TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. TiO2 Nanotube Fabrication
2.1.2. Functionalization of Ti/TiO2 Nanotubes with Icariin
2.1.3. TiO2 Nanotube Characterization
2.1.4. In Vitro Release of Icariin
2.2. Cell Culture
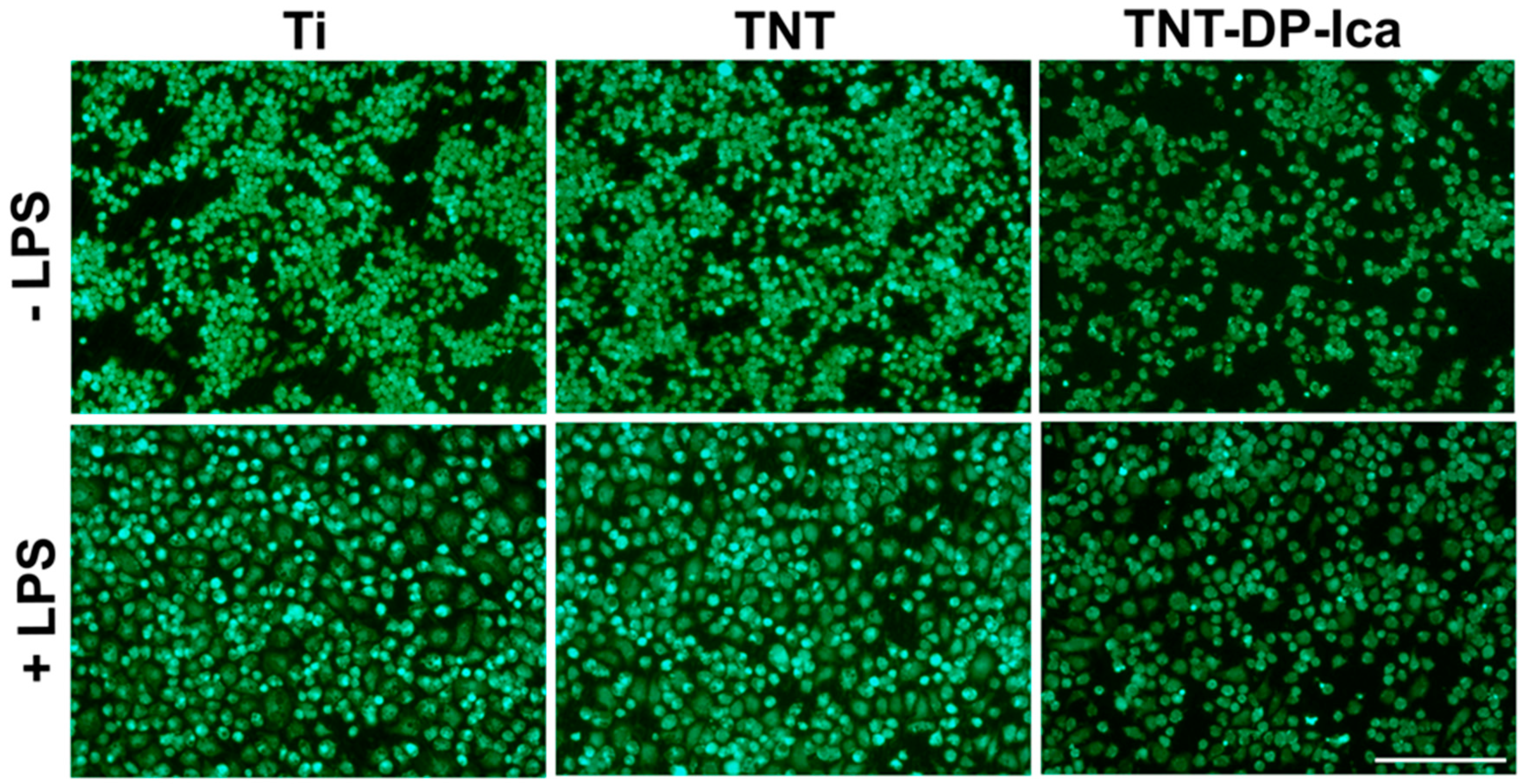
2.3. Live/Dead Assay
2.4. CCK-8 Assay
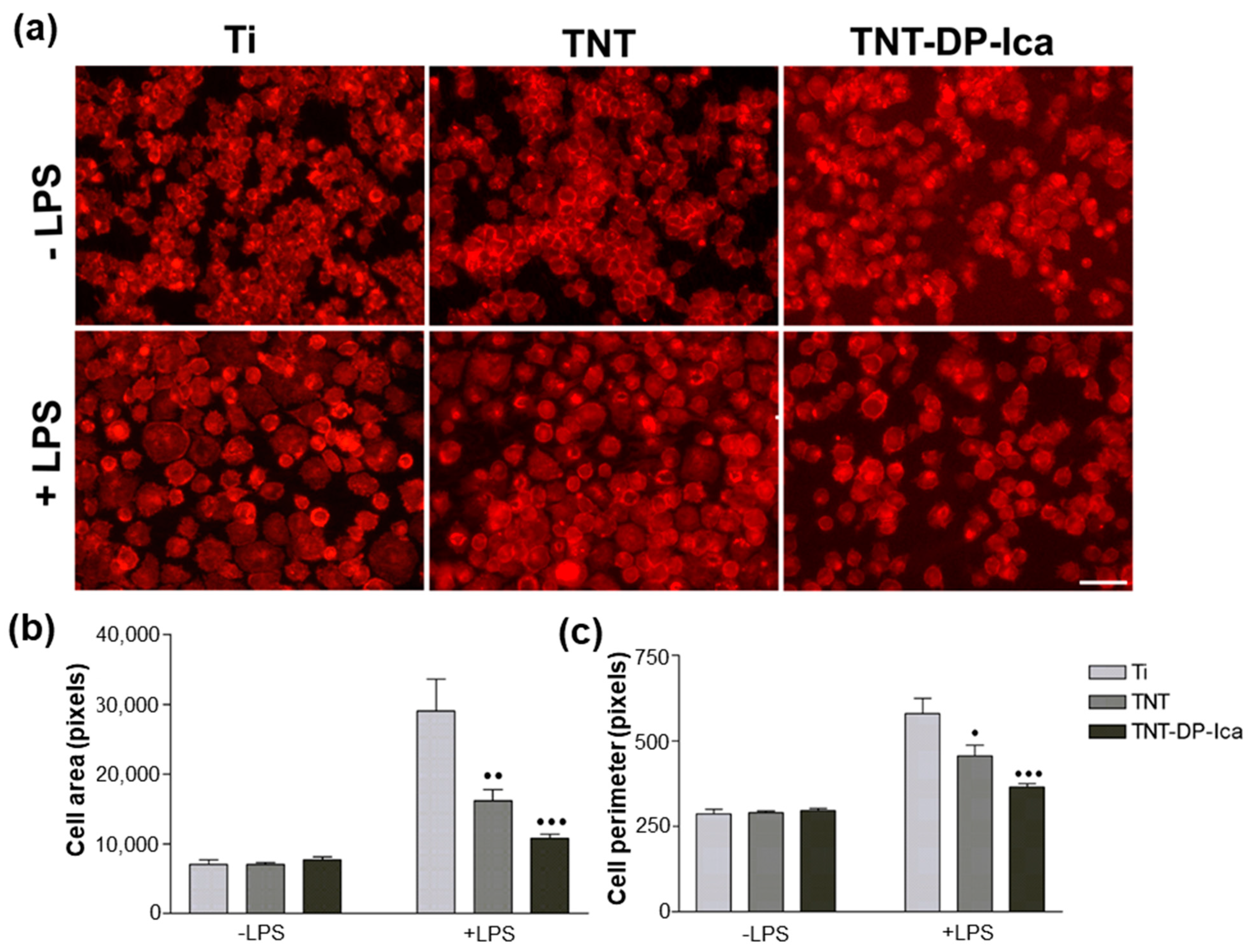
2.5. Cell Morphology Analysis
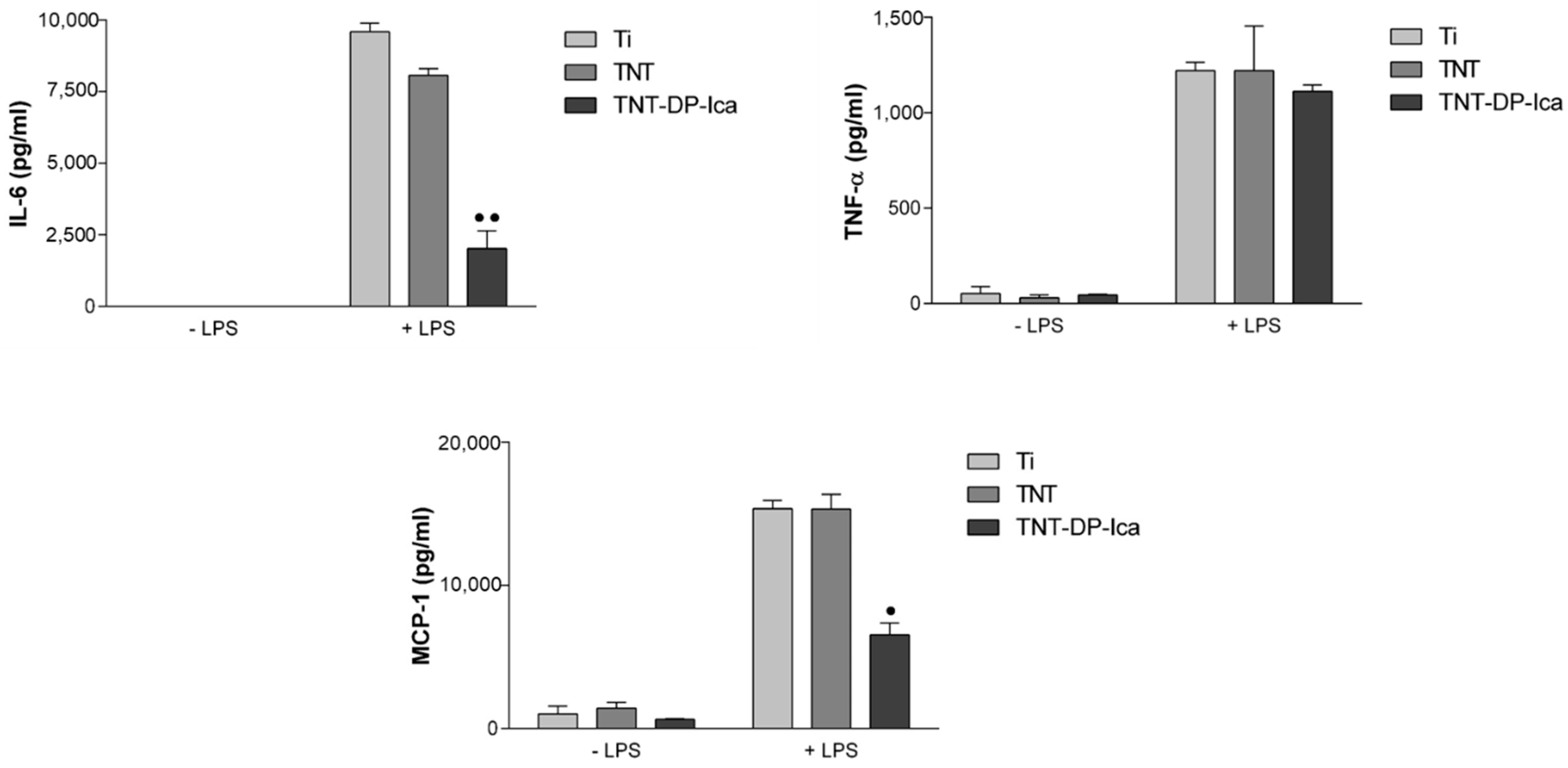
2.6. Quantification of Secreted Cytokines and Chemokines
2.7. Nitric Oxide Assay
2.8. In Vivo Implantation and Histological Analysis
2.8.1. Implant Preparation and Surgery
2.8.2. Histological Preparation and Procedures
2.9. Statistical Analysis
3. Results
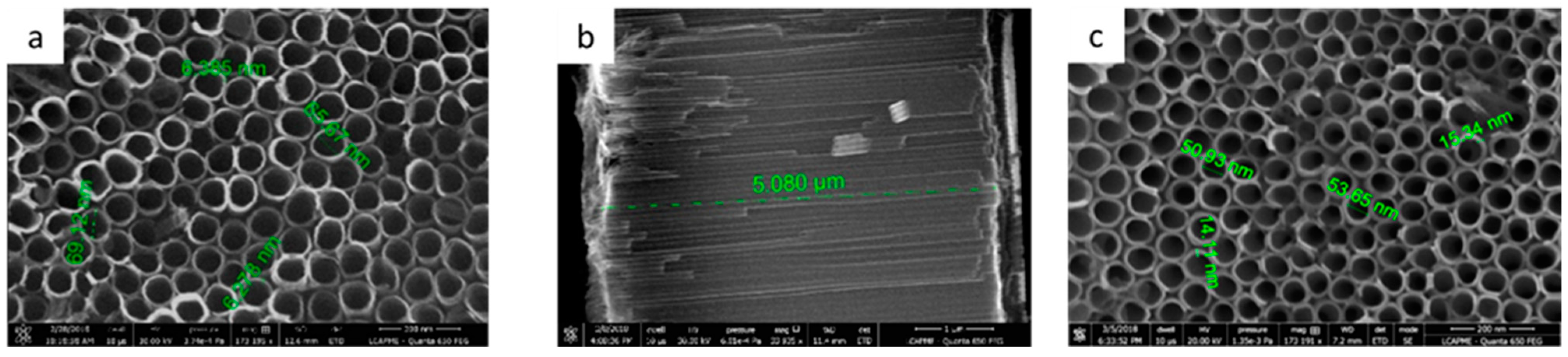
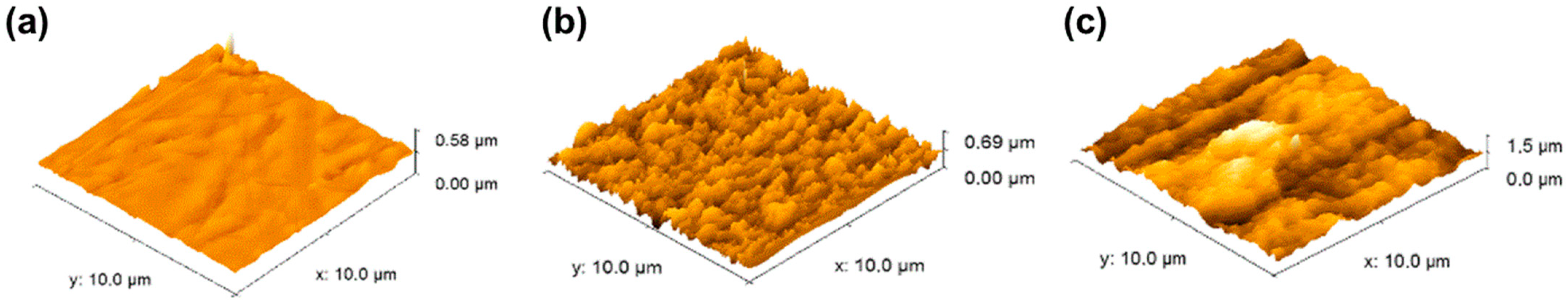
3.1. Surface Characterization
3.1.1. Ti/TiO2 Characterization
3.1.2. Surface Roughness
3.1.3. Surface Energy Determination
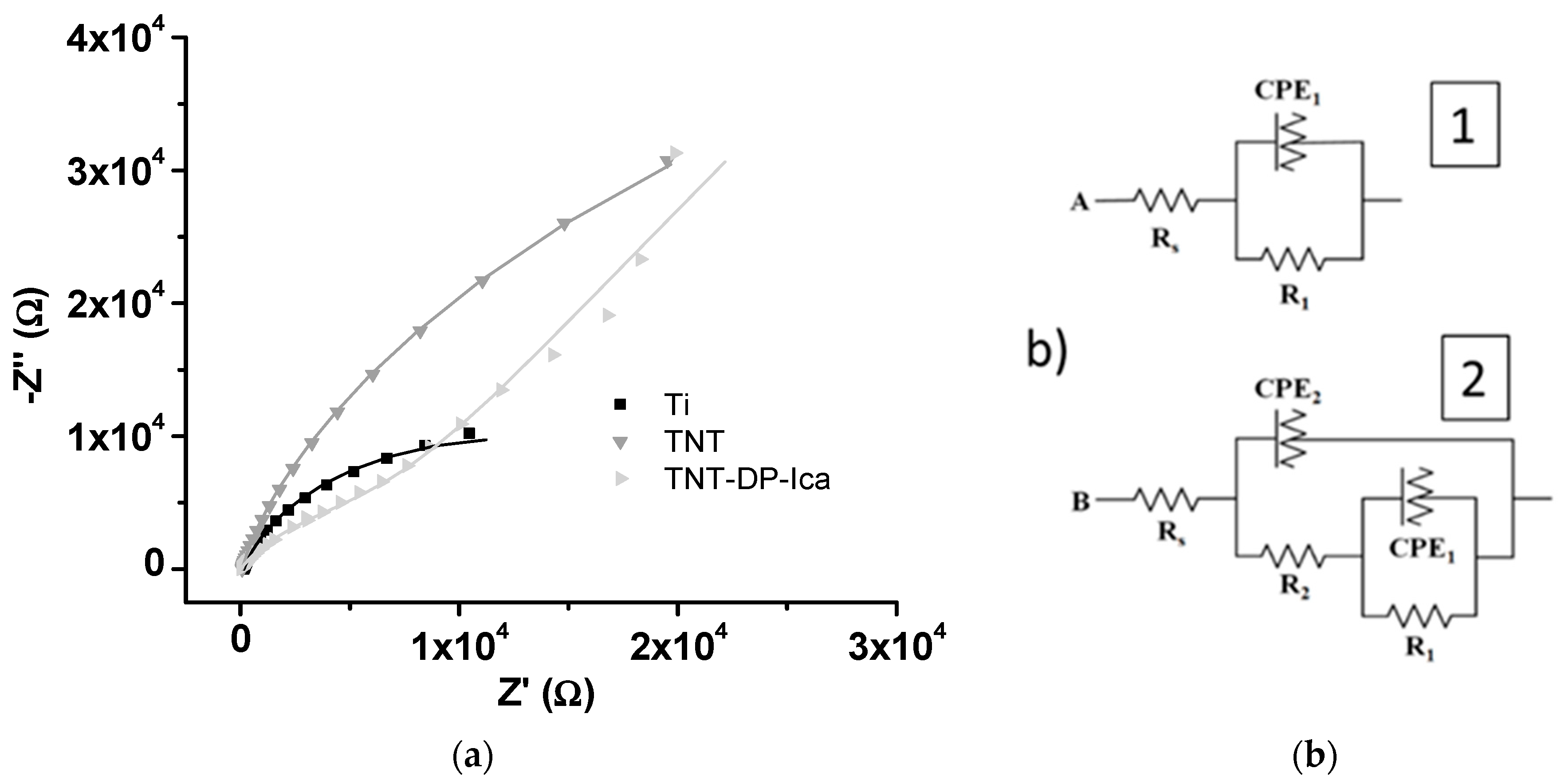
3.1.4. Electrochemical Impedance Spectroscopy
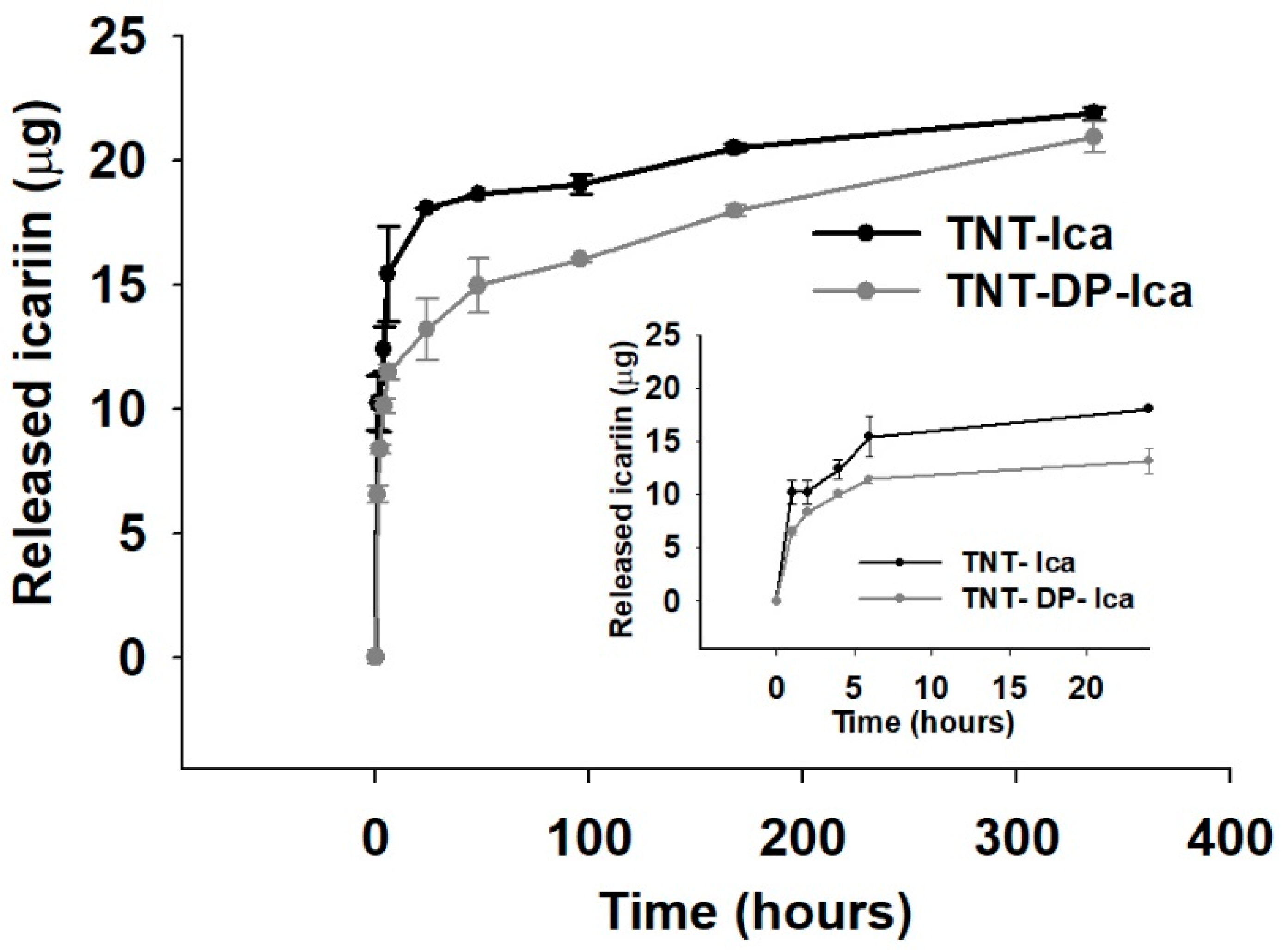
3.1.5. In Vitro Release of Icariin
3.2. In Vitro Responses of Macrophages
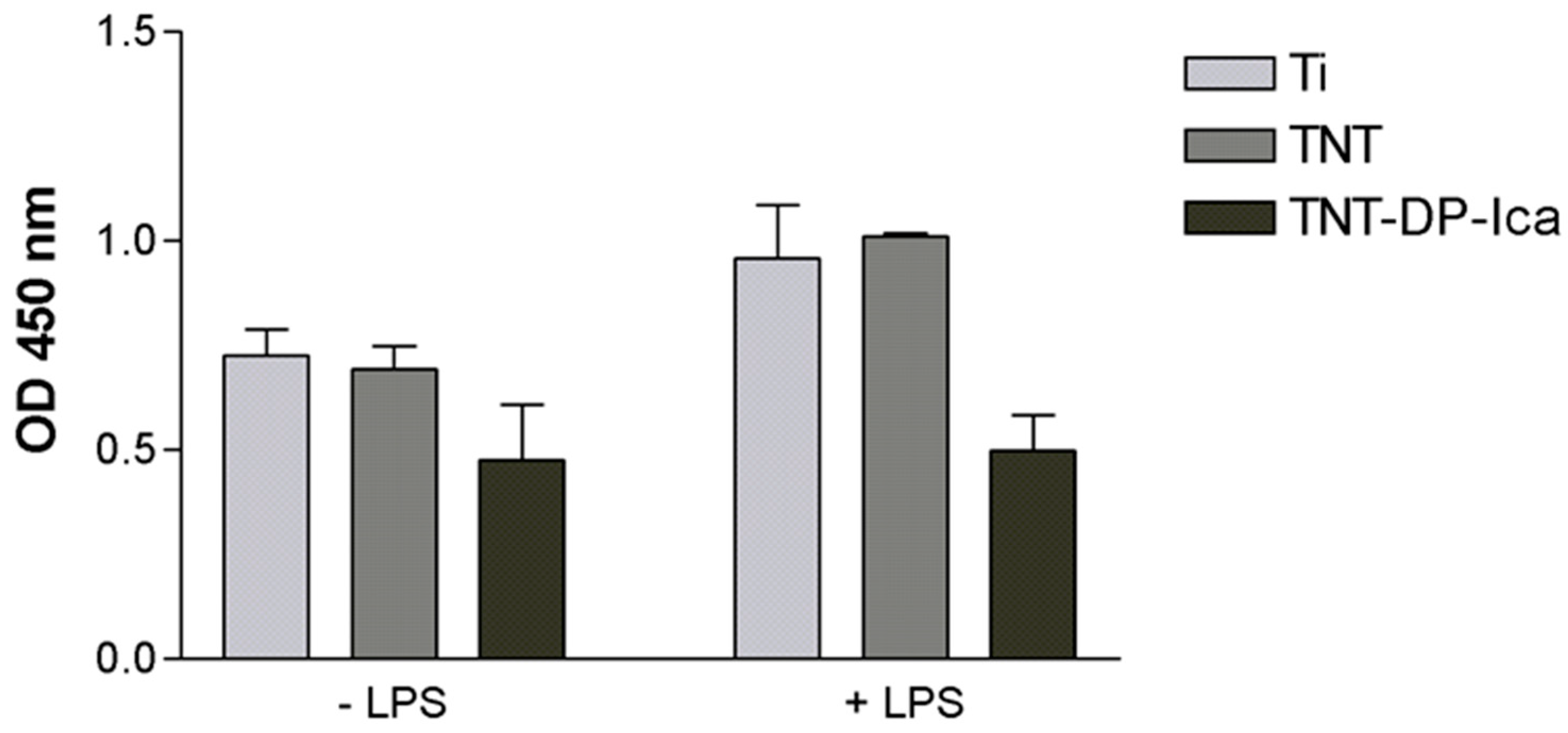
3.2.1. Cell Viability and Proliferation
3.2.2. Cell Morphological Features
3.2.3. Extracellular Release of Inflammatory Mediators
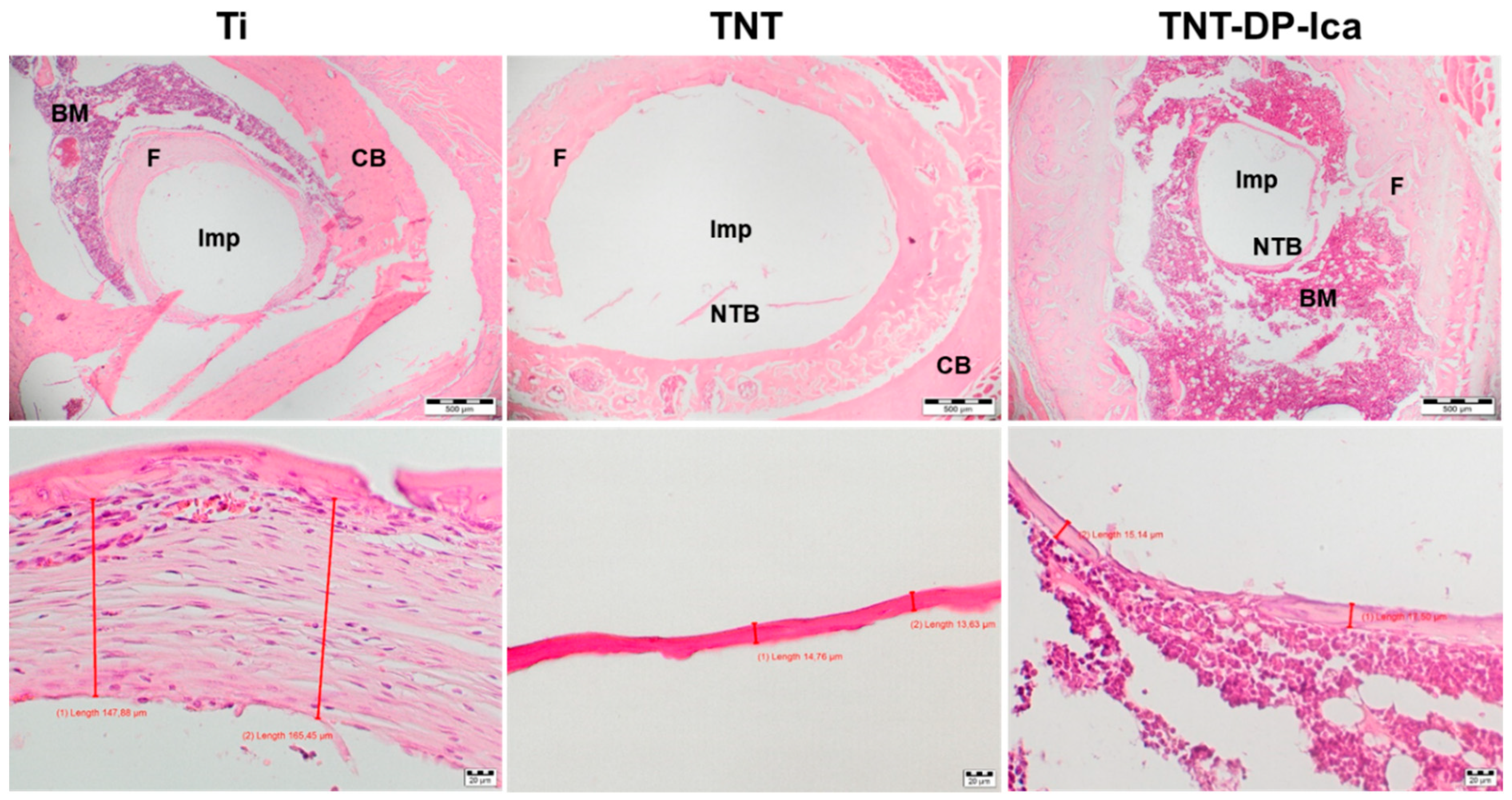
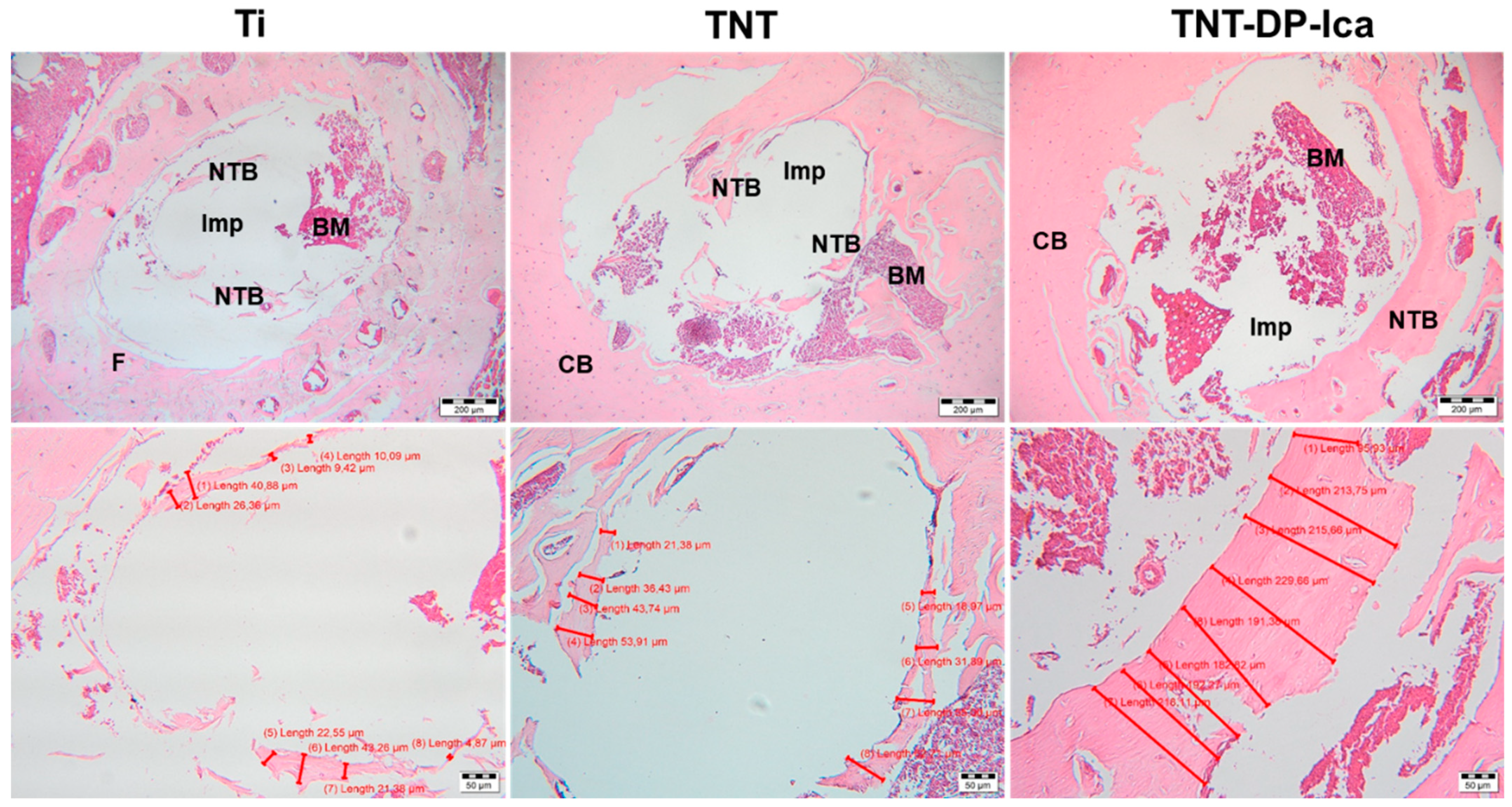
3.3. In Vivo Biological Performance Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglic, V.; Milosev, I.; Scmuki, P.; Iglic, A.; Mozetic, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Civantos, A.; Martinez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abbarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [Green Version]
- Swami, N.; Cui, Z.; Nair, L.S. Titania Nanotubes: Novel Nanostructures for Improper Osseointegration. J. Heat Transf. 2010, 132, 034002. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone-implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Keshavarz, M.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Metal-oxide surface-enhanced Raman biosensor template toeards point-of-care EGFR detection and cancer diagnostic. Nanoscale Horiz. 2020, 5, 294–307. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Seelective apoptosis induced by polymorphic alteration of Self-assembled Silica Nano-webs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qi, X.; Miao, Y.; Wu, H.