Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases
Abstract
:1. Introduction
2. Blood-Brain Barrier (BBB)
2.1. Passive and Active Diffusion across the BBB
2.2. Transcytosis across the BBB
3. Biopolymer Nanoparticles (NPs) in the Treatment of CNS Diseases
Biopolymer NP Penetration Mechanisms

| Ligand | Penetration Mechanism | Ref. |
|---|---|---|
| Albumin | Adsorption-mediated endocytosis | [63,69] |
| TAT peptide | Adsorption-mediated endocytosis | [63,64,65,66,67] |
| Insulin | Receptor-mediated endocytosis | [12,63,70,71] |
| ApoE | Receptor-mediated endocytosis | [63,72,73] |
| Transferrin | Receptor-mediated endocytosis | [63,74] |
| LDL | Receptor-mediated endocytosis | [63,75] |
| Glutathione | Receptor-mediated endocytosis | [63,76,77] |
| OX26 | Receptor-mediated endocytosis | [63,78,79] |
4. Nose-to-Brain (NtB) Drug Delivery
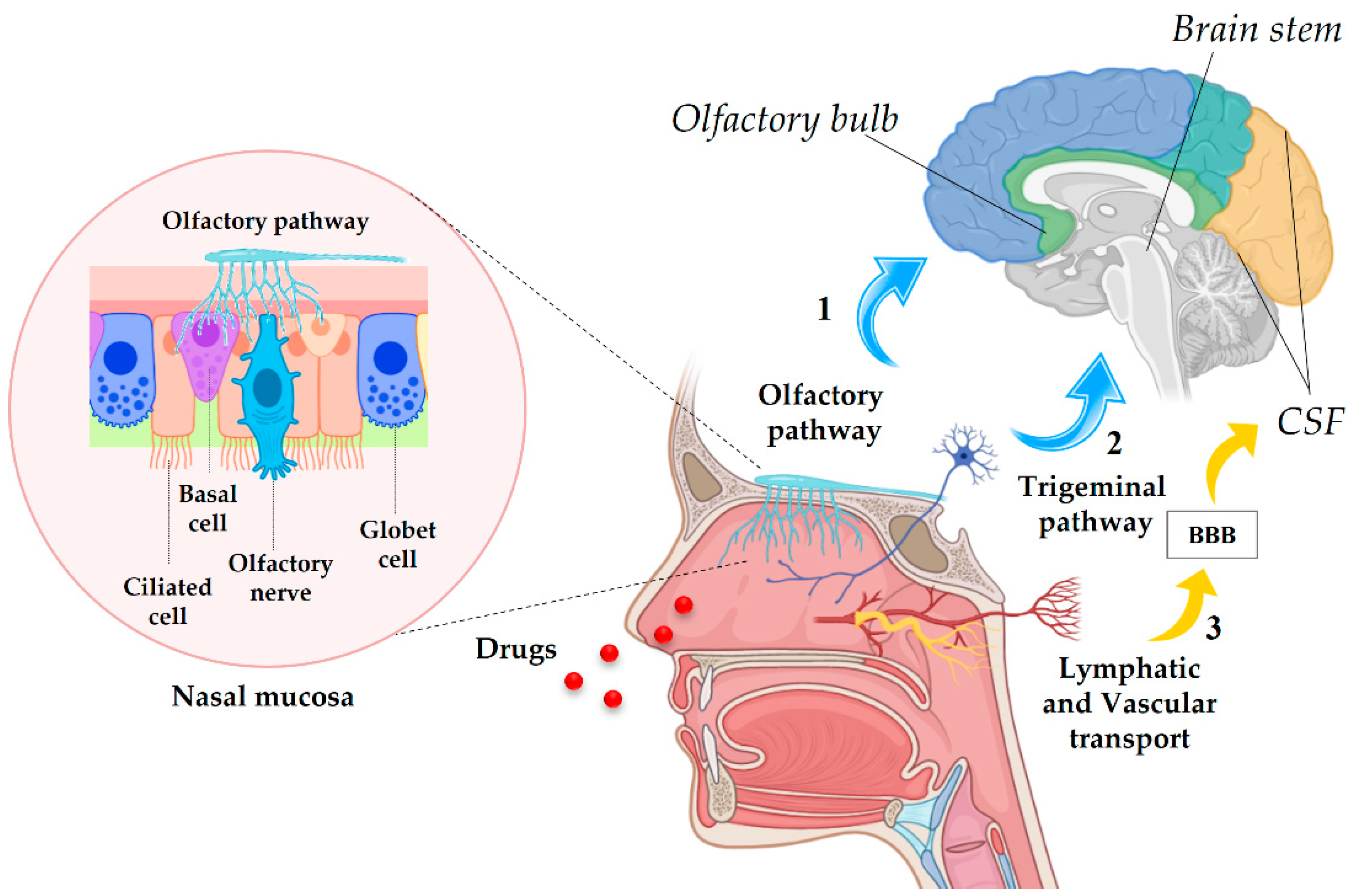
4.1. Anatomy of the Nasal Cavity and Drug Delivery Pathways
4.2. NPs through the NtB Route
5. NtB Drug Delivery for the Treatment of Neurological Diseases
6. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central Nervous System Delivery of Molecules across the Blood–Brain Barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern Methods for Delivery of Drugs across the Blood–Brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Polchi, A.; Magini, A.; Mazuryk, J.; Tancini, B.; Gapiński, J.; Patkowski, A.; Giovagnoli, S.; Emiliani, C. Rapamycin Loaded Solid Lipid Nanoparticles as a New Tool to Deliver MTOR Inhibitors: Formulation and in Vitro Characterization. Nanomaterials 2016, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid Lipid Nanoparticles as a Drug Delivery System for the Treatment of Neurodegenerative Diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for Drug Delivery: The Need for Precision in Reporting Particle Size Parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart Polymers for the Controlled Delivery of Drugs—A Concise Overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Khanal, S.; Adhikari, U.; Rijal, N.P.; Bhattarai, S.R.; Sankar, J.; Bhattarai, N. PH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. J. Funct. Biomater. 2016, 7, 21. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; Van Gool, S.W.; De Vleeschouwer, S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers 2013, 5, 1020–1048. [Google Scholar] [CrossRef]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in Brain Targeting Drug Delivery System by Nasal Route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Akel, H.; Ismail, R.; Csóka, I. Progress and Perspectives of Brain-Targeting Lipid-Based Nanosystems via the Nasal Route in Alzheimer’s Disease. Eur. J. Pharm. Biopharm. 2020, 148, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-Brain Drug Delivery: An Update on Clinical Challenges and Progress towards Approval of Anti-Alzheimer Drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Egleton, R.D. Pathophysiology of the Blood–Brain Barrier: Animal Models and Methods. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 80, pp. 277–309. [Google Scholar]
- Zlokovic, B.V. The Blood–Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of Drugs across the Blood–Brain Barrier by Nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood–Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Singh, N.; Ecker, G.F. Insights into the Structure, Function, and Ligand Discovery of the Large Neutral Amino Acid Transporter 1, LAT1. Int. J. Mol. Sci. 2018, 19, 1278. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood–Brain Barrier Active Efflux Transporters: ATP-Binding Cassette Gene Family. NeuroRX 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug Resistance in Brain Diseases and the Role of Drug Efflux Transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative Targeted Absolute Proteomics of Human Blood–Brain Barrier Transporters and Receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, X.; Hu, K. 8-Efflux Pump Inhibition to Enhance Brain Targeting Delivery. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–196. ISBN 978-0-12-814001-7. [Google Scholar]
