Immediate Tooth Autotransplantation with Root Canal Filling and Partially Demineralized Dentin/Cementum Matrix into Congenital Missing Tooth Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Case
2.2. Surgical Planning
2.3. Extraction and Immediate Root Canal Filling
2.4. Preparation of Partially Demineralized Dentin/Cementum Matrix (pDDM)
2.5. Tooth Transplantation and pDDM Graft
2.6. Radiographic Evaluation
3. Results
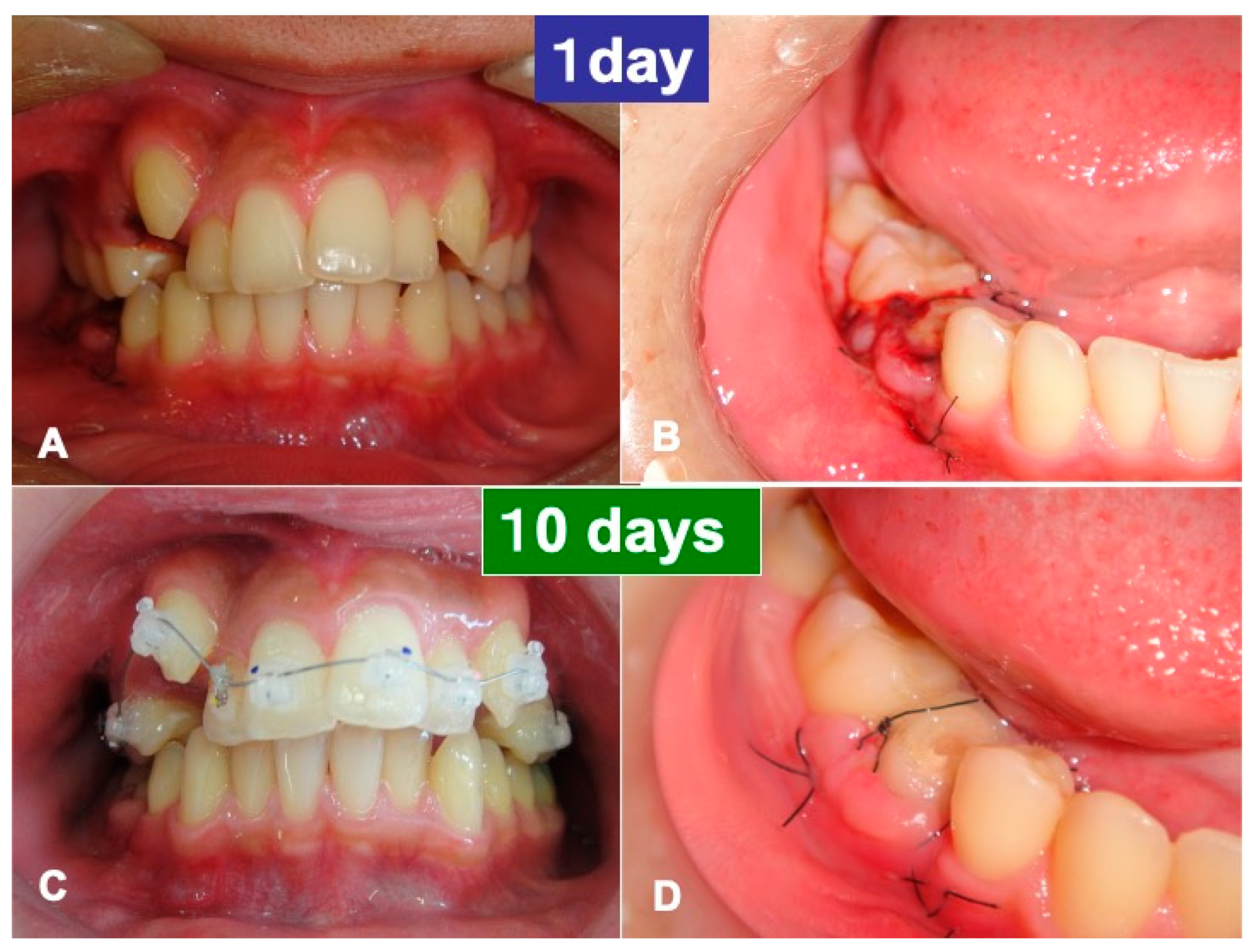
3.1. Gross View and Examinations after Surgery
3.2. Radiographic Evaluation
4. Discussion
4.1. Immediate System for Tooth Transplantation with Root Canal Filling (RCF) and pDDM
4.2. Timing of Root Canal Filling (RCF)
4.3. Surgical Technique for Transplantation
4.4. Root Resorption Subsequent to Transplantation
4.5. Dentin/Cementum as a Biological Graft Material
4.6. Augmentation by Dentin/Cementum Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Considerations
References
- Tsukiboshi, M. Autogenous tooth transplantation: A reevaluation. Int. J. Periodontics Restor. Dent. 1993, 13, 120–149. [Google Scholar]
- Tsukiboshi, M. Autotransplantation of teeth: Requirements for predictable success. Dent. Traumatol. 2002, 18, 157–180. [Google Scholar] [CrossRef]
- Kallu, R.; Vinckier, F.; Politis, C.; Mwalili, S.; Willems, G. Tooth transplantations: A descriptive retrospective study. Int. J. Oral Maxillofac. Surg. 2005, 34, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Paulsen, H.U.; Yu, Z.; Schwartz, O. A long-term study of 370 autotransplanted premolars. Part III. Periodontal healing subsequent to transplantation. Eur. J. Orthod. 1990, 12, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Paulsen, H.U.; Yu, Z.; Bayer, T. A long-term study of 370 autotransplanted premolars. Part IV. Root development subsequent to transplantation. Eur. J. Orthod. 1990, 12, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.U.; Desai, N. Autotransplantation of a mandibular third molar, using a customized reservoir. J. Conserv. Dent. 2020, 23, 206–210. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Sano, K.; Nakamura, M.; Ogasawara, T. Use of third molar transplantation for closure of the oroantral communication after tooth extraction: A report of 2 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cui, N.; Li, X.; Du, X.; Zhang, S.; Wu, C.; Kim, D.H.; Lim, H.K.; Lee, E.S. Effect of Root canal therapy on the success rate of teeth with complete roots in autogenous tooth transplantation. Scanning 2021, 14, 6675604. [Google Scholar] [CrossRef]
- Ravi, K.P.; Jyothi, M.; Sirisha, K.; Racca, K.; Uma, C. Autotransplantation of mandibular third molar: A case report. Case Rep. Dent. 2012, 2012, 629180. [Google Scholar]
- Kim, E.; Jung, J.Y.; Cha, I.H.; Kum, K.Y.; Lee, S.J. Evaluation of the prognosis and causes of failure in 182 cases of autogenous tooth transplantation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 112–119. [Google Scholar] [CrossRef]