-L.; He, N.; Zhu, J.-J. Application of smart nanostrucrtes in medicine. Nanomedicine 2020, 5, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Huang, J.Y.; Li, H.Q.; Zhao, A.Z.J.; Wang, Y.; Zhang, K.Q.; Sun, H.T.; Lai, Y.K. Recent advances on smart TiO2 nanotube platforms for sustainable drug delivery applications. Int. J. Nanomed. 2017, 12, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug delivery systems based on titania nanotubes and active agents for enhanced osseointegration of bone implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Choi, D.S.; Jang, I.; Choi, W.Y. Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with recombinant human bone morphogenetic protein-2: A pilot in vivo study. Int. J. Nanomed. 2015, 10, 1145–1154. [Google Scholar]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface Functionalization of TiO2 Nanotubes with Bone Morphogenetic Protein 2 and Its Synergistic Effect on the Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef]
- Bauer, S.; Park, J.; Pittrof, A.; Song, Y.Y.; Mark, K.; Schmuki, P. Covalent functionalization of TiO2 nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cells. Integr. Biol. 2011, 3, 927–936. [Google Scholar] [CrossRef]
- Shim, I.; Chung, H.J.; Jung, M.R.; Nam, S.Y.; Lee, S.; Lee, H.; Heo, S.J.; Lee, S.J. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J. Biomater. Med. Res. 2014, 102, 3639–3648. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, Y.; Qian, S.; Li, J.; Chang, Q.; Ye, D.; Pan, H.; Zhang, M.; Cao, H.; Liu, X.; et al. Vacuum extraction enhances rhPDGF-BB immobilization on nanotubes to improve implant osseointegration in ovariectomized rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1809–1818. [Google Scholar] [CrossRef]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin: A Potential Osteoinductive Compound for Bone Tissue Engineering. Tissue Eng. A 2009, 16, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Zhou, J.; Ge, B.F.; Zhen, P.; Ma, H.P.; Shi, W.G.; Chang, K.; Xian, C.J.; Chen, K.M. Icariin induces Osteoblast Differentiation and Mineralization without Dexamethasone in vitro. Planta Med. 2013, 79, 1501–1508. [Google Scholar] [CrossRef]
