Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AgNPs
2.3. Synthesis of Ag/HA
2.4. Physicochemical Characterization of Ag/HA
2.5. Antibacterial Activity Test of Ag/HA
3. Results
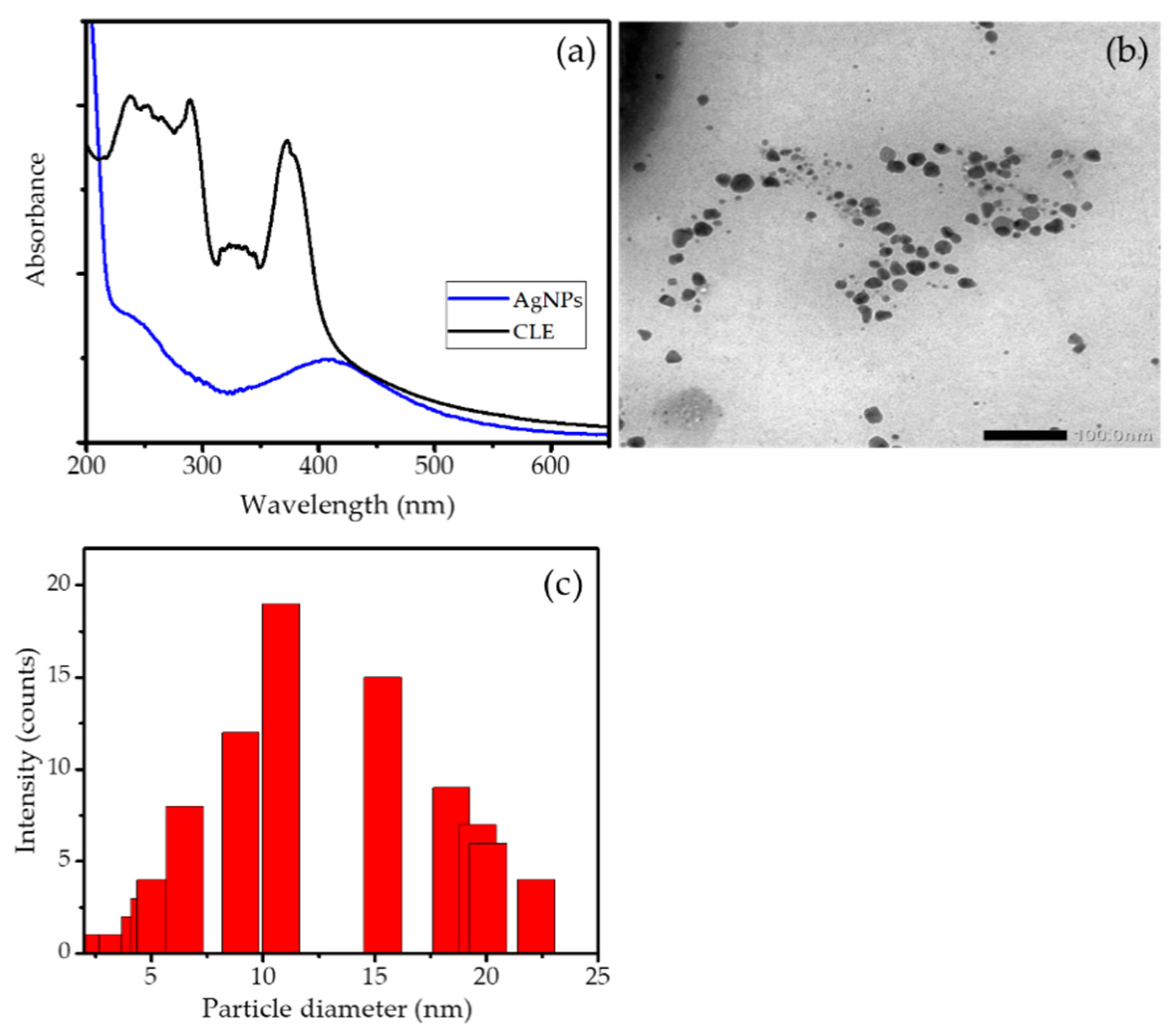
3.1. Synthesis of AgNPs
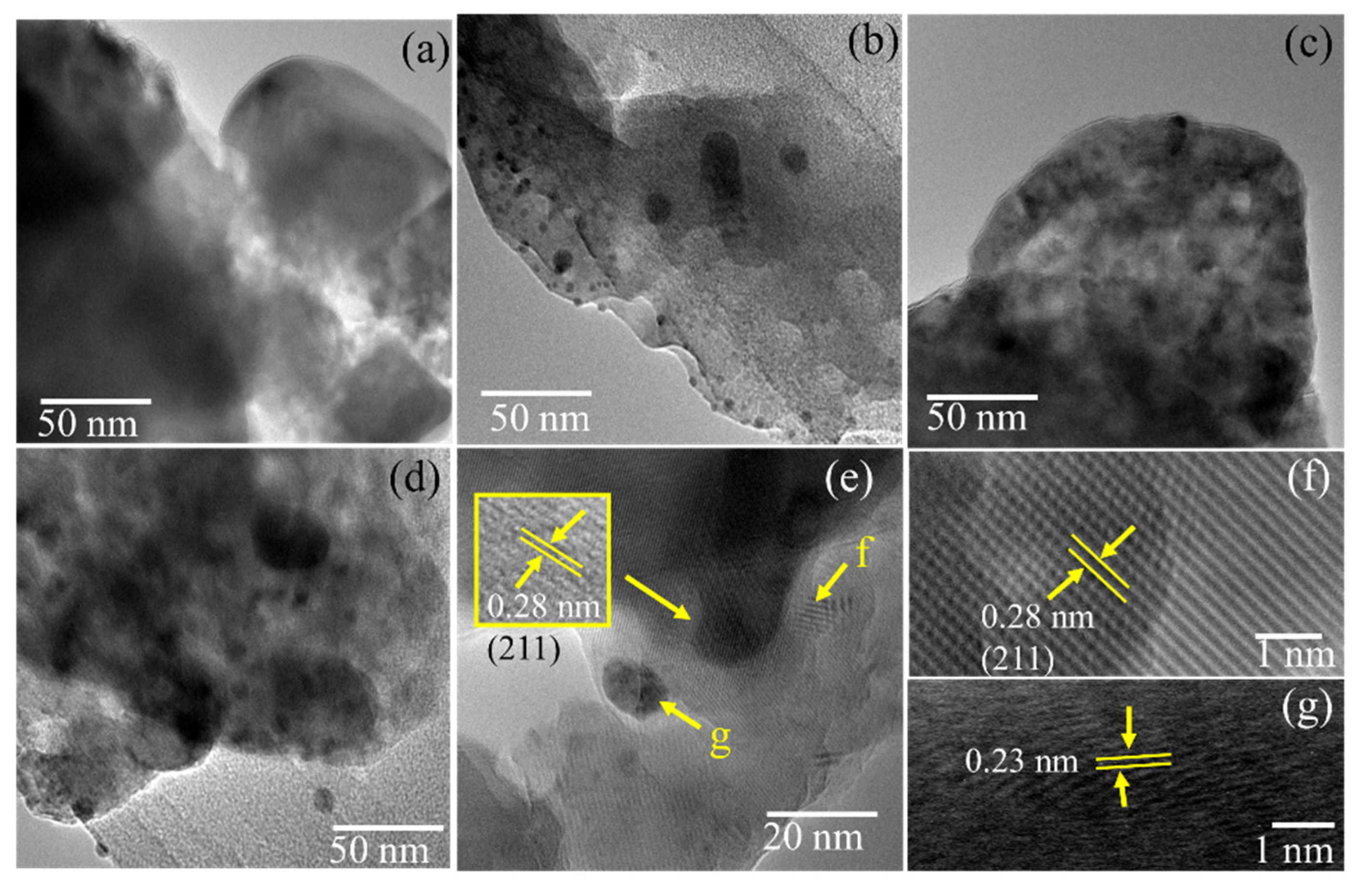
3.2. Characterization of Ag/HA
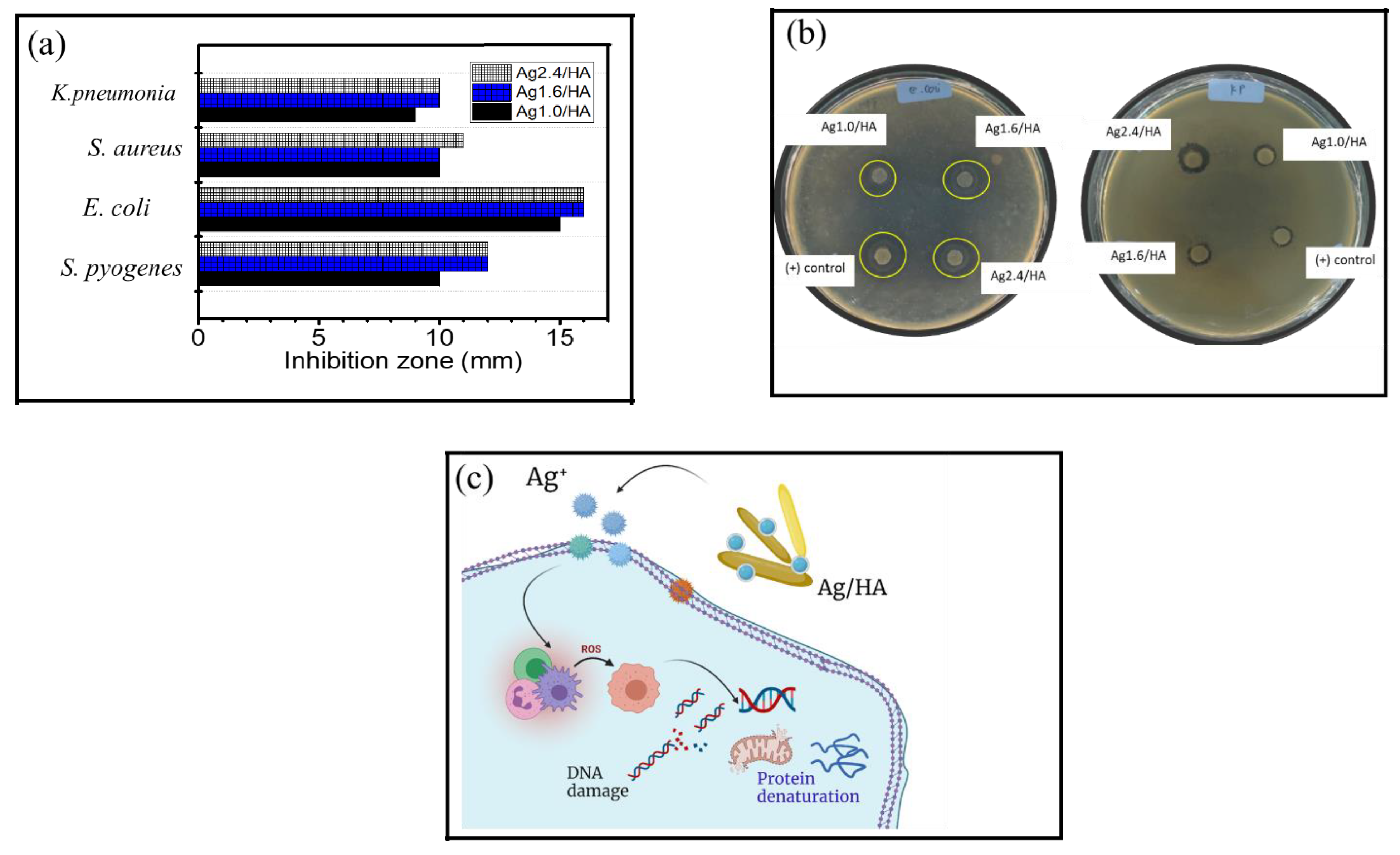
3.3. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Huang, C.; Cai, Y.; Chen, X.; Ke, Y. Silver-based nanocomposite for fabricating high performance value-added cotton. Cellulose 2021, 29, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Rey-Aira, B.; Gómez-Gratacòs, A.; Freire-Dapena, A.; Blanco-Méndez, J.; Luzardo-Álvarez, A.; Otero-Espinar, F. Hydroxyapatite functionalized with 1,2-hydroxypropyl-β-cyclodextrin as scaffolds for antibiotic release in bone tissue. Int. J. Clin. Pharmacol. Pharmacother. 2018, 3, IJCPP-135. [Google Scholar] [CrossRef] [Green Version]
- Che Hashim, N.; Nordin, D. Nano-hydroxyapatite powder for biomedical implant coating. Mater. Today Proc. 2019, 19, 1562–1571. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of silver doped hydroxyapatite thin films for biomedical applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Lett, J.A.; Sagadevan, S.; Paiman, S.; Mohammad, F.; Schirhagl, R.; Léonard, E.; Alshahateet, S.F.; Oh, W.C. Exploring the thumbprints of Ag-hydroxyapatite composite as a surface coating bone material for the implants. J. Mater. Res. Technol. 2020, 9, 12824–12833. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V. Bioceramic layers with antifungal properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef] [Green Version]
- Pongkitdachoti, U.; Unob, F. Silver-doped hydroxyapatite for formaldehyde determination by digital-image colorimetry. Anal. Methods 2022, 14, 926–934. [Google Scholar] [CrossRef]
