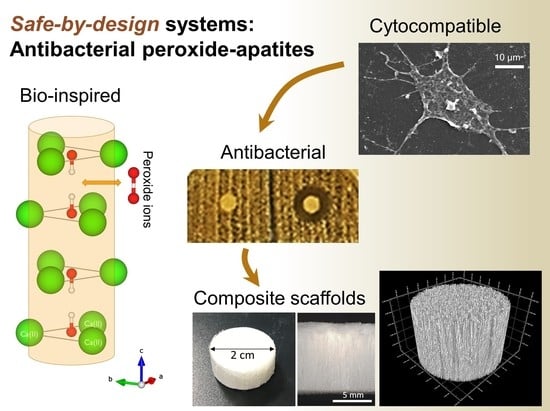
Safe-by-Design Antibacterial Peroxide-Substituted Biomimetic Apatites: Proof of Concept in Tropical Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Apatite Powders
2.2. Materials Fabrication
2.3. Physicochemical Characterization
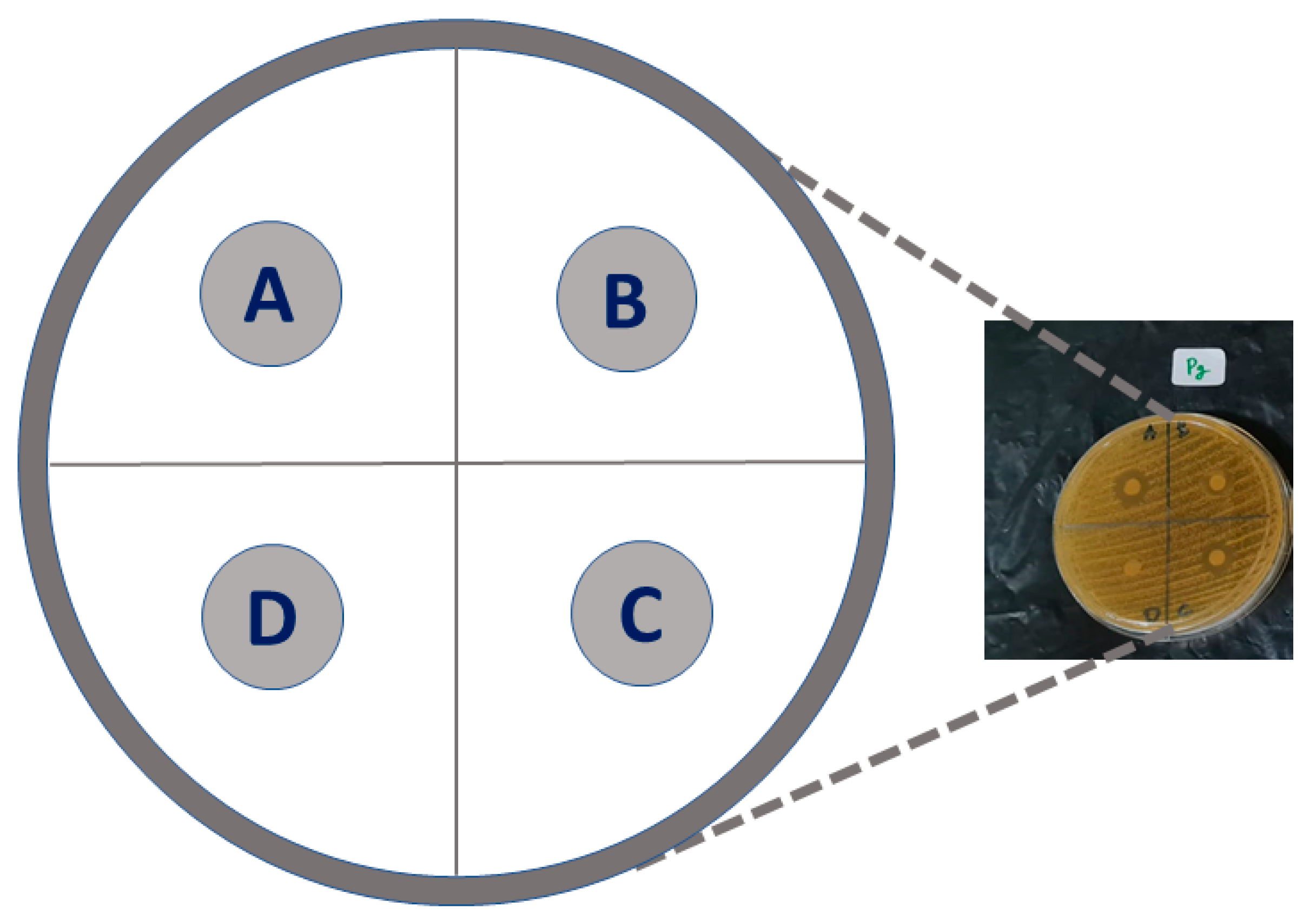