- Song, J.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2017, 29, 535–544. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840. [Google Scholar] [CrossRef]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE2 synthesis. Phytomedicine 2011, 18, 176–185. [Google Scholar] [CrossRef]
- Chung, B.H.; Kim, J.D.; Kim, C.K.; Kim, J.W.; Won, M.H.; Lee, H.S.; Dong, M.S.; Ha, K.S.; Kwon, Y.G.; Kim, Y.M. Icariin stimulates angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways in human endothelial cells. Biochem. Biophys. Res. Commun. 2008, 376, 404–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, L.; Mao, Z.; Wang, N.; Wang, X.; Huang, X.; Hu, Y.; Shou, D.; Wen, C. Icariin Enhances Bone Repair in Rabbits with Bone Infection during Post-infection Treatment and Prevents Inhibition of Osteoblasts by Vancomycin. Front. Pharmacol. 2017, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Annabi, N.; Fathi, A.; Mithieux, A.M.; Weiss, A.S.; Dehghani, F. Fabrication of porous PCL/elastin composite scaffolds for tissue engineering applications. J. Supercrit. Fluids 2011, 59, 157–167. [Google Scholar] [CrossRef]
- Fathi, M.; Akbari, B.; Taheriazam, A. Antibiotics drug release controlling and osteoblast adhesion from Titania nanotubes arrays using silk fibroin coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109743. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Mussel-inspired surface modification of titania nanotubes as a novel drug delivery system. Mater. Sci. Eng. C 2017, 77, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.; Tang, L.; Wang, J.; Zhao, Y.; Tu, Q.; Weng, Y.; Shen, R.; Huang, N. Improved immobilization of biomolecules to quinone-rich polydopamine for efficient surface functionalization. Colloid Surf. B Biointerfaces 2013, 106, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 5077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.G.; Pang, L.; Chen, Z.R.; Tan, X.P. Dual-delivery of vancomycin and icariin from an injectable calcium phosphate cement-release system for controlling infection and improving bone healing. Mol. Med. Rep. 2013, 8, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xia, L.; Zhou, Y.; Ma, W.; Zhang, N.; Chang, J.; Lin, K.; Xu, Y.; Jiang, X. Evaluation of osteogenesis and angiogenesis of icariin loaded on micro/nano hybrid structured hydroxyapatite granules as a local drug delivery system for femoral defect repair. J. Mater. Chem. B 2015, 3, 4871–4883. [Google Scholar] [CrossRef]
