ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
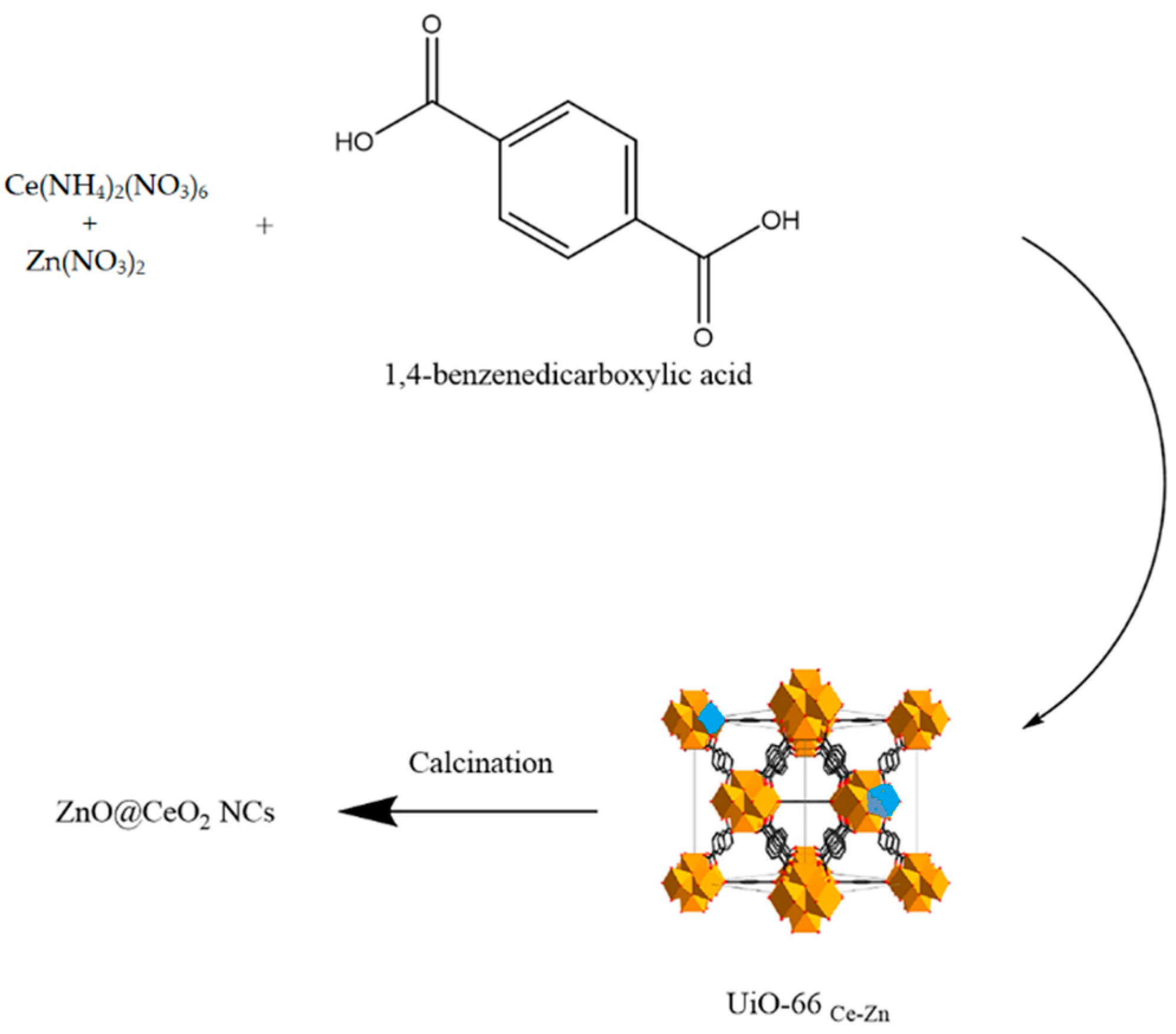
2.2. The Synthesis of the ZnO@CeO2 NCs
2.3. Characterization
2.4. In Vitro Cell Toxicity
2.5. Statistical Analysis
3. Result and Discussion
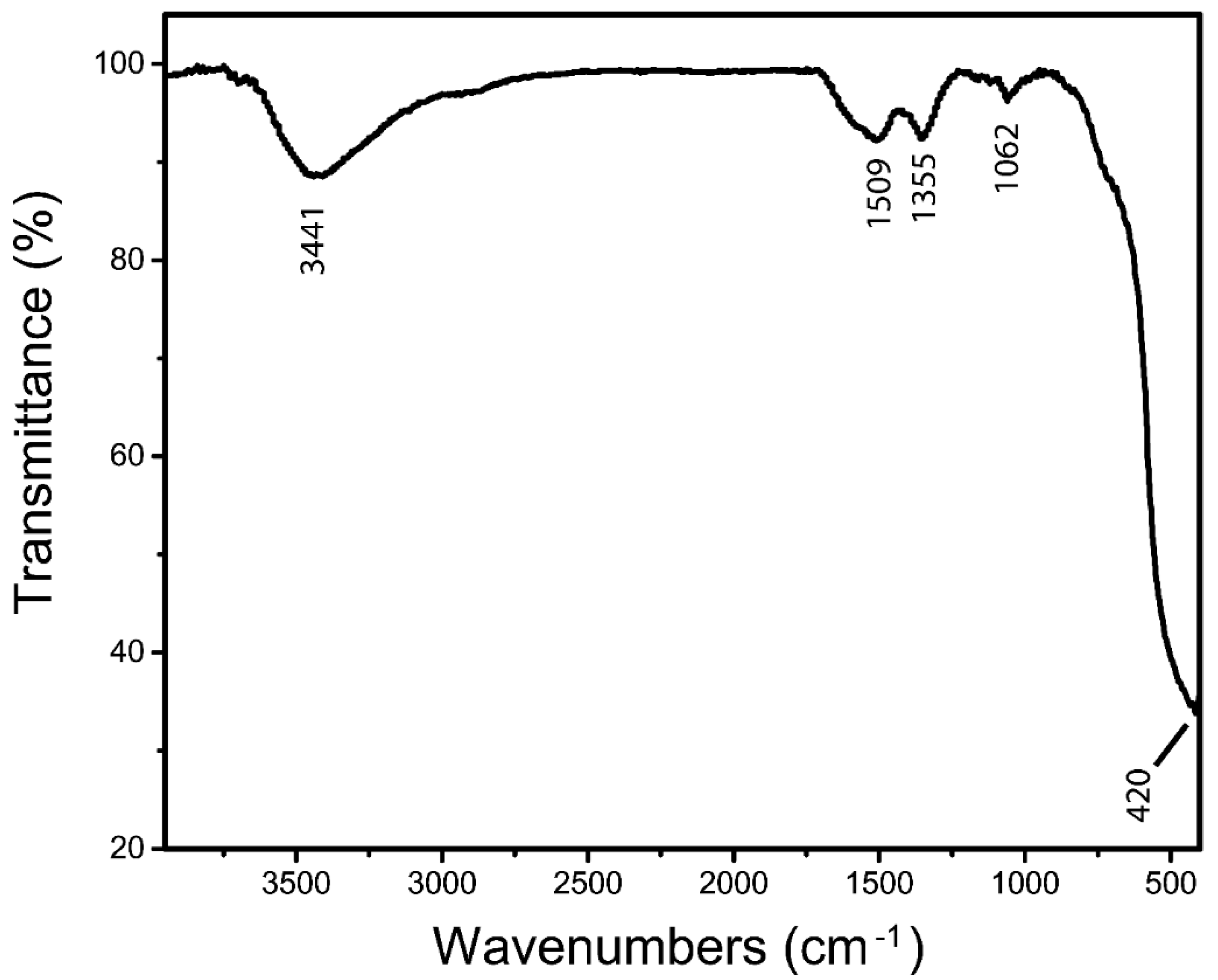
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
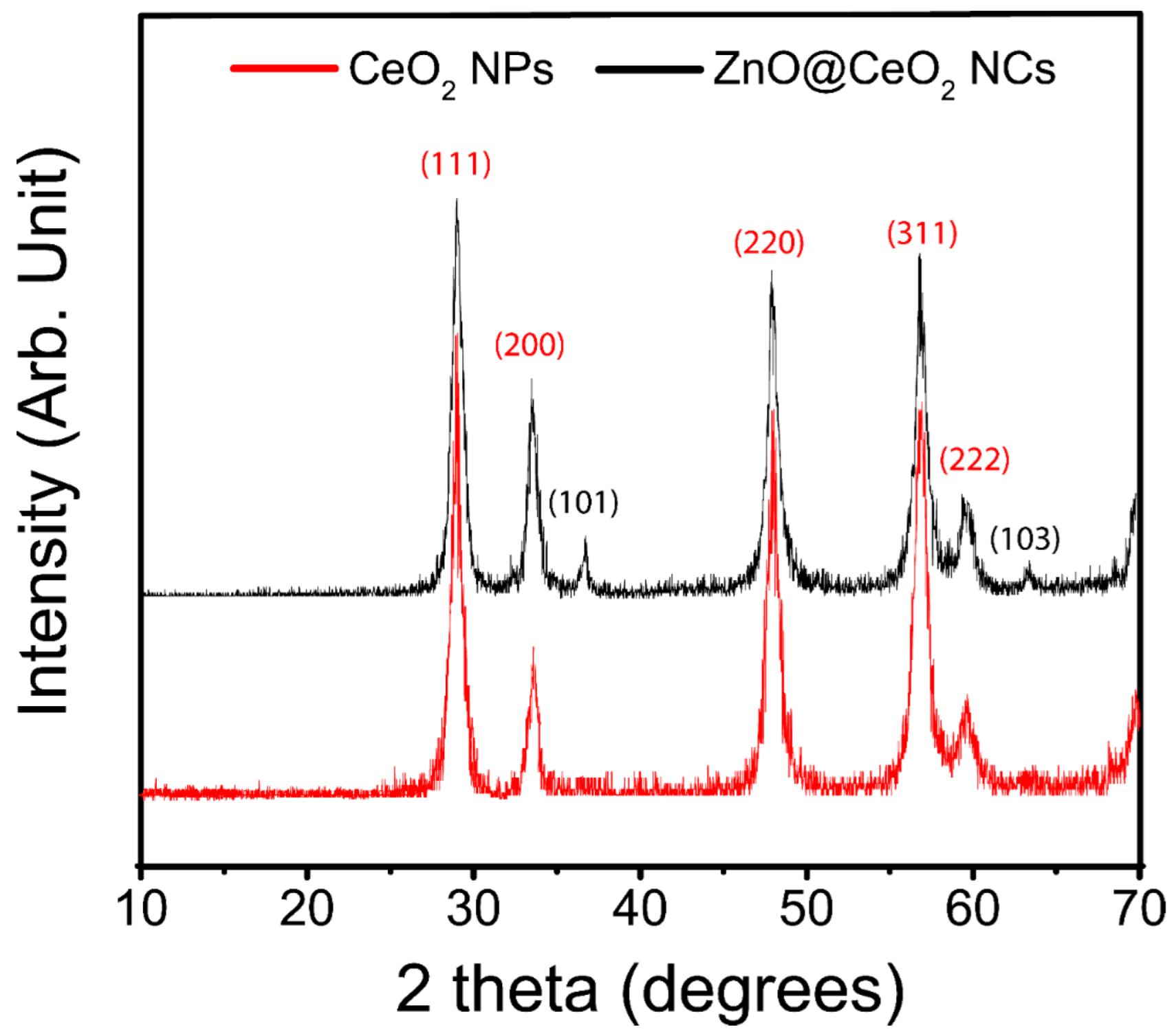
3.2. Powder X-ray Diffraction (PXRD)
3.3. Field Emission Scanning Electron Microscopy (FESEM)
3.4. Transmission Electron Microscopy (TEM)
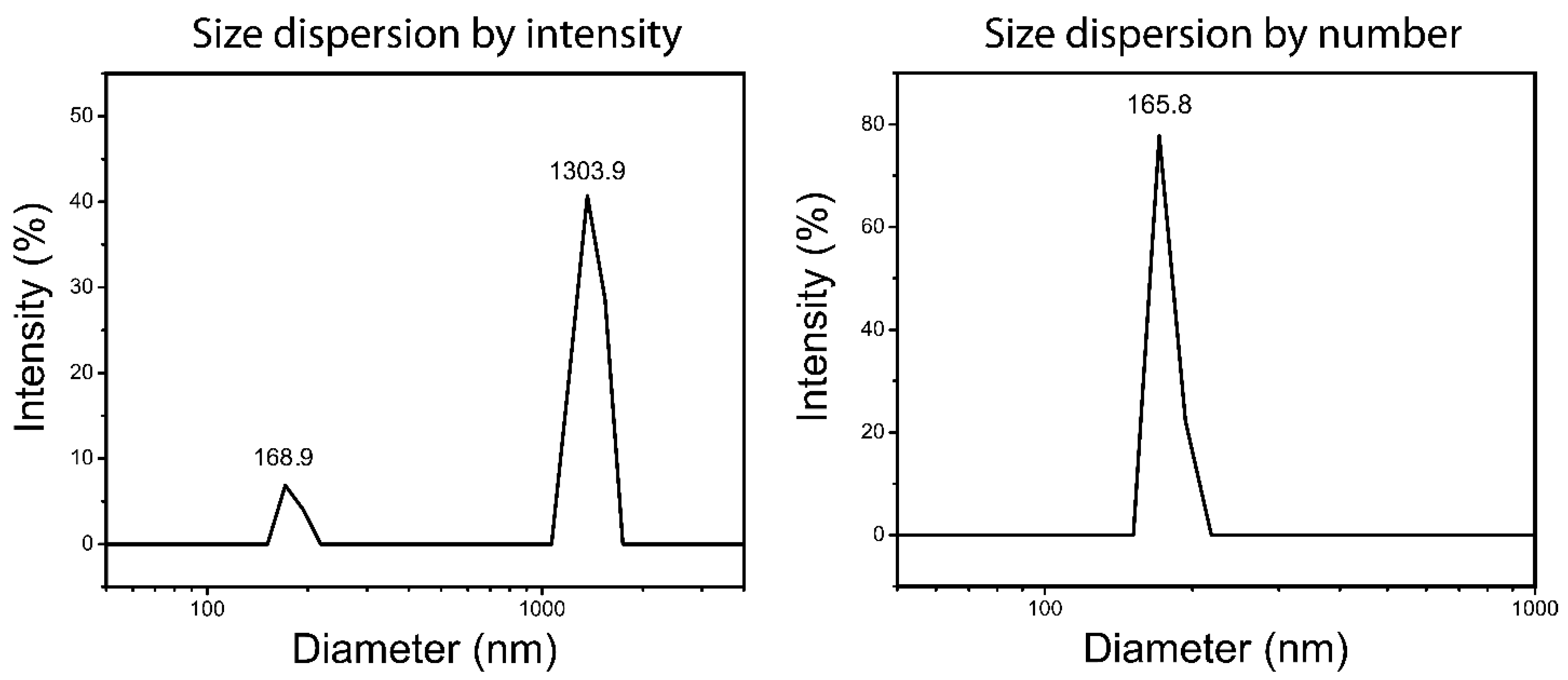
3.5. Dynamic Light Scattering (DLS) and ξ-Potential
3.6. Cell Toxicity Effect of ZnO/CeO2 NCs towards HepG2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thakur, N.; Kumar, K.; Thakur, V.K.; Soni, S.; Kumar, A.; Samant, S.S. Antibacterial and Photocatalytic Activity of Undoped and (Ag, Fe) Co-Doped CuO Nanoparticles via Microwave-Assisted Method. Nanofabrication 2022, 7. [Google Scholar] [CrossRef]
- Hashemzadeh, M.R.; Yazdi, M.E.T.; Amiri, M.S.; Mousavi, S.H. Stem Cell Therapy in the Heart: Biomaterials as A Key Route. Tissue Cell 2021, 71, 101504. [Google Scholar] [CrossRef] [PubMed]
- Taghavizadeh Yazdi, M.E.; Darroudi, M.; Amiri, M.S.; Zarrinfar, H.; Hosseini, H.A.; Mashreghi, M.; Mozafari, H.; Ghorbani, A.; Mousavi, S.H. Antimycobacterial, Anticancer, Antioxidant and Photocatalytic Activity of Biosynthesized Silver Nanoparticles Using Berberis Integerrima. Iran. J. Sci. Tech. Trans. A Sci. 2021, 46, 1–11. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Ghorani-Azam, A.; Mottaghipisheh, J.; Amiri, M.S.; Mashreghi, M.; Hashemzadeh, A.; Haddad-Mashadrizeh, A.; Nourbakhsh, F.; Nadaf, M.; Qayoomian, M.; Taghavizadeh Yazdi, M.E.; et al. Resveratrol-Mediated Gold-Nanoceria Synthesis as Green Nanomedicine for Phytotherapy of Hepatocellular Carcinoma. Front. Biosci. Landmark 2022, 27, 227. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zhao, Y.; Guo, H.; Lu, A.; Zhang, X.; Wang, L.; Chen, M.; Peng, D. Facile Preparation of Well-Dispersed CeO2–ZnO Composite Hollow Microspheres with Enhanced Catalytic Activity for CO Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 421–428. [Google Scholar] [CrossRef]