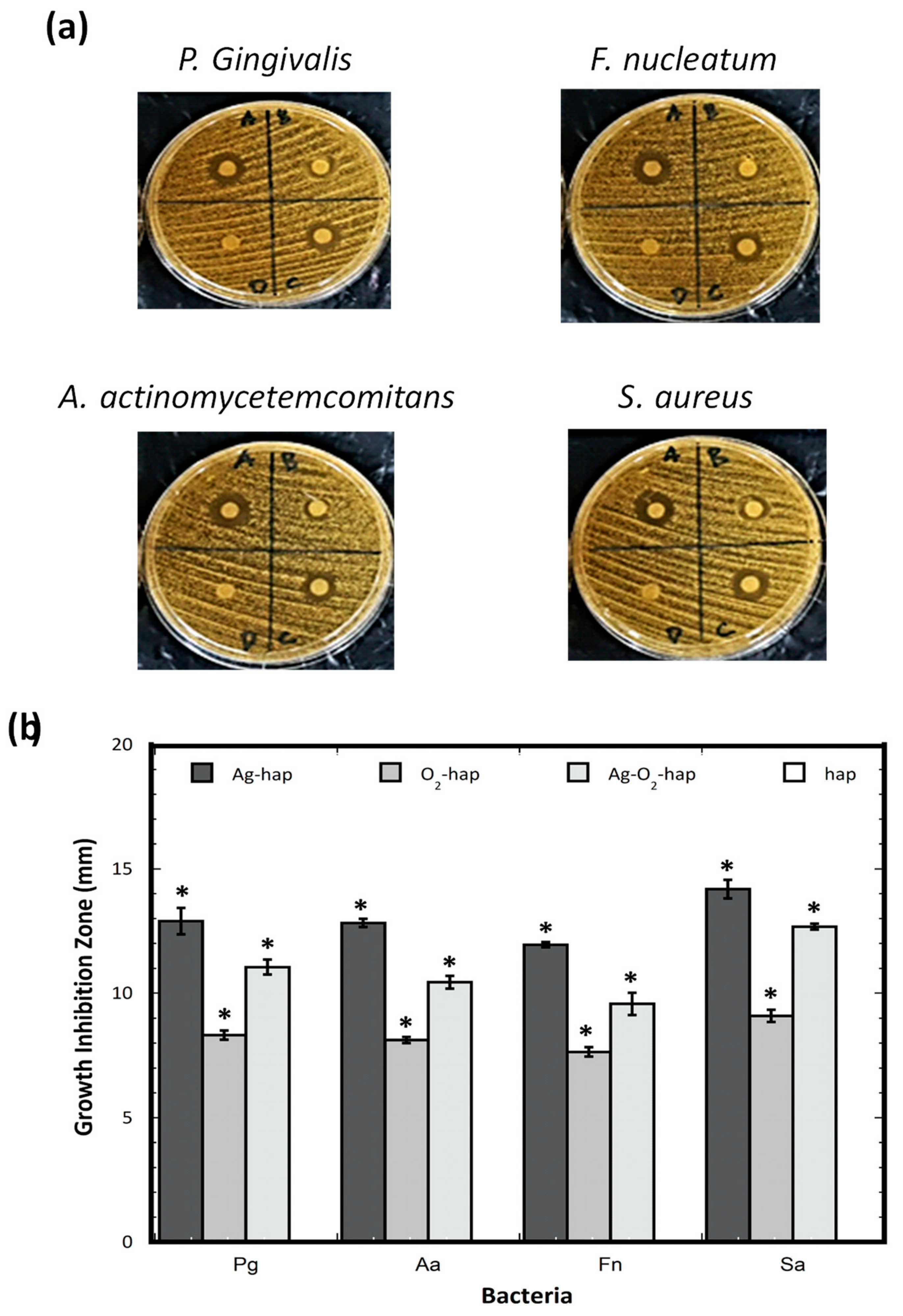
2.4. Antibacterial Properties
