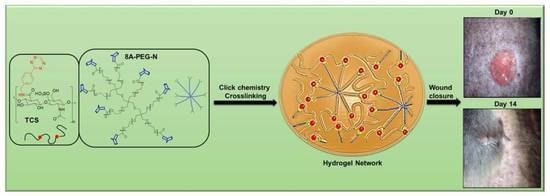
Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tetrazine Chondroitin Sulfate (TCS) Synthesis
2.3. Preparation of Click TCS-A-PEG Hydrogel
2.4. Characterization of Hydrogel Properties
2.5. Scanning Electron Microscopy (SEM) Evaluation
2.6. Swelling Behavior Evaluation
2.7. Adhesion Assay
2.8. Animals
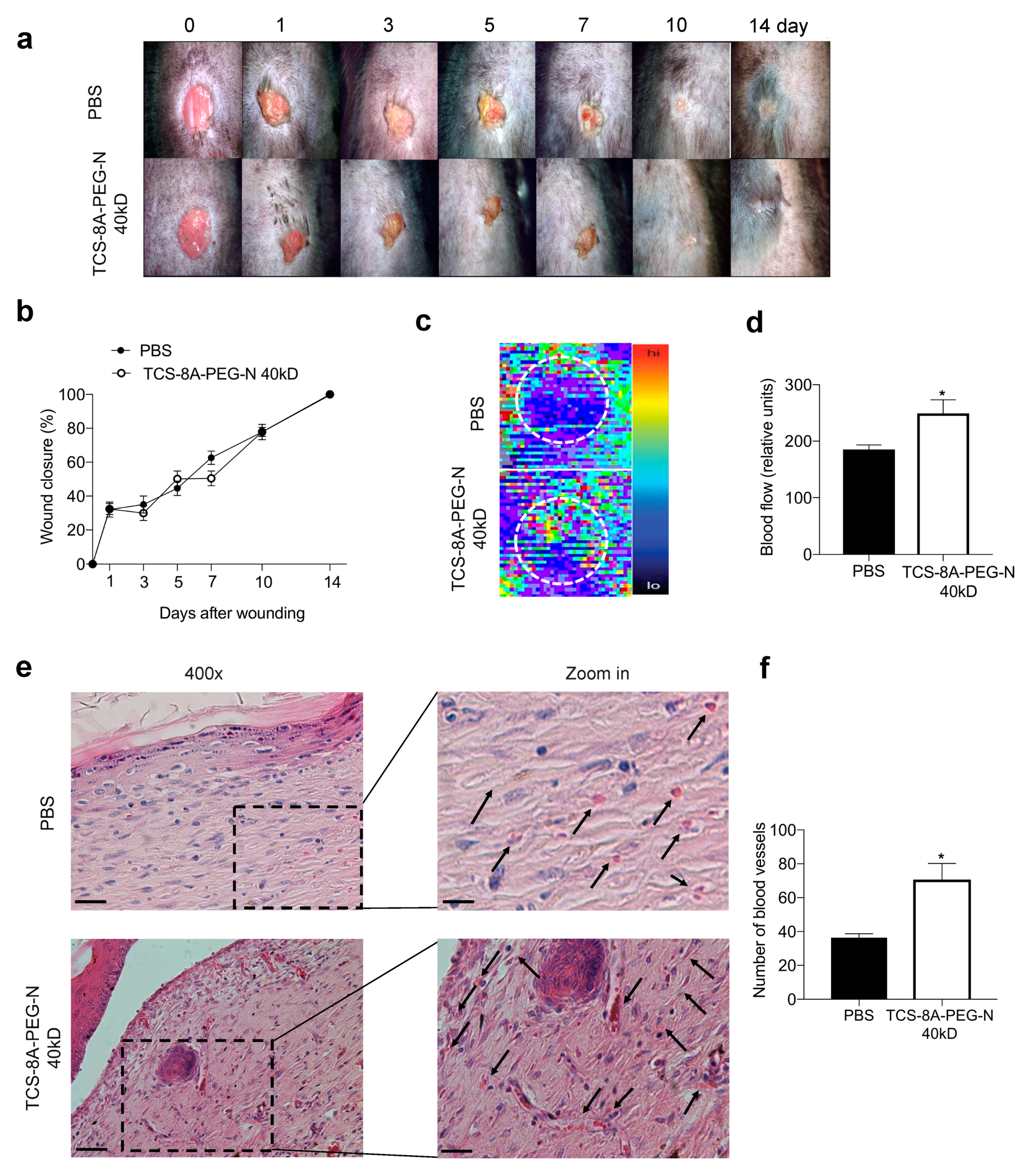
2.9. Excisional Wound Model and Treatment
2.10. Blood Flow Evaluation
2.11. Histological Analysis
2.12. Collagen Deposition Evaluation
2.13. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garg, T.; Goyal, A.K. Biomaterial-based scaffolds—Current status and future directions. Expert Opin. Drug Deliv. 2014, 11, 767–789. [Google Scholar] [CrossRef] [PubMed]
- Uludağ, H. Grand Challenges in Biomaterials. Front. Bioeng. Biotechnol. 2014, 2, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Jain, E.; Hill, L.; Canning, E.; Sell, S.A.; Zustiak, S.P. Control of gelation, degradation and physical properties of polyethylene glycol hydrogels through the chemical and physical identity of the crosslinker. J. Mater. Chem. B 2017, 5, 2679–2691. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Alge, D.L.; Azagarsamy, M.A.; Donohue, D.F.; Anseth, K.S. Synthetically Tractable Click Hydrogels for Three-Dimensional Cell Culture Formed Using Tetrazine–Norbornene Chemistry. Biomacromolecules 2013, 14, 949–953. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, K.; Liu, Q.; Eelkema, R. Self-Healing Injectable Polymer Hydrogel via Dynamic Thiol-Alkynone Double Addition Cross-Links. ACS Macro Lett. 2020, 9, 776–780. [Google Scholar] [CrossRef]
- Griveau, L.; Lafont, M.; le Goff, H.; Drouglazet, C.; Robbiani, B.; Berthier, A.; Sigaudo-Roussel, D.; Latif, N.; Visage, C.L.; Gache, V.; et al. Design and characterization of an in vivo injectable hydrogel with effervescently generated porosity for regenerative medicine applications. Acta Biomater. 2022, 140, 324–337. [Google Scholar] [CrossRef]
- Xin, S.; Chimene, D.; Garza, J.E.; Gaharwar, A.K.; Alge, D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019, 7, 1179–1187. [Google Scholar] [CrossRef]
- Stocco, T.D.; Bassous, N.J.; Zhao, S.; Granato, A.E.; Webster, T.J.; Lobo, A.O. Nanofibrous scaffolds for biomedical applications. Nanoscale 2018, 10, 12228–12255. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Bassous, N.; Harb, S.V.; Corat, M.A.F.; Maharjan, S.; Ruiz-Esparza, G.U.; de Paula, M.M.M.; Webster, T.J.; Tim, C.R.; Viana, B.C.; et al. Engineering multifunctional bactericidal nanofibers for abdominal hernia repair. Commun. Biol. 2021, 4, 233. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Schultz, K.M. Correlation of Bulk Degradation and Molecular Release from Enzymatically Degradable Polymeric Hydrogels. Biomacromolecules 2021, 22, 4489–4500. [Google Scholar] [CrossRef] [PubMed]