- Rajgure, A.V.; Tarwal, N.L.; Patil, J.Y.; Chikhale, L.P.; Pawar, R.C.; Lee, C.S.; Mulla, I.S.; Suryavanshi, S.S. Gas sensing performance of hydrothermally grown CeO2–ZnO composites. Ceram. Int. 2014, 40, 5837–5842. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.; Li, T.; Yan, L.; Yang, B. Preparation of CeO2/ZnO nanostructured microspheres and their catalytic properties. Powder Technol. 2006, 166, 72–76. [Google Scholar] [CrossRef]
- Taufik, A.; Shabrany, H.; Saleh, R. Different heat treatment of CeO2 nanoparticle composited with ZnO to enhance photocatalytic performance. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012038. [Google Scholar] [CrossRef]
- Hassanpour, M.; Salavati-Niasari, M.; Mousavi, S.A.; Safardoust-Hojaghan, H.; Hamadanian, M. CeO2/ZnO ceramic nanocomposites, synthesized via microwave method and used for decolorization of dye. J. Nanomater. 2018, 8, 97–106. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Yazdi, M.E.T.; Meshkat, Z.; Toosi, M.S.; Hosseini, S.M. Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Process Biochem. 2021, 111, 167–177. [Google Scholar] [CrossRef]
- Javad Farhangi, M.; Es-Haghi, A.; Taghavizadeh Yazdi, M.E.; Rahdar, A.; Baino, F. MOF-mediated synthesis of CuO/CeO2 composite nanoparticles: Characterization and estimation of the cellular toxicity against breast cancer cell line (MCF-7). J. Funct. Biomater. 2021, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.; Vijayalakshmi, G. Effect of Zn doping on structural, optical and thermal properties of CeO2 nanoparticles. Superlattices Microstruct. 2015, 85, 510–521. [Google Scholar] [CrossRef]