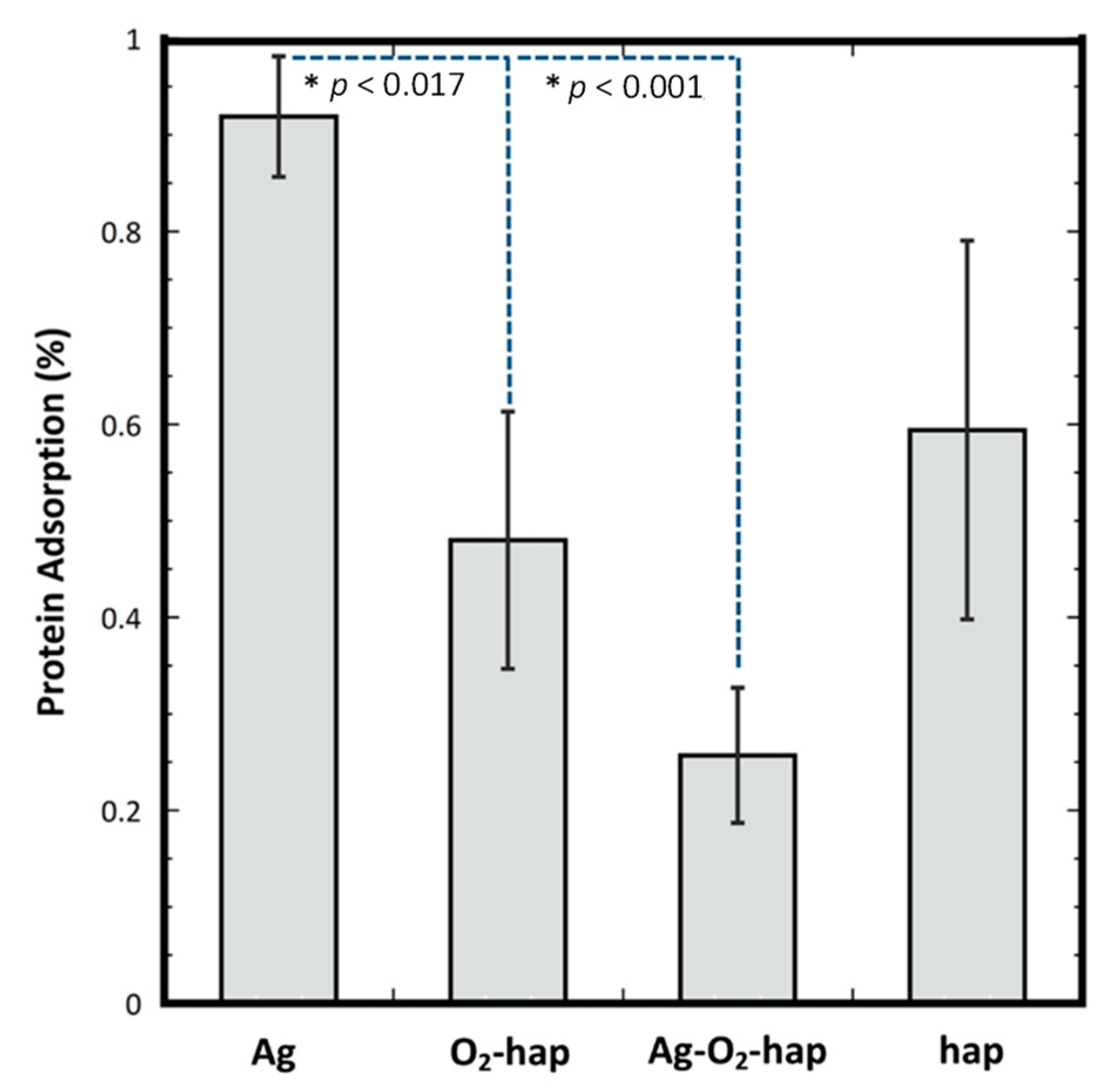
2.5. Protein Adsorption
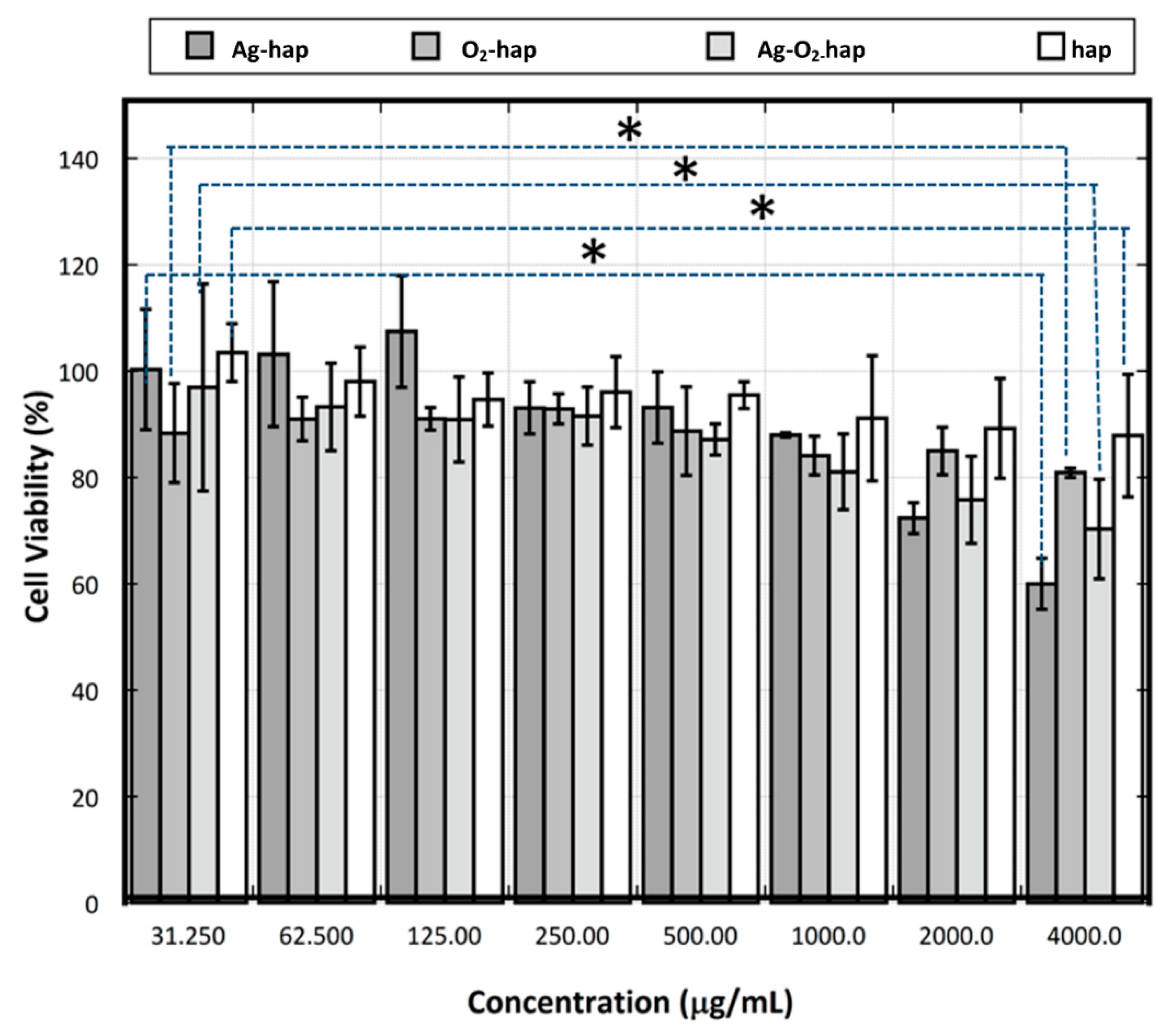