- Beria, L.; Gevrek, T.N.; Erdog, A.; Sanyal, R.; Pasini, D.; Sanyal, A. ‘Clickable’ hydrogels for all: Facile fabrication and functionalization. Biomater. Sci. 2014, 2, 67–75. [Google Scholar] [CrossRef]
- Delattre, C.; Louis, F.; Akashi, M.; Matsusaki, M.; Michaud, P.; Pierre, G. Fabrication Methods of Sustainable Hydrogels. In Sustainable Polymer Composites and Nanocomposites; Inamuddin, T.S., Kumar Mishra, R., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 355–386. [Google Scholar] [CrossRef]
- Konieczynska, M.D.; Grinstaff, M.W. On-Demand Dissolution of Chemically Cross-Linked Hydrogels. Acc. Chem. Res. 2017, 50, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 15012. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, F.; Mao, C.; Ming, X. Multiarm Nanoconjugates for Cancer Cell-Targeted Delivery of Photosensitizers. Mol. Pharm. 2018, 15, 2559–2569. [Google Scholar] [CrossRef]
- Bu, Y.; Zhang, L.; Liu, J.; Zhang, L.; Li, T.; Shen, H.; Wang, X.; Yang, F.; Tang, P.; Wu, D. Synthesis and Properties of Hemostatic and Bacteria-Responsive in Situ Hydrogels for Emergency Treatment in Critical Situations. ACS Appl. Mater. Interfaces 2016, 8, 12674–12683. [Google Scholar] [CrossRef]
- Golas, P.L.; Matyjaszewski, K. Marrying click chemistry with polymerization: Expanding the scope of polymeric materials. Chem. Soc. Rev. 2010, 39, 1338–1354. [Google Scholar] [CrossRef]
- Garner, A.L. cat-ELCCA: Catalyzing drug discovery through click chemistry. Chem. Commun. 2018, 54, 6531–6539. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, P.M.; da Silva, R.M.P.; Strehin, I.; Kouwer, P.H.J.; Leeuwenburgh, S.C.G.; Messersmith, P.B. Self-Healing Hydrogels Formed by Complexation between Calcium Ions and Bisphosphonate-Functionalized Star-Shaped Polymers. Macromolecules 2017, 50, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Donahoe, C.D.; Cohen, T.L.; Li, W.; Nguyen, P.K.; Fortner, J.D.; Mitra, R.D.; Elbert, D.L. Ultralow Protein Adsorbing Coatings from Clickable PEG Nanogel Solutions: Benefits of Attachment under Salt-Induced Phase Separation Conditions and Comparison with PEG/Albumin Nanogel Coatings. Langmuir 2013, 29, 4128–4139. [Google Scholar] [CrossRef] [PubMed]
- Madl, C.M.; Heilshorn, S.C. Rapid Diels–Alder Cross-linking of Cell Encapsulating Hydrogels. Chem. Mater. 2019, 31, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Mazunin, D.; Broguiere, N.; Zenobi-Wong, M.; Bode, J.W. Synthesis of Biocompatible PEG Hydrogels by pH-Sensitive Potassium Acyltrifluoroborate (KAT) Amide Ligations. ACS Biomater. Sci. Eng. 2015, 1, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Christman, K.L.; Schopf, E.; Broyer, R.M.; Li, R.C.; Chen, Y.; Maynard, H.D. Positioning Multiple Proteins at the Nanoscale with Electron Beam Cross-Linked Functional Polymers. J. Am. Chem. Soc. 2009, 131, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Chun, Y.H.; Park, S.