- Rodwihok, C.; Wongratanaphisan, D.; Tam, T.V.; Choi, W.M.; Hur, S.H.; Chung, J.S. Cerium-oxide-nanoparticle-decorated zinc oxide with enhanced photocatalytic degradation of methyl orange. Appl. Sci. 2020, 10, 1697. [Google Scholar] [CrossRef]
- Shah, N.; Bhangaonkar, K.; Pinjari, D.V.; Mhaske, S.T. Ultrasound and conventional synthesis of CeO2/ZnO Nanocomposites and their application in the photocatalytic degradation of Rhodamine B dye. J. Adv. Nanomater. 2017, 2, 133–145. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Hamidi, A.; Amiri, M.S.; Kazemi Oskuee, R.; Hosseini, H.A.; Hashemzadeh, A.; Darroudi, M. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Mater. Technol. 2019, 34, 490–497. [Google Scholar] [CrossRef]
- Forest, V. Experimental and computational nanotoxicology—Complementary approaches for nanomaterial hazard assessment. Nanomaterials 2022, 12, 1346. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Amiri, M.S.; Nourbakhsh, F.; Rahnama, M.; Forouzanfar, F.; Mousavi, S.H. Bio-indicators in cadmium toxicity: Role of HSP27 and HSP70. Environ. Sci. Pollut. Res. 2021, 28, 26359–26379. [Google Scholar] [CrossRef]
- Sinha, S.; Sibuh, B.Z.; Mishra, A.; Pant, K.; Tomar, S.; Anand, J.; Gupta, P.K. Synthesis, Characterization, and Remedial Action of Biogenic p-Ag Nanoparticles. Nanofabrication 2022, 7. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Darroudi, M.; Amiri, M.S.; Hosseini, H.A.; Nourbakhsh, F.; Mashreghi, M.; Farjadi, M.; Mousavi, S.H. Anticancer, antimicrobial, and dye degradation activity of biosynthesised silver nanoparticle using Artemisia kopetdaghensis. Micro Nano Lett. 2020, 15, 1046–1050. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; William Andrew: Norwich, NY, USA, 2012. [Google Scholar]
- Carter, C.B.; Norton, M.G. (Eds.) Sols, Gels, and Organic Chemistry. In Carter, Ceramic Materials: Science and Engineering; Springer: New York, NY, USA, 2007; pp. 400–411. [Google Scholar]