- Alorku, K.; Manoj, M.; Yuan, A. A plant-mediated synthesis of nanostructured hydroxyapatite for biomedical applications: A review. RSC Adv. 2020, 10, 40923–40939. [Google Scholar] [CrossRef]
- Prasad, R.; Swamy, V.S. Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J. Nanoparticles 2013, 2013, 431218. [Google Scholar] [CrossRef] [Green Version]
- Moradi, F.; Sedaghat, S.; Arab-Salmanabadi, S.; Moradi, O. Biosynthesis of silver-montmorillonite nanocomposites using Ocimum Basilicum and Teucrium Polium; A comparative study. Mater. Res. Express 2019, 6, 125008. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Silver nanoparticles: Biosynthesis and antimicrobial potentialities. Int. J. Pharmacol. 2017, 13, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.; Amsari, A.; Siddiqui, N.N.; Ibrahim, F.; Abro, M.I.; Aman, A. Biosynthesis of silver nanoparticles for the fabrication of non cytotoxic and antibacterial metallic polymer based nanocomposite system. Sci. Rep. 2021, 11, 10500. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Laonapakul, T. Synthesis of hydroxyapatite from biogenic wastes. KKU Eng. J. 2015, 42, 269–275. [Google Scholar] [CrossRef]
- Kumar, G.S.; Sathish, L.; Govindan, R.; Girija, E.K. Utilization of snail shells to synthesise hydroxyapatite nanorods for orthopedic applications. RSC Adv. 2015, 5, 39544–39548. [Google Scholar] [CrossRef]
- Maghimaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111806. [Google Scholar] [CrossRef]
- Sankar, R.; Rahman, P.K.S.M.; Varunkumar, K.; Anusha, C.; Kalaiarasi, A.; Shivashangari, K.S.; Ravikumar, V. Facile synthesis of Curcuma longa tuber powder engineered metal nanoparticles for bioimaging applications. J. Mol. Struct. 2017, 1129, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Fatimah, I.; Aulia, G.R.; Puspitasari, W.; Nurillahi, R.; Sopia, L.; Herianto, R. Microwave-synthesized hydroxyapatite from paddy field snail (Pila ampullacea) shell for adsorption of bichromate ion. Sustain. Environ. Res. 2018, 28, 462–471. [Google Scholar] [CrossRef]