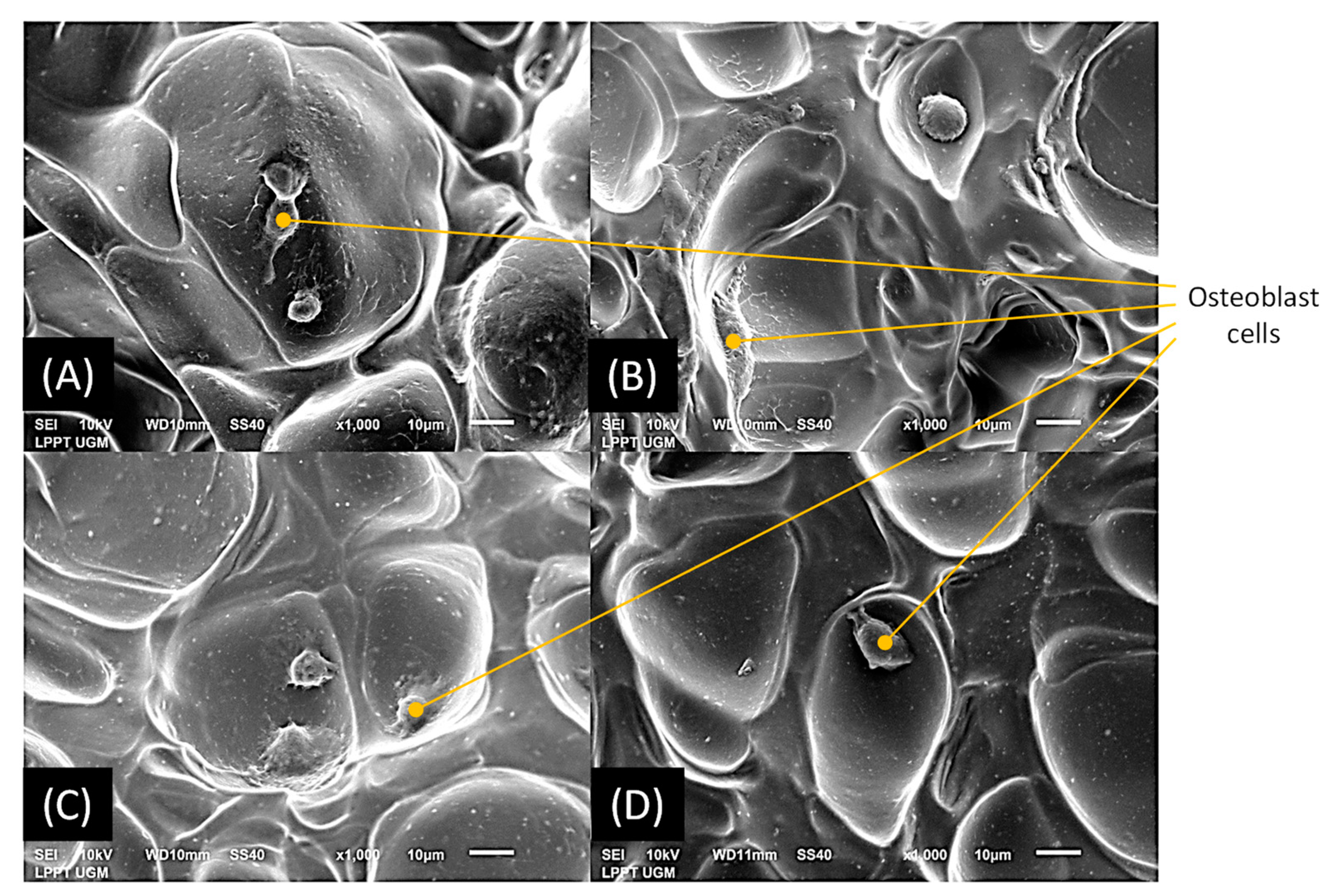
2.6. Osteoblast Cell Viability
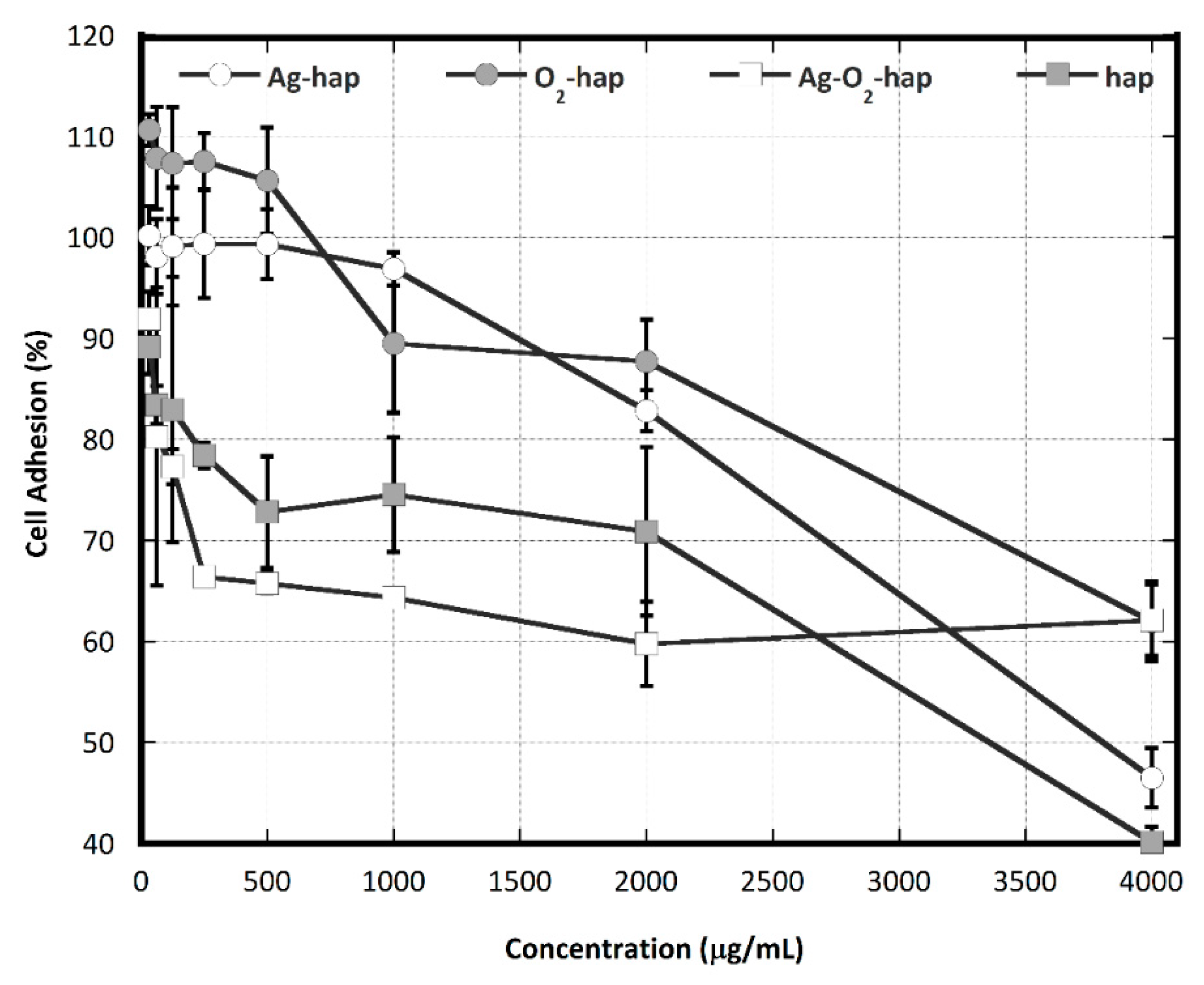