- Wu, T.; Nan, K.; Chen, J.; Jin, D.; Jiang, S.; Zhao, P.; Xu, J.; Du, H.; Zhang, X.; Li, J.; et al. A new bone repair scaffold combined with chitosan/hydroxyapatite and sustained releasing icariin. Chin. Sci. Bull. 2009, 54, 2953–2961. [Google Scholar] [CrossRef]
- Xie, X.; Pei, F.; Wang, H.; Tan, Z.; Yang, Z.; Kang, P. Icariin: A promising osteoinductive compound for repairing bone defect and osteonecrosis. J. Biomater. Appl. 2015, 30, 290–299. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Li, D.X.; Wang, R.; Fan, H.S.; Xiao, Y.M.; Zhang, L.; Zhang, X.D. The effect of loading icariin on biocompatibility and bioactivity of porous β-TCP ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wen, W.; Chen, S.; Zhou, C.; Luo, B. Preparation of Icariin and Deferoxamine Functionalized Poly(l-lactide)/chitosan Micro/Nanofibrous Membranes with Synergistic Enhanced Osteogenesis and Angiogenesis. ACS Appl. Bio Mater. 2018, 1, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Chen, L.; Chen, A.; Feng, X.; Shao, L. Icariin-Loaded TiO2 Nanotubes for Regulation of the Bioactivity of Bone Marrow Cells. J. Nanomater. 2018, 2018, 1810846. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, L.; Liu, C.; Feng, X.; Wei, L.; Shao, L. Self-assembly chitosan/gelatin composite coating on icariin-modified TiO2 nanotubes for the regulation of osteoblast bioactivity. Mater. Des. 2016, 92, 471–479. [Google Scholar] [CrossRef]
- Albu, A.M.; Draghicescu, W.; Munteanu, T.; Ion, R.; Mitran, V.; Cimpean, A.; Popescu, S.; Pirvu, C. Nitrodopamine vs. dopamine as an intermediate layer for bone regeneration applications. Mater. Sci. Eng. C 2019, 98, 461–471. [Google Scholar] [CrossRef]
- Ungureanu, C.; Dumitriu, C.; Popescu, S.; Enculescu, M.; Tofan, V.; Popescu, M.; Pirvu, C. Enhancing antimicrobial activity of TiO2/Ti by torularhodin bioinspired surface modification. Bioelectrochemistry 2016, 107, 14–24. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Minculescu, F.; et al. Characterization and In Vitro and In Vivo Assesment of a Novel Cellulose Acetate-Coated Mg-Based Alloy for Orthopeduc Applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Hylton, D.M.; Shalaby, S.W.; Latour, R.A. Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J. Biomed. Mater. Res. A 2005, 73, 349–358. [Google Scholar] [CrossRef]