-K.; Kim, E.J.; Lee, H.J.; Kim, H.; Koh, W.-G.; Cunha, G.F.; Myung, D.; Na, K.-S. In vivo biocompatibility evaluation of in situ-forming polyethylene glycol-collagen hydrogels in corneal defects. Sci. Rep. 2021, 11, 23913. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Kang, E.H.; Choi, S.; Jeon, E.J.; Cho, J.H.; Kang, D.; Lee, H.; Yun, I.S.; Cho, S.-W. Tissue-Adhesive Chondroitin Sulfate Hydrogel for Cartilage Reconstruction. ACS Biomater. Sci. Eng. 2021, 7, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.; Johnson, M.; Wang, X.; Lyu, J.; Li, Y.; McMahon, S.; Greiser, U.; Sigen, A.; Wang, W. A chondroitin sulfate based injectable hydrogel for delivery of stem cells in cartilage regeneration. Biomater. Sci. 2021, 9, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; He, Y.; Wang, Y.J.; Zhao, Y.; Chen, L. Chondroitin sulfate hydrogels based on electrostatic interactions with enhanced adhesive properties: Exploring the bulk and interfacial contributions. Soft Matter 2020, 16, 6128–6137. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef] [Green Version]
- Mirjafari, A. Ionic liquid syntheses via click chemistry: Expeditious routes toward versatile functional materials. Chem. Commun. 2018, 54, 2944–2961. [Google Scholar] [CrossRef]
- Steinmetz, N.J.; Bryant, S.J. Chondroitin sulfate and dynamic loading alter chondrogenesis of human MSCs in PEG hydrogels. Biotechnol. Bioeng. 2012, 109, 2671–2682. [Google Scholar] [CrossRef]
- Bhowmick, S.; Thanusha, A.V.; Kumar, A.; Scharnweber, D.; Rother, S.; Koul, V. Nanofibrous artificial skin substitute composed of mPEG–PCL grafted gelatin/hyaluronan/chondroitin sulfate/sericin for 2nd degree burn care: In vitro and in vivo study. RSC Adv. 2018, 8, 16420–16432. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.G.; Neufurth, M.; Ackermann, M.; Tolba, E.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. Fabrication of a new physiological macroporous hybrid biomaterial/bioscaffold material based on polyphosphate and collagen by freeze-extraction. J. Mater. Chem. B 2017, 5, 3823–3835. [Google Scholar] [CrossRef]
- Nandini, C.D.; Sugahara, K. Role of the Sulfation Pattern of Chondroitin Sulfate in its Biological Activities and in the Binding of Growth Factors. Adv. Pharmacol. 2006, 53, 253–279. [Google Scholar]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Santos, G.R.C.; Piquet, A.A.; Glauser, B.F.; Tovar, A.M.F.; Pereira, M.S.; Vilanova, E.; Mourão, P.A.S. Systematic Analysis of Pharmaceutical Preparations of Chondroitin Sulfate Combined with Glucosamine. Pharmaceuticals 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Foong, W.C.; Cao, T.; Bay, B.H.; Ouyang, H.W.; Yip, G.W. Chondroitin Sulfate in Palatal Wound Healing. J. Dent. Res. 2004, 83, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, BTRI.S38670. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.-C.; Kuo, S.-W.; Chang, F.-C. Synthesis of the Organic/Inorganic Hybrid Star Polymers and Their Inclusion Complexes with Cyclodextrins. Macromolecules 2005, 38, 3099–3107. [Google Scholar] [CrossRef] [Green Version]