2.7. Osteoblast Cell Adhesion
3. Results and Discussion
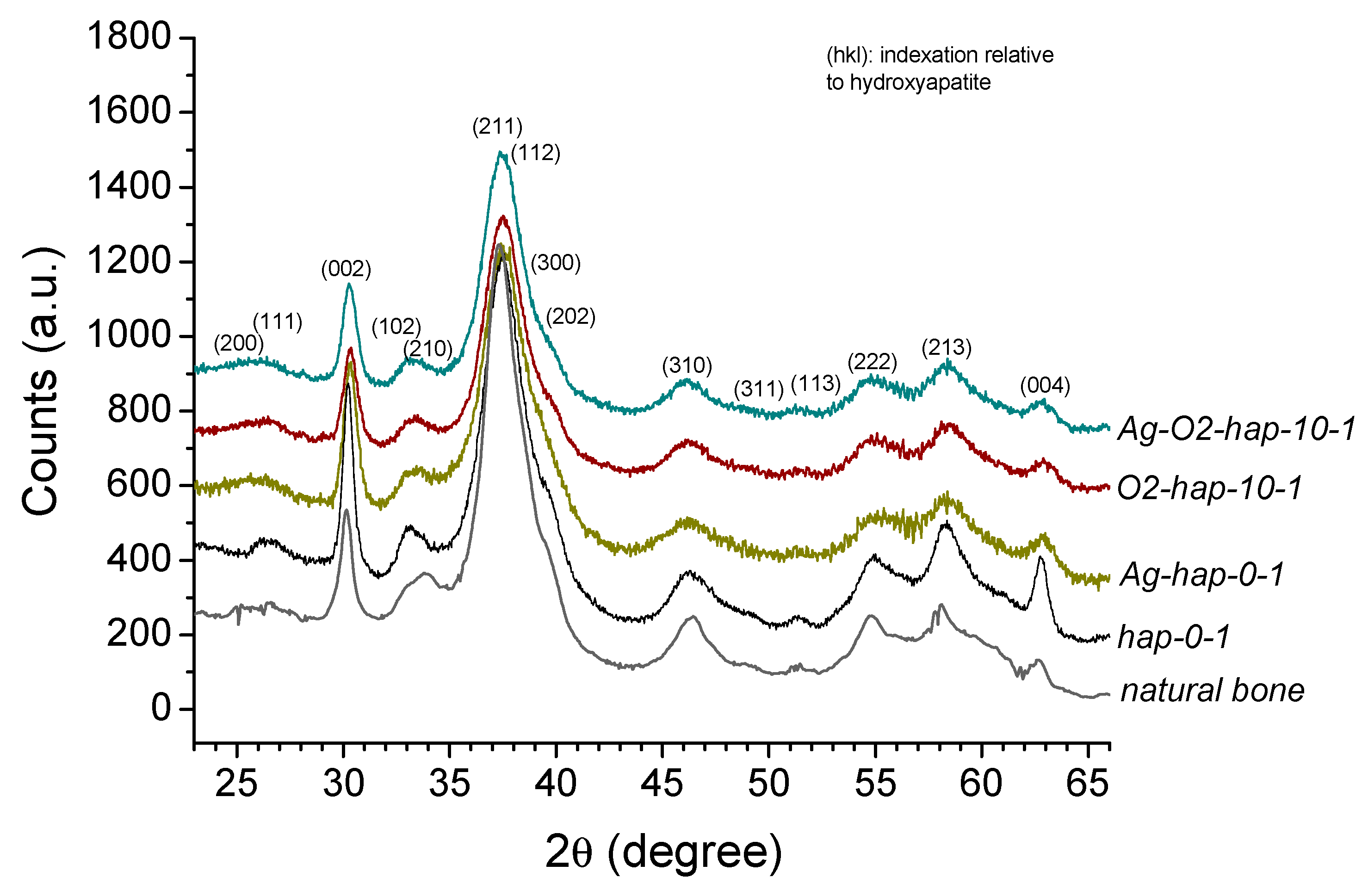