- Derakhshani, M.; Hashamzadeh, A.; Amini, M.M. High surface area mesoporous alumina nanosheets and nanorolls from an aluminum based metal organic framework. Ceram. Int. 2016, 42, 17742–17748. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Wei, C.; Fu, J.; Xu, F.; Tan, H.; Tang, J.; Wang, L. A green strategy to prepare metal oxide superstructure from metal-organic frameworks. Sci. Rep. 2015, 5, 8401. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, M.; Hashamzadeh, A.; Amini, M.M. Novel synthesis of mesoporous crystalline γ-alumina by replication of MOF-5-derived nanoporous carbon template. Ceram. Int. 2018, 44, 17102–17106. [Google Scholar] [CrossRef]
- Liu, J.; Mukherjee, S.; Wang, F.; Fischer, R.A.; Zhang, J. Homochiral metal–organic frameworks for enantioseparation. Chem. Soc. Rev. 2021, 50, 5706–5745. [Google Scholar] [CrossRef]
- Shaabani, A.; Mohammadian, R.; Hooshmand, S.E.; Hashemzadeh, A.; Amini, M.M. Zirconium metal-organic framework (UiO-66) as a robust catalyst toward solvent-free synthesis of remarkable heterocyclic rings. Chem. Sel. 2017, 2, 11906–11911. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Sun, C.; Huang, Z.; Xu, H.; Shen, W. Insights into the pyrolysis processes of Ce-MOFs for preparing highly active catalysts of toluene combustion. Catalysts 2019, 9, 682. [Google Scholar] [CrossRef]
- Waitschat, S.; Fröhlich, D.; Reinsch, H.; Terraschke, H.; Lomachenko, K.A.; Lamberti, C.; Kummer, H.; Helling, T.; Baumgartner, M.; Henninger, S.; et al. Synthesis of M-UiO-66 (M = Zr, Ce or Hf) employing 2,5-pyridinedicarboxylic acid as a linker: Defect chemistry, framework hydrophilisation and sorption properties. Dalton Trans. 2018, 47, 1062–1070. [Google Scholar] [CrossRef]
- Hou, X.; Yang, L.; Bian, Y. One Step Synthesis of Transition Metal Modified Uio-66-Ce Metal-Organic Framework: Catalytic Oxidation of Toluene and Investigation of the Mechanism. SSRN 2022. [Google Scholar] [CrossRef]