- Fernandes, C.; Martins, C.; Fonseca, A.; Nunes, R.; Matos, M.J.; Silva, R.; Garrido, J.; Sarmento, B.; Remião, F.; Otero-Espinar, F.J.; et al. PEGylated PLGA Nanoparticles As a Smart Carrier to Increase the Cellular Uptake of a Coumarin-Based Monoamine Oxidase B Inhibitor. ACS Appl. Mater. Interfaces 2018, 10, 39557–39569. [Google Scholar] [CrossRef] [PubMed]
- Irudayanathan, F.J.; Wang, N.; Wang, X.; Nangia, S. Architecture of the Paracellular Channels Formed by Claudins of the Blood–Brain Barrier Tight Junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into Rather Unexplored Terrain—Transcellular Transport across the Blood–Brain Barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef]
- Freskgård, P.-O.; Urich, E. Antibody Therapies in CNS Diseases. Neuropharmacology 2017, 120, 38–55. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, Y.; Tan, Y.-Z.; Hu, K.-L.; Jiang, X.-G.; Fu, S.-K. Cationic Albumin-Conjugated Pegylated Nanoparticles as Novel Drug Carrier for Brain Delivery. J. Control. Release 2005, 107, 428–448. [Google Scholar] [CrossRef]
- Kaksonen, M.; Roux, A. Mechanisms of Clathrin-Mediated Endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Omori, K.; Tachikawa, M.; Hirose, S.; Taii, A.; Akanuma, S.; Hosoya, K.; Terasaki, T. Developmental Changes in Transporter and Receptor Protein Expression Levels at the Rat Blood–Brain Barrier Based on Quantitative Targeted Absolute Proteomics. Drug Metab. Pharmacokinet. 2020, 35, 117–123. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Y.; Li, C.; Qian, M.; Huang, R. Receptor-Mediated Drug Delivery Systems Targeting to Glioma. Nanomaterials 2016, 6, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood–Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug Delivery across the Blood–Brain Barrier: Recent Advances in the Use of Nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The Blood–Brain Barrier: Physiology and Strategies for Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery across the Blood–Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated Brain Drug Delivery: Overcoming Blood–Brain Barrier to Treat Neurodegenerative Diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Bhatt, P.; Lalani, R.; Mashru, R.; Misra, A. Abstract 2065: Anti-FSHR Antibody Fab’ Fragment Conjugated Immunoliposomes Loaded with Cyclodextrin-Paclitaxel Complex for Improved in Vitro Efficacy on Ovarian Cancer Cells. Cancer Res. 2016, 76, 2065. [Google Scholar] [CrossRef]
- Vhora, I.; Patil, S.; Bhatt, P.; Misra, A. Chapter One—Protein- and Peptide-Drug Conjugates: An Emerging Drug Delivery Technology. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 98, pp. 1–55. [Google Scholar]
- Letchford, K.; Burt, H. A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, L.; Chao, Z.; Shao, C.; Chen, Z.; Wei, Z.; Lu, J.; Huang, Y.; Li, L.; Liu, Q.; et al. Organic Spherical Nucleic Acids for the Transport of a NIR-II-Emitting Dye across the Blood–Brain Barrier. Angew. Chem. Int. Ed. 2020, 59, 9702–9710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tian, D.; Gao, P.; Wang, K.; Li, Y.; Shu, X.; Zhu, J.; Zhao, Q. Cell-Penetrating Peptides Transport Noncovalently Linked Thermally Activated Delayed Fluorescence Nanoparticles for Time-Resolved Luminescence Imaging. J. Am. Chem. Soc. 2018, 140, 17484–17491. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, K.A.; Cu, Y.; Booth, C.J.; Saucier-Sawyer, J.K.; Wood, M.J.; Mark Saltzman, W. Intravaginal Gene Silencing Using Biodegradable Polymer Nanoparticles Densely Loaded with Small-Interfering RNA. Nat. Mater. 2009, 8, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, M.J.; Mark Saltzman, W.; Mellman, I.; Ledizet, M.; Fikrig, E.; Flavell, R.A.; et al. Inflammasome-Activating Nanoparticles as Modular Systems for Optimizing Vaccine Efficacy. Vaccine 2009, 27, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Emiliani, C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. J. Funct. Biomater. 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Pellegrino, R.M.; Emiliani, C. HexA-Enzyme Coated Polymer Nanoparticles for the Development of a Drug-Delivery System in the Treatment of Sandhoff Lysosomal Storage Disease. J. Funct. Biomater. 2022, 13, 37. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Torchilin, V.P. Drug Targeting. Eur. J. Pharm. Sci. 2000, 11, S81–S91. [Google Scholar] [CrossRef]