- Afewerki, S.; Wang, X.; Ruiz-Esparza, G.U.; Tai, C.-W.; Kong, X.; Zhou, S.; Welch, K.; Huang, P.; Bengtsson, R.; Xu, C.; et al. Combined Catalysis for Engineering Bioinspired, Lignin-Based, Long-Lasting, Adhesive, Self-Mending, Antimicrobial Hydrogels. ACS Nano 2020, 14, 17004–17017. [Google Scholar] [CrossRef] [PubMed]
- Canesso, M.C.C.; Vieira, A.T.; Castro, T.B.R.; Schirmer, B.G.A.; Cisalpino, D.; Martins, F.S.; Rachid, M.A.; Nicoli, J.R.; Teixeira, M.M.; Barcelos, L.S. Skin Wound Healing Is Accelerated and Scarless in the Absence of Commensal Microbiota. J. Immunol. 2014, 193, 5171–5180. [Google Scholar] [CrossRef] [Green Version]
- Kloppenberg, F.W.H.; Beerthuizen, G.I.J.M.; ten Duis, H.J. Perfusion of burn wounds assessed by Laser Doppler Imaging is related to burn depth and healing time. Burns 2001, 27, 359–363. [Google Scholar] [CrossRef]
- Moreira, C.F.; Cassini-Vieira, P.; Canesso, M.C.C.; Felipetto, M.; Ranfley, H.; Teixeira, M.M.; Nicoli, J.R.; Martins, F.S.; Barcelos, L.S. Lactobacillus rhamnosus CGMCC 1.3724 (LPR) Improves Skin Wound Healing and Reduces Scar Formation in Mice. Probiotics Antimicrob. Proteins 2021, 13, 709–719. [Google Scholar] [CrossRef]
- Mendes, J.B.; Campos, P.P.; Rocha, M.A.; Andrade, S.P. Cilostazol and pentoxifylline decrease angiogenesis, inflammation, and fibrosis in sponge-induced intraperitoneal adhesion in mice. Life Sci. 2009, 84, 537–543. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Canny, J.F. A computation approach to edge detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 8, 670–700. [Google Scholar]
- De Sousa Araújo, E.; Domingues Stocco, T.; Fernandes de Sousa, G.; Afewerki, S.; Marciano, F.R.; Alexandre Finzi Corat, M.; Michelle Machado de Paula, M.; Ferreira Cândido Lima Verde, T.; Cristina Moreira Silva, M.; Oliveira Lobo, A. Oxygen-generating microparticles in chondrocytes-laden hydrogels by facile and versatile click chemistry strategy. Colloids Surf. B Biointerfaces 2021, 205, 111850. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Jung, U.-W.; Noh, I. Synthesis and Biocompatibility Characterizations of in Situ Chondroitin Sulfate–Gelatin Hydrogel for Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 25–35. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Ardalani, H.; Zhang, J.; Hou, Z.; Nguyen, E.H.; Swanson, S.; Nguyen, B.K.; Bolin, J.; Elwell, A.; Bischel, L.L.; et al. Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater. 2016, 35, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Lake, R.; Park, S.; Edwards, S.; Jones, C.; Jeong, K.J. Injectable Macroporous Hydrogel Formed by Enzymatic Cross-Linking of Gelatin Microgels. ACS Appl. Bio. Mater. 2018, 1, 1430–1439. [Google Scholar] [CrossRef]