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration function. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, W.F.; Silva, G.M.M.; Filho, P.E.C.; Fontes, A.; Oliveira, M.D.L.; Andrade, C.A.S.; Silva, M.V.; Coelho, L.C.B.B.; Machado, G.; Correia, M.T.S. Titanium dioxide nanotubes functionalised with Cratylia mollis seed lectin, Cramoll, enhanced osteoblast-like cells adhesion and proliferation. Mater. Sci. Eng. C 2018, 90, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bhattarai, G.; Park, I.S.; Kim, G.R.; Kim, G.E.; Lee, M.H.; Yi, H.K. Bone regeneration around N-acetyl cysteine-loaded nanotube titanium dental implant in rat mandible. Biomaterials 2013, 34, 10199–10208. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schliephake, H.; Scharnweber, D. Chemical and biological functonalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Montali, A. Antibacterial coating systems. Injury 2006, 37, 81–86. [Google Scholar] [CrossRef]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. A 2010, 92, 1218–1224. [Google Scholar]
- Alhoshan, M.S.; Baqais, A.A.; Al-Hazza, M.I.; Al-Mayouf, A.M. Heat treatment and electrochemical activation of titanium oxide nanotubes: The effect of hydrogen doping on electrochemical behavior. Electrochim. Acta 2012, 62, 390–395. [Google Scholar] [CrossRef]
- Al-Mobarak, N.A.; Al-Swayih, A.A. Development of titanium surgery implants for improving osseointegration through formation of a titanium nanotube layer. Int. J. Electrochem. Sci. 2014, 9, 32–45. [Google Scholar]
- Lee, H.C.; Zhang, L.F.; Lin, J.L.; Chin, Y.L.; Sun, T.P. Development of anodic titania nanotubes for application in high sensitivity amperometric glucose and uric acid biosensors. Sensors 2013, 13, 14161–14174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay between Bone and the Immune System. Front. Immunol. 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, A.; You, Y.; Chen, B.; Wang, W.; Liu, J.; Qi, H.; Ling, Y.; Li, Y.; Li, C. Icariin/Aspririn Composite Coating in TiO2 Nanotubes Surface Induce Immunomodulatory Effect of Macrophage and Improve Osteoblast Activity. Coatings 2020, 10, 427. [Google Scholar] [CrossRef]