- Paulsen, H.U.; Andreasen, J.O.; Schwartz, O. Pulp and periodontal healing, root development and root resorption subsequent to transplantation and orthodontic rotation: A long-term study of autotransplanted premolars. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 630–640. [Google Scholar] [CrossRef]
- Murata, M.; Akazawa, T.; Mitsugi, M.; Kabir, M.A.; Um, I.W.; Minamida, Y.; Kim, K.W.; Kim, Y.K.; Sun, Y.; Qin, C. Autograft of dentin materials for bone regeneration. In Advances in Biomaterials Sciences and Biomedical Applications, 1st ed.; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2013; pp. 391–403. [Google Scholar]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone regeneration using dentin matrix depends on the degree of demineralization and particle size. PLoS ONE 2016, 11, e0147235. [Google Scholar]
- Lin, P.Y.; Chiang, Y.C.; Hsu, L.Y.; Chang, H.J.; Chi, L.Y. Endodontic considerations of survival rate for autotransplanted third molars: A nationwide population-based study. Int. Endod. J. 2020, 53, 733–741. [Google Scholar] [CrossRef]
- Bae, J.H.; Choi, Y.H.; Cho, B.H.; Kim, Y.K.; Kim, S.G. Autotransplantation of teeth with complete root formation: A case series. J. Endod. 2010, 36, 1422–1426. [Google Scholar] [CrossRef]
- Raabe, C.; Bornstein, M.M.; Ducommun, J.; Sendi, P.; von Arx, T.; Janner, S.F.M. A retrospective analysis of autotransplanted teeth including an evaluation of a novel surgical technique. Clin. Oral Investig. 2021, 25, 3513–3525. [Google Scholar] [CrossRef]
- Hwang, L.A.; Chang, C.Y.; Su, W.C.; Chang, C.W.; Huang, C.Y. Rapid prototyping-assisted tooth autotransplantation is associated with a reduced root canal treatment rate: A retrospective cohort study. BMC Oral Health 2022, 22, 25. [Google Scholar] [CrossRef]
- Murata, M. Collagen biology for bone regenerative surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2012, 38, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Huggins, C.B.; Reddi, A.H. Coagulation of blood plasma of guinea pig by the bone matrix. Proc. Natl. Acad. Sci. USA 1973, 70, 929–933. [Google Scholar] [CrossRef] [Green Version]
- Butler, W.T.; Mikulski, A.; Urist, M.R.; Bridges, G.; Uyeno, S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J. Dent. Res. 1977, 56, 228–232. [Google Scholar] [CrossRef]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J. Biomed. Mater. Res. A. 2012, 100, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Um, I.W.; Ku, J.K.; Kim, Y.K.; Lee, B.K.; Leem, D.H. Histological review of demineralized dentin matrix as a carrier of rhBMP-2. Tissue Eng. Part B Rev. 2020, 26, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Um, I.W.; Kim, Y.K.; Park, J.C.; Lee, J.H. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: A case series. Clin. Implant Dent. Relat. Res. 2019, 21, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.K.; Park, Y.H.; Park, J.C.; Ku, J.K.; Um, I.W.; Kim, J.Y. Evaluation of the healing potential of demineralized dentin matrix fixed with recombinant human bone morphogenetic protein-2 in bone grafts. Materials 2017, 10, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, M.A.; Murata, M.; Akazawa, T.; Kusano, K.; Yamada, K.; Ito, M. Evaluation of perforated dentin scaffold on bone regeneration in critical-size sheep iliac defects. Clin. Oral Implant. Res. 2017, 28, e227–e235. [Google Scholar] [CrossRef]