- Xia, G.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Surface fluid-swellable chitosan fiber as the wound dressing material. Carbohydr. Polym. 2016, 136, 860–866. [Google Scholar] [CrossRef]
- Tamura, M.; Yanagawa, F.; Sugiura, S.; Takagi, T.; Sumaru, K.; Matsui, H.; Kanamori, T. Optical cell separation from three-dimensional environment in photodegradable hydrogels for pure culture techniques. Sci. Rep. 2014, 4, 4793. [Google Scholar] [CrossRef] [Green Version]
- Toda, S.; Fattah, A.; Asawa, K.; Nakamura, N.; Ekdahl, K.N.; Nilsson, B.; Teramura, Y. Optimization of Islet Microencapsulation with Thin Polymer Membranes for Long-Term Stability. Micromachines 2019, 10, 755. [Google Scholar] [CrossRef] [Green Version]
- Sparks, H.D.; Anjum, F.; Vallmajo-Martin, Q.; Ehrbar, M.; Abbasi, S.; Kallos, M.S.; Biernaskie, J. Flowable Polyethylene Glycol Hydrogels Support the in Vitro Survival and Proliferation of Dermal Progenitor Cells in a Mechanically Dependent Manner. ACS Biomater. Sci. Eng. 2019, 5, 950–958. [Google Scholar] [CrossRef]
- Kim, S.-H.; An, Y.-H.; Kim, H.D.; Kim, K.; Lee, S.-H.; Yim, H.-G.; Kim, B.-G.; Hwang, N.S. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 2018, 110, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, G.F.; Afewerki, S.; Dittz, D.; Santos, F.E.P.; Gontijo, D.O.; Scalzo, S.R.A.; Santos, A.L.C.; Guimaraes, L.C.; Pereira, E.M.; Barcelos, L.S.; et al. Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering. J. Funct. Biomater. 2022, 13, 45. https://doi.org/10.3390/jfb13020045
Sousa GF, Afewerki S, Dittz D, Santos FEP, Gontijo DO, Scalzo SRA, Santos ALC, Guimaraes LC, Pereira EM, Barcelos LS, et al. Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering. Journal of Functional Biomaterials. 2022; 13(2):45. https://doi.org/10.3390/jfb13020045
Chicago/Turabian StyleSousa, Gustavo F., Samson Afewerki, Dalton Dittz, Francisco E. P. Santos, Daniele O. Gontijo, Sérgio R. A. Scalzo, Ana L. C. Santos, Lays C. Guimaraes, Ester M. Pereira, Luciola S. Barcelos, and et al. 2022. "Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering" Journal of Functional Biomaterials 13, no. 2: 45. https://doi.org/10.3390/jfb13020045
APA StyleSousa, G. F., Afewerki, S., Dittz, D., Santos, F. E. P., Gontijo, D. O., Scalzo, S. R. A., Santos, A. L. C., Guimaraes, L. C., Pereira, E. M., Barcelos, L. S., Do Monte, S. J. H., Guimaraes, P. P. G., Marciano, F. R., & Lobo, A. O. (2022). Catalyst-Free Click Chemistry for Engineering Chondroitin Sulfate-Multiarmed PEG Hydrogels for Skin Tissue Engineering. Journal of Functional Biomaterials, 13(2), 45. https://doi.org/10.3390/jfb13020045