- Chi, L.; Gao, W.; Shu, X.; Lu, X. A natural flavonoid glucoside, icariin, regulates Th17 and alleviates rheumatoid arthritis in a murine model. Mediat. Inflamm. 2014, 2014, 392062. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 13, 4029–4043. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Deng, Y.; Ye, Z.; Liang, S.; Tang, Z.; Wei, S. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surf. B Biointerfaces 2013, 111, 107–116. [Google Scholar] [CrossRef]
- Li, L.; Xie, C.; Xiao, X. Polydopamine modified TiO2 nanotube arrays as local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 1011537. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Takebe, J.; Itoh, S.; Okada, J.; Ishibashi, K. Anodic oxidation and hydrothermal treatment of titanium results in a surface that causes increased attachment and altered cytoskeletal morphology of rat bone marrow stromal cells in vitro. J. Biomed. Mater. Res. B 2000, 51, 398–407. [Google Scholar] [CrossRef]
- Yang, C.R.; Di Chen, J. Preparation and biological evaluation of chitosan-collagen-icariin composite scaffolds for neuronal regeneration. Neurol. Sci. 2013, 34, 941–947. [Google Scholar] [CrossRef]
- Kindle, L.; Rothe, L.; Kriss, M.; Osdoby, P.; Collin-Osdoby, P. Humanmicrovascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J. Bone Miner. Res. 2006, 21, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Nagalla, R.R.; Chen, E.Y.; Liu, W.F. Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliver. Rev. 2017, 114, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Sem. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone Remodelling In Vitro: Where are We Headed? Bone 2018, 110, 38–46. [Google Scholar] [CrossRef]
- Nakahama, K. Cellular communications in bone homeostasis and repair. Cell. Mol. Life Sci. 2010, 67, 4001–4009. [Google Scholar] [CrossRef]
- Chen, S.R.; Xu, X.Z.; Wang, Y.H.; Chen, J.W.; Xu, S.W.; Gu, L.Q.; Liu, P.Q. Icariin derivative inhibits inflammation through suppression of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways. Biol. Pharm. Bull. 2010, 33, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Ito, H. Chemokines in mesenchymal stem cell therapy for bone repair: A novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod. Rheumatol. 2011, 21, 113–121. [Google Scholar] [CrossRef]
- Goodman, S.B.; Ma, T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials 2010, 31, 5045–5050. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Thiede, M.; Seibert, K.; Mielke, C.; Phippard, D.; Svagr, B.; Cullinane, D.A.; Einhorn, T. Differential Inhibition of Fracture Healing by Non-selective and Cyclooxygenase-2 Selective Non-Steroidal Anti-Inflammatory Drugs. J. Orthop. Res. 2003, 21, 670–675. [Google Scholar] [CrossRef]