- Claudio, P.; Reatul, K.; Brigitte, E.; Geraldine, P. Chapter 10—Drug-Delivery Nanocarriers to Cross the Blood–Brain Barrier. In Nanobiomaterials in Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 333–370. ISBN 978-0-323-42866-8. [Google Scholar]
- Sabbagh, F.; Kim, B.S. Recent Advances in Polymeric Transdermal Drug Delivery Systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef]
- Lammers, T.; Subr, V.; Ulbrich, K.; Hennink, W.E.; Storm, G.; Kiessling, F. Polymeric Nanomedicines for Image-Guided Drug Delivery and Tumor-Targeted Combination Therapy. Nano Today 2010, 5, 197–212. [Google Scholar] [CrossRef]
- Grund, S.; Bauer, M.; Fischer, D. Polymers in Drug Delivery—State of the Art and Future Trends. Adv. Eng. Mater. 2011, 13, B61–B87. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for Diagnostics, Drug Delivery and Targeted Treatment across Blood–Brain Barrier: Perspectives on Tracking and Neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the Blood–Brain Barrier: Advances in Nanoparticle Technology for Drug Delivery in Neuro-Oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for Small Molecule Delivery to the Central Nervous System across the Blood–Brain Barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef]
- Nelson, C.E.; Kintzing, J.R.; Hanna, A.; Shannon, J.M.; Gupta, M.K.; Duvall, C.L. Balancing Cationic and Hydrophobic Content of PEGylated SiRNA Polyplexes Enhances Endosome Escape, Stability, Blood Circulation Time, and Bioactivity in Vivo. ACS Nano 2013, 7, 8870–8880. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle Transport across the Blood Brain Barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef]
- Pulicherla, K.K.; Verma, M.K. Targeting Therapeutics across the Blood Brain Barrier (BBB), Prerequisite towards Thrombolytic Therapy for Cerebrovascular Disorders—An Overview and Advancements. AAPS PharmSciTech 2015, 16, 223–233. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS Delivery via Adsorptive Transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Cano, A.; Sánchez-López, E.; Ettcheto, M.; López-Machado, A.; Espina, M.; Souto, E.B.; Galindo, R.; Camins, A.; García, M.L.; Turowski, P. Current Advances in the Development of Novel Polymeric Nanoparticles for the Treatment of Neurodegenerative Diseases. Nanomedicine 2020, 15, 1239–1261. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Lu, W. Adsorptive-Mediated Brain Delivery Systems. Curr. Pharm. Biotechnol. 2012, 13, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Kucharz, K.; Fernandes, E.F.A.; Strømgaard, K.; Nielsen, M.S.; Helms, H.C.C.; Bach, A.; Tofte-Hansen, M.U.; Garcia, B.I.A.; Lauritzen, M.; et al. Conjugation of Therapeutic PSD-95 Inhibitors to the Cell-Penetrating Peptide Tat Affects Blood–Brain Barrier Adherence, Uptake, and Permeation. Pharmaceutics 2020, 12, 661. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Feng, Y.; Guan, Y. A Novel Botulinum Toxin TAT-EGFP-HCS Fusion Protein Capable of Specific Delivery through the Blood–Brain Barrier to the Central Nervous System. CNS Neurol. Disord.—Drug Targets 2019, 18, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics across the Blood–Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef]
- Lozić, I.; Hartz, R.V.; Bartlett, C.A.; Shaw, J.A.; Archer, M.; Naidu, P.S.R.; Smith, N.M.; Dunlop, S.A.; Iyer, K.S.; Kilburn, M.R.; et al. Enabling Dual Cellular Destinations of Polymeric Nanoparticles for Treatment Following Partial Injury to the Central Nervous System. Biomaterials 2016, 74, 200–216. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Motiei, M.; Popovtzer, R. Insulin-Coated Gold Nanoparticles as an Effective Approach for Bypassing the Blood–Brain Barrier. In Proceedings of the Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XVI, SPIE, San Francisco, CA, USA, 5 March 2019; Volume 10891, pp. 183–192. [Google Scholar]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The Effect of Nanoparticle Size on the Ability to Cross the Blood–Brain Barrier: An in Vivo Study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Wagner, S.; Zensi, A.; Wien, S.L.; Tschickardt, S.E.; Maier, W.; Vogel, T.; Worek, F.; Pietrzik, C.U.; Kreuter, J.; von Briesen, H. Uptake Mechanism of ApoE-Modified Nanoparticles on Brain Capillary Endothelial Cells as a Blood–Brain Barrier Model. PLoS ONE 2012, 7, e32568. [Google Scholar] [CrossRef]