- Kabir, M.A.; Murata, M.; Shakya, M.; Yamada, K.; Akazawa, T. Bio-absorption of human dentin-derived biomaterial in sheep critical-size iliac defects. Materials 2021, 14, 223. [Google Scholar] [CrossRef]
- Um, I.W.; Lee, J.K. Familial tooth bone graft. In Advances in Oral Tissue Engineering; Murata, M., Um, I.W., Eds.; Quintessence Publishing Co, Inc.: Chicago, IL, USA, 2014; pp. 67–72. [Google Scholar]
- Park, S.M.; Kim, D.H.; Pang, E.K. Bone formation of demineralized human dentin block graft with different demineralization time: In vitro and in vivo study. J. Cranio-Maxillo-Facial Surg. 2017, 45, 903–912. [Google Scholar] [CrossRef]
- Moon, Y.S.; Sohn, D.S.; Kim, G.; Park, I. Comparative histomorphometric evaluation of bone regeneration with different preparations of xenogeneic tooth block bone. Int. J. Oral Maxillofac. Implants 2019, 34, 1413–1422. [Google Scholar] [CrossRef]
- Zhu, B.; Yokozeki, K.; Kabir, M.A.; Todoh, M.; Akazawa, T.; Murata, M. Chemical properties of human dentin blocks and vertical augmentation by ultrasonically demineralized dentin matrix blocks on scratched skull without periosteum of adult-aged rats. Materials 2021, 15, 105. [Google Scholar] [CrossRef]
- Okubo, N.; Ishikawa, M.; Shakya, M.; Hosono, H.; Maehara, O.; Ohkawara, T.; Ohnishi, S.; Akazawa, T.; Murata, M. Autograft of demineralized dentin matrix prepared immediately after extraction for horizontal bone augmentation of the anterior atrophic maxilla: A first case of non-vital tooth-derived dentin. J. Hard Tissue Biol. 2022, 23, 363–370. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, M.; Nakanishi, Y.; Kusano, K.; Hirose, Y.; Tazaki, J.; Akazawa, T.; Mizoguchi, I. Immediate Tooth Autotransplantation with Root Canal Filling and Partially Demineralized Dentin/Cementum Matrix into Congenital Missing Tooth Region. J. Funct. Biomater. 2022, 13, 82. https://doi.org/10.3390/jfb13020082
Murata M, Nakanishi Y, Kusano K, Hirose Y, Tazaki J, Akazawa T, Mizoguchi I. Immediate Tooth Autotransplantation with Root Canal Filling and Partially Demineralized Dentin/Cementum Matrix into Congenital Missing Tooth Region. Journal of Functional Biomaterials. 2022; 13(2):82. https://doi.org/10.3390/jfb13020082
Chicago/Turabian StyleMurata, Masaru, Yasuhiro Nakanishi, Kaoru Kusano, Yukito Hirose, Junichi Tazaki, Toshiyuki Akazawa, and Itaru Mizoguchi. 2022. "Immediate Tooth Autotransplantation with Root Canal Filling and Partially Demineralized Dentin/Cementum Matrix into Congenital Missing Tooth Region" Journal of Functional Biomaterials 13, no. 2: 82. https://doi.org/10.3390/jfb13020082
APA StyleMurata, M., Nakanishi, Y., Kusano, K., Hirose, Y., Tazaki, J., Akazawa, T., & Mizoguchi, I. (2022). Immediate Tooth Autotransplantation with Root Canal Filling and Partially Demineralized Dentin/Cementum Matrix into Congenital Missing Tooth Region. Journal of Functional Biomaterials, 13(2), 82. https://doi.org/10.3390/jfb13020082






