0.0 | 1.0 |
| Zn-1-nHAp | 0.0 | 1.0 | 1.0 |
| Zn-2.5-nHAp | 0.0 | 2.5 | 1.0 |
| Zn-4-nHAp | 0.0 | 4.0 | 1.0 |
| Sr/Zn-1-nHAp | 1.0 | 1.0 | 1.0 |
| Sr/Zn-2.5-nHAp | 2.5 | 2.5 | 1.0 |
| Sr/Zn-4-nHAp | 4.0 | 4.0 | 1.0 |
| Bone Scaffolds | PLGA (mol %) | Sr (mol %) | Zn (mol %) | nHAp (mol %) |
|---|---|---|---|---|
| Scaffold 1 (S1) | 3.0 | 0.0 | 0.0 | 1.0 |
| Scaffold 2 (S2) | 3.0 | 1.0 | 1.0 | 1.0 |
| Scaffold 3 (S3) | 3.0 | 2.5 | 2.5 | 1.0 |
| Scaffold 4 (S4) | 3.0 | 4.0 | 4.0 | 1.0 |
| Samples | Particle Size (nm) | ||
|---|---|---|---|
| d10 | d50 | d90 | |
| nHAp | 58.6 | 92.3 | 196.4 |
| Sr-1-nHAp | 52.2 | 86.0 | 190.3 |
| Zn-1-nHAp | 48.4 | 89.0 | 185.4 |
| Sr-2.5-nHAp | 43.7 | 86.0 | 167.6 |
| Zn-2.5-nHAp | 44.5 | 86.0 | 163.0 |
| Sr-4-nHAp | 40.3 | 84.4 | 152.6 |
| Zn-4-nHAp | 42.3 | 85.0 | 150.8 |
| Sr/Zn-1-nHAp | 45.2 | 79.4 | 146.8 |
| Sr/Zn-2.5-nHAp | 47.5 | 80.2 | 148.2 |
| Sr/Zn-4-nHAp | 46.4 | 76.6 | 149.0 |
| Sample | T2% (°C) | T 10% (°C) | T 50% (°C) | T 90% (°C) | Residue (wt%) |
|---|---|---|---|---|---|
| nHAp | 532 | - | - | 95 | |
| PLGA | 333 | 338 | 369 | 425 | 0 |
| PLGA nHAp | 345 | 344 | 380 | 432 | 5 |
| PLGA nHAp 1% Zn Sr | 350 | 360 | 402 | 522 | 15 |
| PLGA nHAp 2.5% Zn Sr | 358 | 365 | 410 | 555 | 17 |
| PLGA nHAp 4% Zn Sr | 369 | 372 | 421 | 572 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.u.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. https://doi.org/10.3390/jfb13010013
Hassan M, Sulaiman M, Yuvaraju PD, Galiwango E, Rehman Iu, Al-Marzouqi AH, Khaleel A, Mohsin S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. Journal of Functional Biomaterials. 2022; 13(1):13. https://doi.org/10.3390/jfb13010013
Chicago/Turabian StyleHassan, Mozan, Mohsin Sulaiman, Priya Dharshini Yuvaraju, Emmanuel Galiwango, Ihtesham ur Rehman, Ali H. Al-Marzouqi, Abbas Khaleel, and Sahar Mohsin. 2022. "Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration" Journal of Functional Biomaterials 13, no. 1: 13. https://doi.org/10.3390/jfb13010013