- Hartl, N.; Adams, F.; Merkel, O.M. From Adsorption to Covalent Bonding: Apolipoprotein E Functionalization of Polymeric Nanoparticles for Drug Delivery Across the Blood–Brain Barrier. Adv. Ther. 2021, 4, 2000092. [Google Scholar] [CrossRef]
- Chang, J.; Jallouli, Y.; Kroubi, M.; Yuan, X.; Feng, W.; Kang, C.; Pu, P.; Betbeder, D. Characterization of Endocytosis of Transferrin-Coated PLGA Nanoparticles by the Blood–Brain Barrier. Int. J. Pharm. 2009, 379, 285–292. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate Systems for Brain Delivery of Drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Grover, A.; Hirani, A.; Pathak, Y.; Sutariya, V. Brain-Targeted Delivery of Docetaxel by Glutathione-Coated Nanoparticles for Brain Cancer. AAPS PharmSciTech 2014, 15, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Meszaros, M.; Kiss, L.; Kota, Z.; Pali, T.; Hoyk, Z.; Bozso, Z.; Fulop, L.; Toth, A.; Rakhely, G.; et al. Biotin and Glutathione Targeting of Solid Nanoparticles to Cross Human Brain Endothelial Cells. Curr. Pharm. Des. 2017, 23, 4198–4205. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, K.; Couvreur, P. Polyalkylcyanoacrylate Nanoparticles for Delivery of Drugs across the Blood–Brain Barrier. WIREs Nanomed. Nanobiotechnol. 2009, 1, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, L.; Deng, G.; Liu, J.; Chen, Q.; Chen, Z. Systemic Delivery to Central Nervous System by Engineered PLGA Nanoparticles. Am. J. Transl. Res. 2016, 8, 749–764. [Google Scholar] [PubMed]
- Sawyer, A.J.; Saucier-Sawyer, J.K.; Booth, C.J.; Liu, J.; Patel, T.; Piepmeier, J.M.; Saltzman, W.M. Convection-Enhanced Delivery of Camptothecin-Loaded Polymer Nanoparticles for Treatment of Intracranial Tumors. Drug Deliv. Transl. Res. 2011, 1, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiong, S.; Liu, P.; Liu, W.; Wang, Q.; Liu, Y.; Tan, H.; Chen, X.; Shi, X.; Wang, Q.; et al. Polymeric Nanoparticles-Based Brain Delivery with Improved Therapeutic Efficacy of Ginkgolide B in Parkinson’s Disease. Int. J. Nanomed. 2020, 15, 10453–10467. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Mittal, G.; Carswell, H.; Brett, R.; Currie, S.; Kumar, M.N.V.R. Development and Evaluation of Polymer Nanoparticles for Oral Delivery of Estradiol to Rat Brain in a Model of Alzheimer’s Pathology. J. Control. Release 2011, 150, 220–228. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, C.; Wang, L.; Xiang, Y.; Zhang, W.; Li, Y.; Zhuang, X. Comparable Intestinal and Hepatic First-Pass Effect of YL-IPA08 on the Bioavailability and Effective Brain Exposure, a Rapid Anti-PTSD and Anti-Depression Compound. Front. Pharmacol. 2020, 11, 588127. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The Nasal Approach to Delivering Treatment for Brain Diseases: An Anatomic, Physiologic, and Delivery Technology Overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef]
- Warnken, Z.N.; Smyth, H.D.C.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and Device Design to Increase Nose to Brain Drug Delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Emad, N.A.; Ahmed, B.; Alhalmi, A.; Alzobaidi, N.; Al-Kubati, S.S. Recent Progress in Nanocarriers for Direct Nose to Brain Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 64, 102642. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal Delivery of Biologics to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.D.; Frey, W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal Treatment of Central Nervous System Dysfunction in Humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef]
- Thorne, R.G.; Emory, C.R.; Ala, T.A.; Frey, W.H. Quantitative Analysis of the Olfactory Pathway for Drug Delivery to the Brain. Brain Res. 1995, 692, 278–282. [Google Scholar] [CrossRef]
- Frey, W.H.; Liu, J.; Chen, X.; Thorne, R.G.; Fawcett, J.R.; Ala, T.A.; Rahman, Y.-E. Delivery of 125I-NGF to the Brain via the Olfactory Route. Drug Deliv. 1997, 4, 87–92. [Google Scholar] [CrossRef]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Bernhard, F.; Verleysdonk, S.; Buadze, M.; Lourhmati, A.; Klopfer, T.; Schaumann, F.; Schmid, B.; et al. Therapeutic Efficacy of Intranasally Delivered Mesenchymal Stem Cells in a Rat Model of Parkinson Disease. Rejuvenation Res. 2011, 14, 3–16. [Google Scholar] [CrossRef]