- Fatimah, I.; Fahrani, D.; Harmawantika, T.; Sahroni, I.; Kamari, A.; Rahmatillah, C.S.; Nurillahi, R. Functionalization of hydroxyapatite derived from cockle (Anadara granosa) shells into hydroxyapatite–nano TiO2 for photocatalytic degradation of methyl violet. Sustain. Environ. Res. 2019, 29, 40. [Google Scholar] [CrossRef] [Green Version]
- Ciobanu, G.; Ilisei, S.; Luca, C. Hydroxyapatite-silver nanoparticles coatings on porous polyurethane scaffold. Mater. Sci. Eng. C 2014, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sobczak-Kupiec, A.; Malina, D.; Kijkowska, R.; Florkiewicz, W.; Pluta, K.; Wzorek, Z. Hydroxyapatite/silver nanoparticles powders as antimicrobial agent for bone replacements. Croat. Chem. Acta 2019, 92, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Ghiasi, B.; Sefidbakht, Y.; Rezaei, M. Hydroxyapatite for Biomedicine and Drug Delivery; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 104, ISBN 9783030108342. [Google Scholar]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and applicationfor nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.R.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Y.; Huang, Y.; Zhu, P. Hydrothermal synthesis and biocompatibility study of highly crystalline carbonated hydroxyapatite nanorods. Nanoscale Res. Lett. 2015, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Saleem, O.; Wahaj, M.; Akhtar, M.A.; Ur Rehman, M.A. Fabrication and characterization of Ag-Sr-substituted hydroxyapatite/chitosan coatings deposited via electrophoretic deposition: A design of experiment study. ACS Omega 2020, 5, 22984–22992. [Google Scholar] [CrossRef]