- Chen, X.; Yu, E.; Cai, S.; Jia, H.; Chen, J.; Liang, P. In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J. 2018, 344, 469–479. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, T.; Gao, W.; Ma, K.; Tian, Y.; Li, X. CuO/CeO2 catalysts synthesized from Ce-UiO-66 metal-organic framework for preferential CO oxidation. Int. J. Hydrogen Energy 2017, 42, 17457–17465. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal–organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, R.; Ahangar, H.A.; Kaushal, A.; Zakaria, A.; Zamiri, G.; Tobaldi, D.; Ferreira, J.M.F. Dielectrical properties of CeO2 nanoparticles at different temperatures. PLoS ONE 2015, 10, e0122989. [Google Scholar] [CrossRef]
- Dezfuli, A.S.; Ganjali, M.R.; Naderi, H.R.; Norouzi, P. A high performance supercapacitor based on a ceria/graphene nanocomposite synthesized by a facile sonochemical method. RSC Adv. 2015, 5, 46050–46058. [Google Scholar] [CrossRef]
- Kumar, E.; Selvarajan, P.; Balasubramanian, K. Preparation and studies of cerium dioxide (CeO2) nanoparticles by microwave-assisted solution method. Recent Res. Sci. Technol. 2010, 2, 37–41. [Google Scholar]
- Karimi, A.; Fatehifar, E.; Alizadeh, R. Synthesis and characterization of nanostructured CuO/CeO2 catalysts via ultrasound assisted techniques used for selective oxidation of CO. Iran. J. Chem. Eng. 2013, 10, 51–59. [Google Scholar]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Visible light-induced photo-degradation of methylene blue by n–p heterojunction CeO2/CuS composite based on ribbon-like CeO2 nanofibers via electrospinning. Polyhedron 2019, 170, 160–171. [Google Scholar] [CrossRef]
- Faure, B.; Salazar-Alvarez, G.; Ahniyaz, A.; Villaluenga, I.; Berriozabal, G.; De Miguel, Y.R.; Bergström, L. Dispersion and surface functionalization of oxide nanoparticles for transparent photocatalytic and UV-protecting coatings and sunscreens. Sci. Tech. Adv. Mater. 2013, 14, 023001. [Google Scholar] [CrossRef]