- Reiss, C.S.; Plakhov, I.V.; Komatsu, T. Viral Replication in Olfactory Receptor Neurons and Entry into the Olfactory Bulb and Braina. Ann. N. Y. Acad. Sci. 1998, 855, 751–761. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Intranasal Mucoadhesive Microemulsions of Clonazepam: Preliminary Studies on Brain Targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal Delivery to the Central Nervous System: Mechanisms and Experimental Considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Illum, L. Transport of Drugs from the Nasal Cavity to the Central Nervous System. Eur. J. Pharm. Sci 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Direct Nose to Brain Drug Delivery via Integrated Nerve Pathways Bypassing the Blood–Brain Barrier: An Excellent Platform for Brain Targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for Direct Nose-to-Brain Delivery of Drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chi, N.; Tang, X. Preparation of Estradiol Chitosan Nanoparticles for Improving Nasal Absorption and Brain Targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, K.R.; Bhatnagar, A.; Nishad, D.K.; Singh, T.; Gabrani, R.; Sharma, S.K.; Ali, J.; Dang, S. Nose to Brain Delivery of Midazolam Loaded PLGA Nanoparticles: In Vitro and In Vivo Investigations. Curr. Drug Deliv. 2016, 13, 557–564. [Google Scholar] [CrossRef]
- Haque, S.U.; Nasar, A.; Inamuddin, R.; Rahman, M.M. Applications of Chitosan (CHI)-Reduced Graphene Oxide (RGO)-Polyaniline (PAni) Conducting Composite Electrode for Energy Generation in Glucose Biofuel Cell. Sci. Rep. 2020, 10, 10428. [Google Scholar] [CrossRef]
- Illum, L. Nanoparticulate Systems for Nasal Delivery of Drugs: A Real Improvement over Simple Systems? J. Pharm. Sci. 2007, 96, 473–483. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Asfour, H.Z. Neuroprotective and Antioxidant Effect of Naringenin-Loaded Nanoparticles for Nose-to-Brain Delivery. Brain Sci. 2019, 9, 275. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Naki, T. Chitosan-Based Nanocarriers for Nose to Brain Delivery. Appl. Sci. 2019, 9, 2219. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-Based Drug Nanocarriers: Where Do We Stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Tzeyung, A.S.; Md, S.; Bhattamisra, S.K.; Madheswaran, T.; Alhakamy, N.A.; Aldawsari, H.M.; Radhakrishnan, A.K. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and Chitosan Coating Nanoparticles for the Treatment of Brain Disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Afshari, R.; Khalaji, S.; Rabiee, M.; Bagherzadeh, M.; Fatahi, Y.; Dinarvand, R.; Tahriri, M.; Tayebi, L.; et al. Polymeric Nanoparticles for Nasal Drug Delivery to the Brain: Relevance to Alzheimer’s Disease. Adv. Ther. 2021, 4, 2000076. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, C.; Shao, X.; Liu, Q.; Qian, Y.; Feng, L.; Chen, J.; Zha, Y.; Zhang, Q.; Jiang, X. Enhancement of Nose-to-Brain Delivery of Basic Fibroblast Growth Factor for Improving Rat Memory Impairments Induced by Co-Injection of β-Amyloid and Ibotenic Acid into the Bilateral Hippocampus. Int. J. Pharm. 2012, 423, 226–234. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s Disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Kandil, L.S.; Farid, R.M.; ElGamal, S.S.; Hanafy, A.S. Intranasal Galantamine/Chitosan Complex Nanoparticles Elicit Neuroprotection Potentials in Rat Brains via Antioxidant Effect. Drug Dev. Ind. Pharm. 2021, 47, 735–740. [Google Scholar] [CrossRef]
- Craparo, E.F.; Musumeci, T.; Bonaccorso, A.; Pellitteri, R.; Romeo, A.; Naletova, I.; Cucci, L.M.; Cavallaro, G.; Satriano, C. MPEG-PLGA Nanoparticles Labelled with Loaded or Conjugated Rhodamine-B for Potential Nose-to-Brain Delivery. Pharmaceutics 2021, 13, 1508. [Google Scholar] [CrossRef]