- Mullis, B.H.; Copland, S.T.; Weinhold, P.S.; Miclau, T.; Lester, G.E.; Bos, G.D. Effect of COX-2 Inhibitors and Non-Steroidal Anti-Inflammatory Drugs on a Mouse Fracture Model. Injury 2006, 37, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Cimpean, A.; Ion, R.; Gordin, D.M.; Neacsu, P.; Mitran, V.; Gloriant, T. Biomaterials as modulators of the macrophage inflammatory response. In Biomaterials; Navani, N.K., Sinha, S., Govil, J.N., Eds.; Nanotechnology Series; Studium Press India Pvt. Ltd.: New Delhi, India, 2013; pp. 139–176. [Google Scholar]
- Xu, C.Q.; Liu, B.J.; Wu, J.F.; Xu, Y.C.; Duan, X.H.; Cao, Y.X.; Dong, J.C. Icariin attenuates LPS-induced acute inflammatory responses: Involvement of PI3K/Akt and NF-κB signaling pathway. Eur. J. Pharmacol. 2010, 642, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.S.; Song, S.L.; Liang, H.; Ji, A.G. Icariin inhibits atherosclerosis progress in Apoe null mice by downregulating CX3CR1 in macrophage. Biochem. Biophys. Res. Com. 2016, 470, 845–850. [Google Scholar] [CrossRef]
- Burd, T.A.; Hughes, M.S.; Anglen, J.O. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J. Bone Jt. Surg. 2003, 85, 700–705. [Google Scholar] [CrossRef]
- Giannoudis, P.; MacDonald, D.A.; Matthews, S.; Smith, R.; Furlong, A.J.; De Boer, P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J. Bone Jt. Surg. 2000, 82, 655–658. [Google Scholar] [CrossRef]
- Sagi, H.C.; Jordan, C.J.; Barei, D.P.; Serrano-Riera, R.; Steverson, B. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J. Orthop. Trauma 2014, 28, 377–383. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Impaired fracture healing in the absence of TNF-alpha signaling: The role of TNF-alpha in endochondral cartilage resorption. J. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef]