- Sivaraj, D.; Vijayalakshmi, K. Enhanced antibacterial and corrosion resistance properties of Ag substituted hydroxyapatite/functionalized multiwall carbon nanotube nanocomposite coating on 316L stainless steel for biomedical application. Ultrason. Sonochem. 2019, 59, 104730. [Google Scholar] [CrossRef]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Katas, H.; Lim, C.S.; Nor Azlan, A.Y.H.; Buang, F.; Mh Busra, M.F. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Constantin, L.V.; Predoi, D. Antibacterial activity of silver-doped hydroxyapatite nanoparticles against gram-positive and gram-negative bacteria. Nanoscale Res. Lett. 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcaro, F.; Carlini, L.; Ugolini, A.; Visaggio, D.; Visca, P.; Fratoddi, I.; Venditti, I.; Meneghini, C.; Simonelli, L.; Marini, C.; et al. Synthesis and structural characterization of silver nanoparticles stabilized with 3-mercapto-1-propansulfonate and 1-thioglucose mixed thiols for antibacterial applications. Materials 2016, 9, 1028. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauz, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [Green Version]
- Citradewi, P.W.; Hidayat, H.; Purwiandono, G.; Fatimah, I.; Sagadevan, S. Clitorea ternatea-mediated silver nanoparticle-doped hydroxyapatite derived from cockle shell as antibacterial material. Chem. Phys. Lett. 2021, 769, 138412. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, L.; Yu, X.; Lei, S.; Qi, Y.; Cui, C. Synthesis of silver modified hydroxyapatite nanoparticle and evaluation of its biological properties in vitro for potential biomedical application. J. Ceram. Soc. Jpn. 2021, 129, 443–452. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, H.T.; Pham, N.T. Green synthesis and antibacterial activity of HAp@Ag nanocomposite using Centella asiatica (L.) urban extract and eggshell. Int. J. Biomater. 2020, 2020, 8841221. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Rathi, M.A.; Almaary, K.S.; Alkhattaf, F.S.; Elbadawi, Y.B.; Chang, S.W.; Ravindran, B. Preparation and antibacterial application of hydroxyapatite doped Silver nanoparticles derived from chicken bone. J. King Saud Univ.-Sci. 2022, 34, 101749. [Google Scholar] [CrossRef]
- Silva-Holguín, P.N.; Reyes-López, S.Y. Synthesis of hydroxyapatite-ag composite as antimicrobial agent. Dose-Response 2020, 18, 1559325820951342. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Gu, X.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Synthesis of silver nanoparticles-decorated hydroxyapatite (HA@Ag) poriferous nanocomposites and the study of their antibacterial activities. RSC Adv. 2018, 8, 41722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadalannagari, S.; Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Antimicrobial activity of hemocompatible silver doped hydroxyapatite nanoparticles synthesized by modified sol–gel technique. Appl. Nanosci. 2014, 4, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Wilcock, C.J.; Stafford, G.P.; Miller, C.A.; Ryabenkova, Y.; Fatima, M.; Gentile, P.; Möbus, G.; Hatton, P.V. Preparation and antibacterial properties of silver-doped nanoscale hydroxyapatite pastes for bone repair and augmentation. J. Biomed. Nanotechnol. 2017, 13, 1168–1176. [Google Scholar] [CrossRef] [PubMed]



| Sample | MIC (μg/mL) | ||||
|---|---|---|---|---|---|
| Ag1.0/HA | Ag1.6/HA | Ag2.4/HA | HA | AgNPs | |
| E. coli | 5 | 10 | 10 | 100 | 2 |
| S. aureus | 20 | 10 | 10 | 100 | 2 |
| K. pneumonia | 20 | 20 | 20 | 100 | 5 |
| S. pyogenes | 40 | 20 | 20 | 100 | 5 |
| Synthesis Method of Ag/HA | Remark | Reference |
|---|---|---|
| Ag/HA synthesized by using gallic acid as bioreductor | The material showed an inhibition zone of around 15 mm for E. coli, and 9.5 mm for S. aureus at the concentration of 5mM | [42] |