- Nyoka, M.; Choonara, Y.E.; Kumar, P.; Kondiah, P.P.; Pillay, V. Synthesis of Cerium Oxide Nanoparticles Using Various Methods: Implications for Biomedical Applications. Nanomaterials 2020, 10, 242. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The relationship between pH and zeta potential of ∼30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Amiri, M.S.; Mashreghi, M.; Yazdi, M.E.T. Silver-zinc oxide nanocomposite: From synthesis to antimicrobial and anticancer properties. Ceram. Int. 2021, 47, 21490–21497. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi-Kouhi, S.M.; Beyk-Khormizi, A.; Mohammadzadeh, V.; Ashna, M.; Es-haghi, A.; Mashreghi, M.; Hashemzadeh, A.; Mozafari, H.; Nadaf, M.; Taghavizadeh Yazdi, M.E. Biological synthesis and characterization of gold nanoparticles using Verbascum speciosum Schrad. and cytotoxicity properties toward HepG2 cancer cell line. Res. Chem. Intermed. 2022, 48, 1–12. [Google Scholar] [CrossRef]
- Shakerimanesh, K.; Bayat, F.; Shahrokhi, A.; Baradaran, A.; Yousefi, E.; Mashreghi, M.; Es-haghi, A.; Taghavizadeh Yazdi, M.E. Biomimetic synthesis and characterisation of homogenouse gold nanoparticles and estimation of its cytotoxity against breast cancer cell line. Mater. Tech. 2022, 1–8. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Jahromi, M.; Kasmaie, F.M.; Barghbani, M.; Yazdi, M.E.T.; Meshkat, Z.; Homaee Shandizh, F.; Hosseini, S.M. Apoptotic, antioxidant and cytotoxic properties of synthesized AgNPs using green tea against human testicular embryonic cancer stem cells. Process Biochem. 2022, 119, 106–118. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Es-haghi, A.; Taghavizadeh Yazdi, M.E.; Sharifalhoseini, M.; Baghani, M.; Yousefi, E.; Rahdar, A.; Baino, F. Application of Response Surface Methodology for Optimizing the Therapeutic Activity of ZnO Nanoparticles Biosynthesized from Aspergillus niger. Biomimetics 2021, 6, 34. [Google Scholar] [CrossRef]