- Abd-algaleel, S.A.; Metwally, A.A.; Abdel-Bar, H.M.; Kassem, D.H.; Hathout, R.M. Synchronizing In Silico, In Vitro, and In Vivo Studies for the Successful Nose to Brain Delivery of an Anticancer Molecule. Mol. Pharm. 2021, 18, 3763–3776. [Google Scholar] [CrossRef]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K.; et al. Bioengineered PLGA-Chitosan Nanoparticles for Brain Targeted Intranasal Delivery of Antiepileptic TRH Analogues. Chem. Eng. J. 2018, 346, 630–639. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.; Chen, Z.-T.; Liao, T.-Y.; Lin, C.; Shen, H.C.-H.; Wang, Y.-H.; Chang, C.-W.; Liu, R.-S.; Chen, R.P.-Y.; Tu, P.-H. An Intranasally Delivered Peptide Drug Ameliorates Cognitive Decline in Alzheimer Transgenic Mice. EMBO Mol. Med. 2017, 9, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.L.; Koerber, J.T.; Pack, D.W. A Degradable Polyethylenimine Derivative with Low Toxicity for Highly Efficient Gene Delivery. Bioconjug. Chem. 2003, 14, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-Loaded Nanostructured Lipid Carriers (NLCs) for Nasal Administration: Design, Characterization, and in Vivo Study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of Physicochemical Properties on Intranasal Nanoparticle Transit into Murine Olfactory Epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef]
- Gartziandia, O.; Egusquiaguirre, S.P.; Bianco, J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M.; Préat, V.; Beloqui, A. Nanoparticle Transport across in Vitro Olfactory Cell Monolayers. Int. J. Pharm. 2016, 499, 81–89. [Google Scholar] [CrossRef]
- Calzoni, E.; Argentati, C.; Cesaretti, A.; Montegiove, N.; Tortorella, I.; Bazzucchi, M.; Morena, F.; Martino, S.; Emiliani, C. RNA Modifications in Neurodegenerations. In Epitranscriptomics; Jurga, S., Barciszewski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 23–77. ISBN 978-3-030-71612-7. [Google Scholar]
- Md, S.; Khan, R.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine Loaded Chitosan Nanoparticles Intended for Direct Nose to Brain Delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy Study in Mice Model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J.; Pardeshi, C.V. Nanotechnology-Mediated Nose to Brain Drug Delivery for Parkinson’s Disease: A Mini Review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; Helmy, M.W.; ElGamal, S.S. Pharmacological, Toxicological and Neuronal Localization Assessment of Galantamine/Chitosan Complex Nanoparticles in Rats: Future Potential Contribution in Alzheimer’s Disease Management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Farid, R.M.; ElGamal, S.S. Complexation as an Approach to Entrap Cationic Drugs into Cationic Nanoparticles Administered Intranasally for Alzheimer’s Disease Management: Preparation and Detection in Rat Brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.L.; et al. The Nasal Delivery of Nanoencapsulated Statins—An Approach for Brain Delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, P.Y.; Kondrasheva, I.G.; Severin, E.S.; Guseva, A.A.; Kamensky, A.A. Increasing the Efficiency of Parkinson’s Disease Treatment Using a Poly (Lactic-Co-Glycolic Acid) (PLGA) Based L-DOPA Delivery System. Exp. Neurobiol. 2014, 23, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal Nanoparticles of Basic Fibroblast Growth Factor for Brain Delivery to Treat Alzheimer’s Disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Jafarieh, O.; Md, S.; Ali, M.; Baboota, S.; Sahni, J.K.; Kumari, B.; Bhatnagar, A.; Ali, J. Design, Characterization, and Evaluation of Intranasal Delivery of Ropinirole-Loaded Mucoadhesive Nanoparticles for Brain Targeting. Drug Dev. Ind. Pharm. 2015, 41, 1674–1681. [Google Scholar] [CrossRef]
- Sharma, S.; Lohan, S.; Murthy, R.S.R. Formulation and Characterization of Intranasal Mucoadhesive Nanoparticulates and Thermo-Reversible Gel of Levodopa for Brain Delivery. Drug Dev. Ind. Pharm. 2014, 40, 869–878. [Google Scholar] [CrossRef]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal Delivery of Nanoparticle Encapsulated Tarenflurbil: A Potential Brain Targeting Strategy for Alzheimer’s Disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain Delivery of Vasoactive Intestinal Peptide Enhanced with the Nanoparticles Conjugated with Wheat Germ Agglutinin Following Intranasal Administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-Modified PEG-Co-PCL Nanoparticles for Enhanced Brain Delivery of NAP Peptide Following Intranasal Administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal Delivery of Huperzine A to the Brain Using Lactoferrin-Conjugated N-Trimethylated Chitosan Surface-Modified PLGA Nanoparticles for Treatment of Alzheimer’s Disease. IJN 2018, 13, 705–718. [Google Scholar] [CrossRef]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Enriched Chitosan Nanoparticles Loaded with SiRNA Are Effective in Lowering Huntington’s Disease Gene Expression Following Intranasal Administration. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102119. [Google Scholar] [CrossRef] [PubMed]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Passoni, A.; Favagrossa, M.; Duskey, J.T.; Bombaci, M.; Vandelli, M.A.; Colombo, L.; Bagnati, R.; et al. Insights into Kinetics, Release, and Behavioral Effects of Brain-Targeted Hybrid Nanoparticles for Cholesterol Delivery in Huntington’s Disease. J. Control. Release 2021, 330, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Schuh, R.S.; Bidone, J.; Poletto, E.; Pinheiro, C.V.; Pasqualim, G.; de Carvalho, T.G.; Farinon, M.; da Silva Diel, D.; Xavier, R.M.; Baldo, G.; et al. Nasal Administration of Cationic Nanoemulsions as Nucleic Acids Delivery Systems Aiming at Mucopolysaccharidosis Type I Gene Therapy. Pharm. Res. 2018, 35, 221. [Google Scholar] [CrossRef] [PubMed]
- Bruinsmann, F.A.; Vaz, G.R.; Alves, A.D.C.S.; Aguirre, T.; Pohlmann, A.R.; Guterres, S.S.; Sonvico, F. Nasal Drug Delivery of Anticancer Drugs for the Treatment of Glioblastoma: Preclinical and Clinical Trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, W. Recent Advances in Brain Tumor-Targeted Nano-Drug Delivery Systems. Expert Opin. Drug Deliv. 2012, 9, 671–686. [Google Scholar] [CrossRef]
- Adjei, I.M.; Blanka, S. Modulation of the Tumor Microenvironment for Cancer Treatment: A Biomaterials Approach. J. Funct. Biomater. 2015, 6, 81–103. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Préat, V. Drug Delivery Challenges and Future of Chemotherapeutic Nanomedicine for Glioblastoma Treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef]
- De Oliveira Junior, E.R.; Nascimento, T.L.; Salomão, M.A.; da Silva, A.C.G.; Valadares, M.C.; Lima, E.M. Increased Nose-to-Brain Delivery of Melatonin Mediated by Polycaprolactone Nanoparticles for the Treatment of Glioblastoma. Pharm. Res. 2019, 36, 131. [Google Scholar] [CrossRef]
- Chung, K.; Ullah, I.; Kim, N.; Lim, J.; Shin, J.; Lee, S.C.; Jeon, S.; Kim, S.H.; Kumar, P.; Lee, S.-K. Intranasal Delivery of Cancer-Targeting Doxorubicin-Loaded PLGA Nanoparticles Arrests Glioblastoma Growth. J. Drug Target. 2020, 28, 617–626. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced Anti-Angiogenic Effects of Bevacizumab in Glioblastoma Treatment upon Intranasal Administration in Polymeric Nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of SiRNA-Loaded Chitosan Nanoparticles Targeting Galectin-1 for the Treatment of Glioblastoma Multiforme via Intranasal Administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.T.; Joseph, A.; Shavi, G.; Rao, J.V.; Udupa, N. Development and Evaluation of Carboplatin-Loaded PCL Nanoparticles for Intranasal Delivery. Drug Deliv. 2016, 23, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Sekerdag, E.; Lüle, S.; Bozdağ Pehlivan, S.; Öztürk, N.; Kara, A.; Kaffashi, A.; Vural, I.; Işıkay, I.; Yavuz, B.; Oguz, K.K.; et al. A Potential Non-Invasive Glioblastoma Treatment: Nose-to-Brain Delivery of Farnesylthiosalicylic Acid Incorporated Hybrid Nanoparticles. J. Control. Release 2017, 261, 187–198. [Google Scholar] [CrossRef] [PubMed]
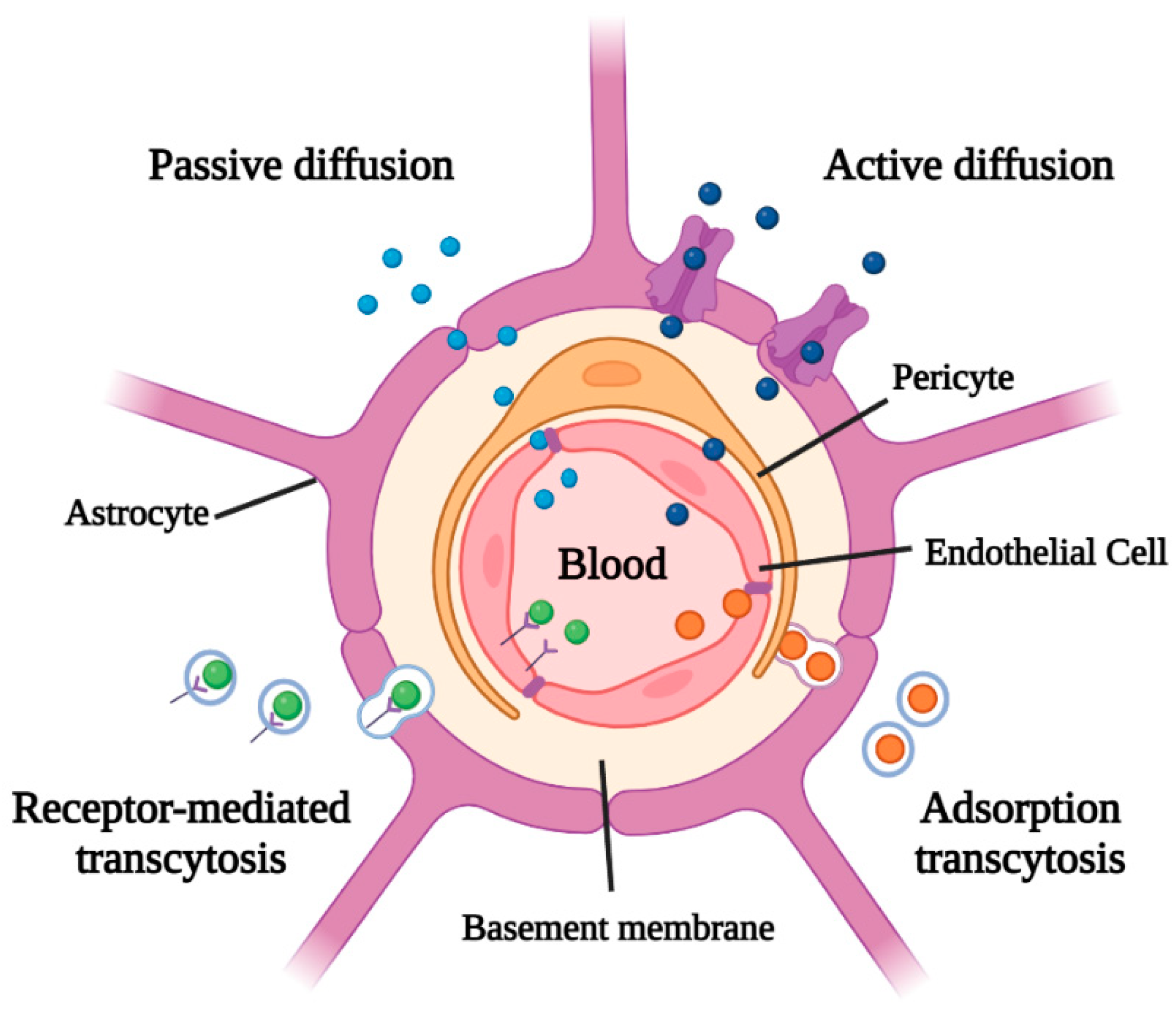

| Strategy | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Passive diffusion | Not require energy (ATP) consumption. | Only small lipophilic molecules (<500 Da) might diffuse. | [1,25] |
| Active diffusion | Transport a variety of molecules with structural diversity. | Require ATP consumption and restrict the passage of some therapeutic drugs. | [1,22] |
| Adsorption transcytosis | Molecules non-specifically bound to the membrane are internalized by endocytosis. | Slow and non-selective process. | [1,26] |
| Receptor-mediated transcytosis | Selective process specific for the largest molecules. | Slow process that requires the presence of specific receptors. | [1,27] |
| Pathology | Drug | NP Composition | NP Size | NP Synthesis Method | Biological Outcomes | Ref. |