- Yang, X.; Ricciardi, B.F.; Hernandez-Soria, A.; Shi, Y.; Camacho, N.P.; Bostrom, M.P.G. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007, 41, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X. Mussel-Inspired Polydopamine Coating: A General Strategy To Enhance Osteogenic Differentiation and Osseointegration for Diverse Implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cao, H.; Wang, X. Porous composite scaffold incorporating osteogenic phytomolecules icariin for promoting skeletal regeneration in a challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shen, J.; Wang, M.; Cui, J.; Zhu, S.; Zhang, W.; Yang, H.; Xu, Y.; Geng, D. Icariin protects against titanium particle-induced osteolysis and inflammatory response in a mouse calvarial model. Biomaterials 2015, 60, 92–99. [Google Scholar] [CrossRef]

| Sample | Water | Ethylene Glycol | Dimethyl Sulfoxide | Free Surface Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|---|
| Angle | S.D. | Angle | S.D. | Angle | S.D. | ||
| Ti | 72 | 0.31 | 34 | 0.94 | 29 | 1.57 | 39.97 |
| TNT | 20 | 0.52 | 14 | 0.51 | 12 | 0.45 | 68.82 |
| TNT-DP-Ica | 35 | 0.69 | 11 | 0.33 | 8 | 0.36 | 59.27 |
| Sample | Rs (Ω) | R1 (Ω) | R2 (Ω) | χ2 |
|---|---|---|---|---|
| Ti | 19.4 | 7.27 × 105 | - | 0.2112 |
| TNT | 18.41 | 4.21 × 105 | 3.19 × 103 | 0.0229 |
| TNT-DP-Ica | 21.84 | 9.84 × 105 | 12.09 × 103 | 0.0636 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrescu, A.-M.; Mitran, V.; Draghicescu, W.; Popescu, S.; Pirvu, C.; Ionascu, I.; Soare, T.; Uzun, S.; Croitoru, S.M.; Cimpean, A. TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration. J. Funct. Biomater. 2022, 13, 43. https://doi.org/10.3390/jfb13020043
Negrescu A-M, Mitran V, Draghicescu W, Popescu S, Pirvu C, Ionascu I, Soare T, Uzun S, Croitoru SM, Cimpean A. TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration. Journal of Functional Biomaterials. 2022; 13(2):43. https://doi.org/10.3390/jfb13020043
Chicago/Turabian StyleNegrescu, Andreea-Mariana, Valentina Mitran, Wanda Draghicescu, Simona Popescu, Cristian Pirvu, Iuliana Ionascu, Teodoru Soare, Seralp Uzun, Sorin Mihai Croitoru, and Anisoara Cimpean. 2022. "TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration" Journal of Functional Biomaterials 13, no. 2: 43. https://doi.org/10.3390/jfb13020043
APA StyleNegrescu, A.-M., Mitran, V., Draghicescu, W., Popescu, S., Pirvu, C., Ionascu, I., Soare, T., Uzun, S., Croitoru, S. M., & Cimpean, A. (2022). TiO2 Nanotubes Functionalized with Icariin for an Attenuated In Vitro Immune Response and Improved In Vivo Osseointegration. Journal of Functional Biomaterials, 13(2), 43. https://doi.org/10.3390/jfb13020043