- Shamasi, Z.; Es-haghi, A.; Taghavizadeh Yazdi, M.E.; Amiri, M.S.; Homayouni-Tabrizi, M. Role of Rubia tinctorum in the synthesis of zinc oxide nanoparticles and apoptosis induction in breast cancer cell line. Nanomed. J. 2021, 8, 65–72. [Google Scholar] [CrossRef]
- Tanino, R.; Amano, Y.; Tong, X.; Sun, R.; Tsubata, Y.; Harada, M.; Fujita, Y.; Isobe, T. Anticancer activity of ZnO nanoparticles against human small-cell lung cancer in an orthotopic mouse model. Mol. Cancer Ther. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Yazdi, M.E.T.; Nourbakhsh, F.; Mashreghi, M.; Mousavi, S.H. Ultrasound-based synthesis of ZnO· Ag2O3 nanocomposite: Characterization and evaluation of its antimicrobial and anticancer properties. Res. Chem. Intermed. 2021, 47, 1285–1296. [Google Scholar] [CrossRef]
- Modarres, M.; Yazdi, M.E.T. Elicitation Improves Phenolic Acid Content and Antioxidant Enzymes Activity in Salvia leriifolia Cell Cultures. Iran. J. Sci. Tech. Trans. A Sci. 2021, 45, 849–855. [Google Scholar] [CrossRef]
- Ashna, M.; Es-Haghi, A.; Karimi Noghondar, M.; Al Amara, D.; Yazdi, M.E.T. Greener synthesis of cerium oxide nanoemulsion using pollen grains of Brassica napus and evaluation of its antitumour and cytotoxicity properties. Mater. Technol. 2020, 37, 525–535. [Google Scholar] [CrossRef]
- Es-haghi, A.; Javadi, F.; Yazdi, M.E.T.; Amiri, M.S. The Expression of Antioxidant Genes and Cytotoxicity of Biosynthesized Cerium Oxide Nanoparticles Against Hepatic Carcinoma Cell Line. Avicenna J. Med. Biochem. 2019, 7, 16–20. [Google Scholar] [CrossRef]
- Alirezaei, M.; Ghobeh, M.; Es-haghi, A. Poly (lactic-co-glycolic acid)(PLGA)-based nanoparticles modified with chitosan-folic acid to delivery of Artemisia vulgaris L. essential oil to HT-29 cancer cells. Process Biochem. 2022, 121, 207–215. [Google Scholar] [CrossRef]