| Ag/HA synthesized by 8%wt. of Ag content | The material exhibited an inhibition zone of 10 mm for E. coli and 18 mm for S. aureus at the concentration of 20 μg/mL | [40] |
| Ag/HA synthesized by using Clitoria ternatea as bioreductor | The material showed an inhibition zone of around 12 mm for E. coli, and 12 mm for S. aureus at the concentration of 20 μg/mL | [37] |
| Ag/HA synthesized by using sol-gel method | The MIC towards E. coli, and S. aureus are 15 and 25 μg/mL, respectively | [44] |
| Ag/HA synthesized by using black Sumatra chicken shell | The MIC towards E. coli, and S. aureus are 72 and 45 μg/mL, respectively | [41] |
| Ag/HA synthesized by rapid mixing method | The MIC towards S. aureus is 2.5 μg/mL for the Ag content of 2 % wt. | [45] |
| Ag/HA synthesized by using hydrothermal method | The MIC towards S. aureus is 7.85 μg/mL and the MIC towards E. coli is 3.9 μg/mL by the Ag:HA molar ratio of 3:10. | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, I.; Hidayat, H.; Purwiandono, G.; Khoirunisa, K.; Zahra, H.A.; Audita, R.; Sagadevan, S. Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste. J. Funct. Biomater. 2022, 13, 84. https://doi.org/10.3390/jfb13020084
Fatimah I, Hidayat H, Purwiandono G, Khoirunisa K, Zahra HA, Audita R, Sagadevan S. Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste. Journal of Functional Biomaterials. 2022; 13(2):84. https://doi.org/10.3390/jfb13020084
Chicago/Turabian StyleFatimah, Is, Habibi Hidayat, Gani Purwiandono, Khoirunisa Khoirunisa, Hasna Azizah Zahra, Rahmania Audita, and Suresh Sagadevan. 2022. "Green Synthesis of Antibacterial Nanocomposite of Silver Nanoparticle-Doped Hydroxyapatite Utilizing Curcuma longa Leaf Extract and Land Snail (Achatina fulica) Shell Waste" Journal of Functional Biomaterials 13, no. 2: 84. https://doi.org/10.3390/jfb13020084