|---|---|---|---|---|---|---|
| PD | BRC | CS | ~160 nm | Ionic gelation | High-uptake of BRC-CS NPs via the NtB route and symptomatology reduction in PD mice. | [124] |
| PD | RH | CS | ~170 nm | Ionic gelation | High accumulation of RH-CS NPs in the brain and higher mucoadhesion of RH-CS NPs than RH solution form in rats. | [131] |
| PD | Levodopa | CS | ~100 nm | Ionic gelation | High accumulation and enhanced residence of levodopa-CS NPs in the brain of Wistar rats. | [132] |
| PD | Levodopa | PLGA | ~250 nm | Emulsion/solvent evaporation | Intranasal levodopa-PLGA NPs provide a lasting motor function recovery with sustained effect in the 6-OHDA-induced PD rat model. | [129] |
| AD | Galantamine | CS | 40–80 nm 180–190 nm | Ionic gelation | Intranasal galantamine-CS NPs improve the distribution of the drugs in different brain areas and ameliorate memory and brain functions in Wistar rats. | [126,127] |
| AD | Tarenflurbil | PLGA | ~140 nm | Emulsification/solvent diffusion | Tarenflurbil-PLGA NPs improve drug bioavailability and brain targeting in SD rats. | [133] |
| AD | VIP | PEG-PLA | 100–120 nm | Emulsion/solvent evaporation | VIP is a promising agent for the AD treatment. VIP-PLA NPs improve drug bioavailability in SD rats and KM mice. | [134] |
| AD | bFGF | PEG-PLGA | ~110 nm | Emulsion/solvent evaporation | bFGF-PEG-PLGA NPs improve cognitive and memory ability in SD rats. | [130] |
| AD | NAP | PEG-co-PCL | 70–90 nm | Emulsion/solvent evaporation | NAP-PEG-co-PCL improves cholinergic function and reduces neurodegeneration in SD rats and AD mice model. | [135] |
| AD | HupA | PLGA | ~150 nm | Emulsion/solvent evaporation | HupA-PLGA NPs have a good sustained-release effect in KM mice. | [136] |
| HD | anti-HTT siRNA | CS | 100–200 nm | Emulsion/solvent evaporation | Anti-HTT-siRNA-CS NPs determine a low expression of HTT mRNA in HD mice models. | [137] |
| HD | Cholesterol | g7-PLGA | ~180 nm | Nanoprecipitation and simple emulsion | Cholesterol-(g7)-PLGA NPs enhance endogenous cholesterol biosynthesis, prevent cognitive decline, and ameliorate motor defects in HD mice. | [138] |
| Drug | NP Composition | NP Size | NP Synthesis Method | Biological Outcomes | Ref. |
|---|---|---|---|---|---|
| MLT | PCL | ~170 nm | Nanoprecipitation | MLT-PCL-NPs exhibit a strong anticancer activity against U87MG cell line and an accumulation in the brain of Wistar rats. | [144] |
| DOX | RGD-PLGA | 180–200 nm | Double emulsion method | DOX-RGD-PLGA NPs induce apoptosis and inhibition of brain tumor growth and in GBM rat model. | [145] |
| Bevacizumab monoclonal antibody | PLGA | ~185 nm | Emulsion/solvent evaporation | Bevacizumab-PLGA NPs induce a reduction of tumor growth and show a higher anti-angiogenic effect in CD-1 mice. | [146] |
| anti-Gal-1 siRNA | CS | ~170 nm | Ionic gelation | anti-Gal-1 siRNA-CS NPs reduce the expression of Gal-1 both in murine and human cells of GBM and in GBM mice. | [147] |
| CPt | PCL | ~300 nm | Double emulsion/solvent evaporation | CPt-PCL NPs show high nasal absorption and high in vitro cytotoxicity in LN229 human GBM cells. | [148] |
| FTA | Lipid-PEG-PLGA | ~160 nm | Emulsion/sonication method | Intranasal administration of FTA-lipid-PEG-PLGA-NP determines the reduction of 55% of the tumor area in GBM rats. | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montegiove, N.; Calzoni, E.; Emiliani, C.; Cesaretti, A. Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases. J. Funct. Biomater. 2022, 13, 125. https://doi.org/10.3390/jfb13030125
Montegiove N, Calzoni E, Emiliani C, Cesaretti A. Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases. Journal of Functional Biomaterials. 2022; 13(3):125. https://doi.org/10.3390/jfb13030125
Chicago/Turabian StyleMontegiove, Nicolò, Eleonora Calzoni, Carla Emiliani, and Alessio Cesaretti. 2022. "Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases" Journal of Functional Biomaterials 13, no. 3: 125. https://doi.org/10.3390/jfb13030125
APA StyleMontegiove, N., Calzoni, E., Emiliani, C., & Cesaretti, A. (2022). Biopolymer Nanoparticles for Nose-to-Brain Drug Delivery: A New Promising Approach for the Treatment of Neurological Diseases. Journal of Functional Biomaterials, 13(3), 125. https://doi.org/10.3390/jfb13030125