- Casals, G.; Perramón, M.; Casals, E.; Portolés, I.; Fernández-Varo, G.; Morales-Ruiz, M.; Puntes, V.; Jiménez, W. Cerium oxide nanoparticles: A new therapeutic tool in liver diseases. Antioxidants 2021, 10, 660. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alaizeri, Z.M.; Alhadlaq, H. Facile synthesis of Zn-doped Bi2O3 nanoparticles and their selective cytotoxicity toward cancer cells. ACS Omega 2021, 6, 17353–17361. [Google Scholar] [CrossRef]
- Mdlovu, N.B.; Lin, K.-S.; Weng, M.-T.; Mdlovu, N.V. Formulation and in-vitro evaluations of doxorubicin loaded polymerized magnetic nanocarriers for liver cancer cells. J. Taiwan Inst. Chem. Eng. 2021, 126, 278–287. [Google Scholar] [CrossRef]
- Krause, B.C.; Kriegel, F.L.; Tartz, V.; Jungnickel, H.; Reichardt, P.; Singh, A.V.; Laux, P.; Shemis, M.; Luch, A. Combinatory Effects of Cerium Dioxide Nanoparticles and Acetaminophen on the Liver—A Case Study of Low-Dose Interactions in Human HuH-7 Cells. Int. J. Mol. Sci. 2021, 22, 6866. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabyadh, T.; Albadri, R.; Es-haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. J. Funct. Biomater. 2022, 13, 139. https://doi.org/10.3390/jfb13030139
Alabyadh T, Albadri R, Es-haghi A, Yazdi MET, Ajalli N, Rahdar A, Thakur VK. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. Journal of Functional Biomaterials. 2022; 13(3):139. https://doi.org/10.3390/jfb13030139
Chicago/Turabian StyleAlabyadh, Toqa, Riyadh Albadri, Ali Es-haghi, Mohammad Ehsan Taghavizadeh Yazdi, Narges Ajalli, Abbas Rahdar, and Vijay Kumar Thakur. 2022. "ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells" Journal of Functional Biomaterials 13, no. 3: 139. https://doi.org/10.3390/jfb13030139
APA StyleAlabyadh, T., Albadri, R., Es-haghi, A., Yazdi, M. E. T., Ajalli, N., Rahdar, A., & Thakur, V. K. (2022). ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. Journal of Functional Biomaterials, 13(3), 139. https://doi.org/10.3390/jfb13030139