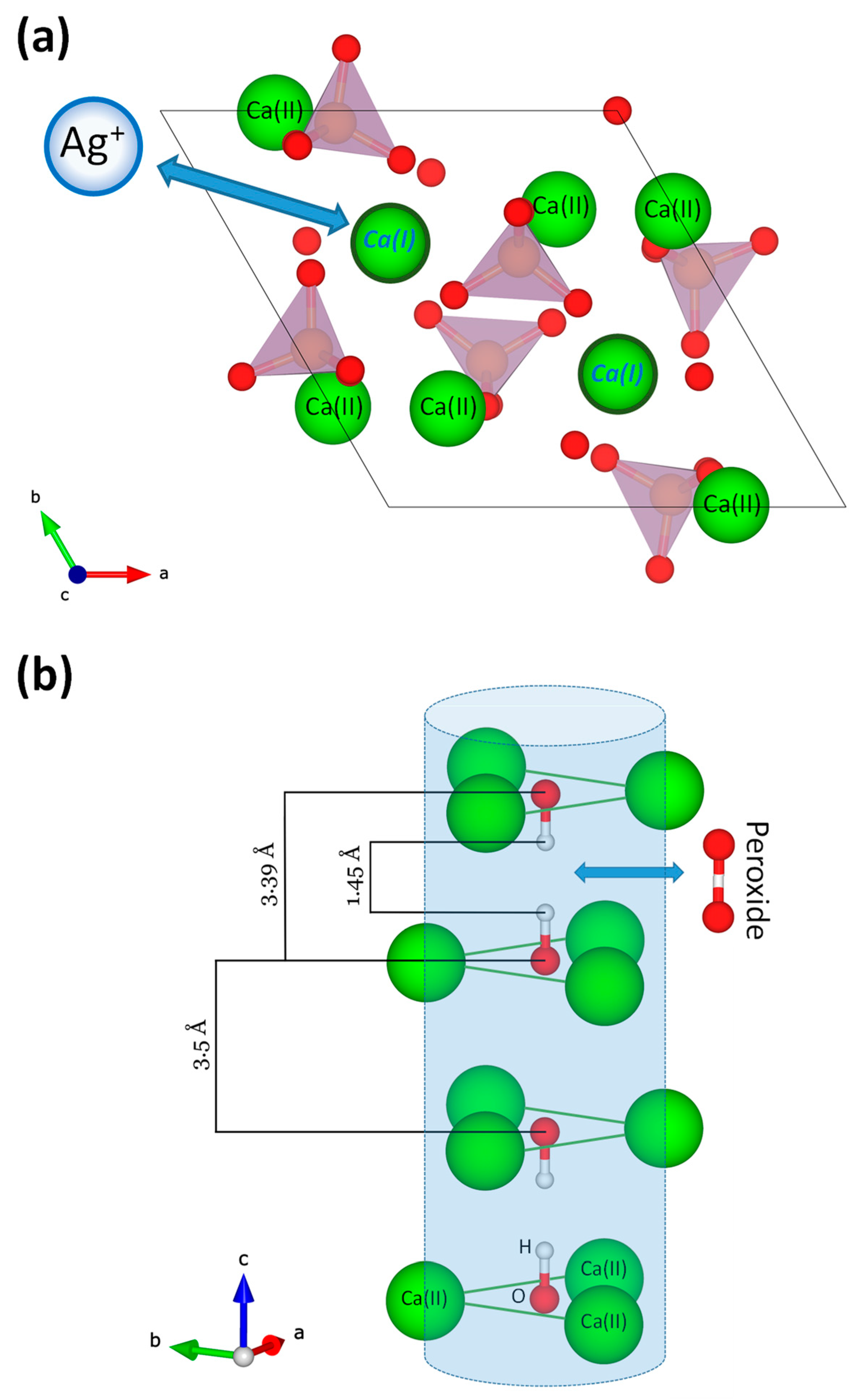
3.1. Preparation and Characterization of Doped Biomimetic Apatites
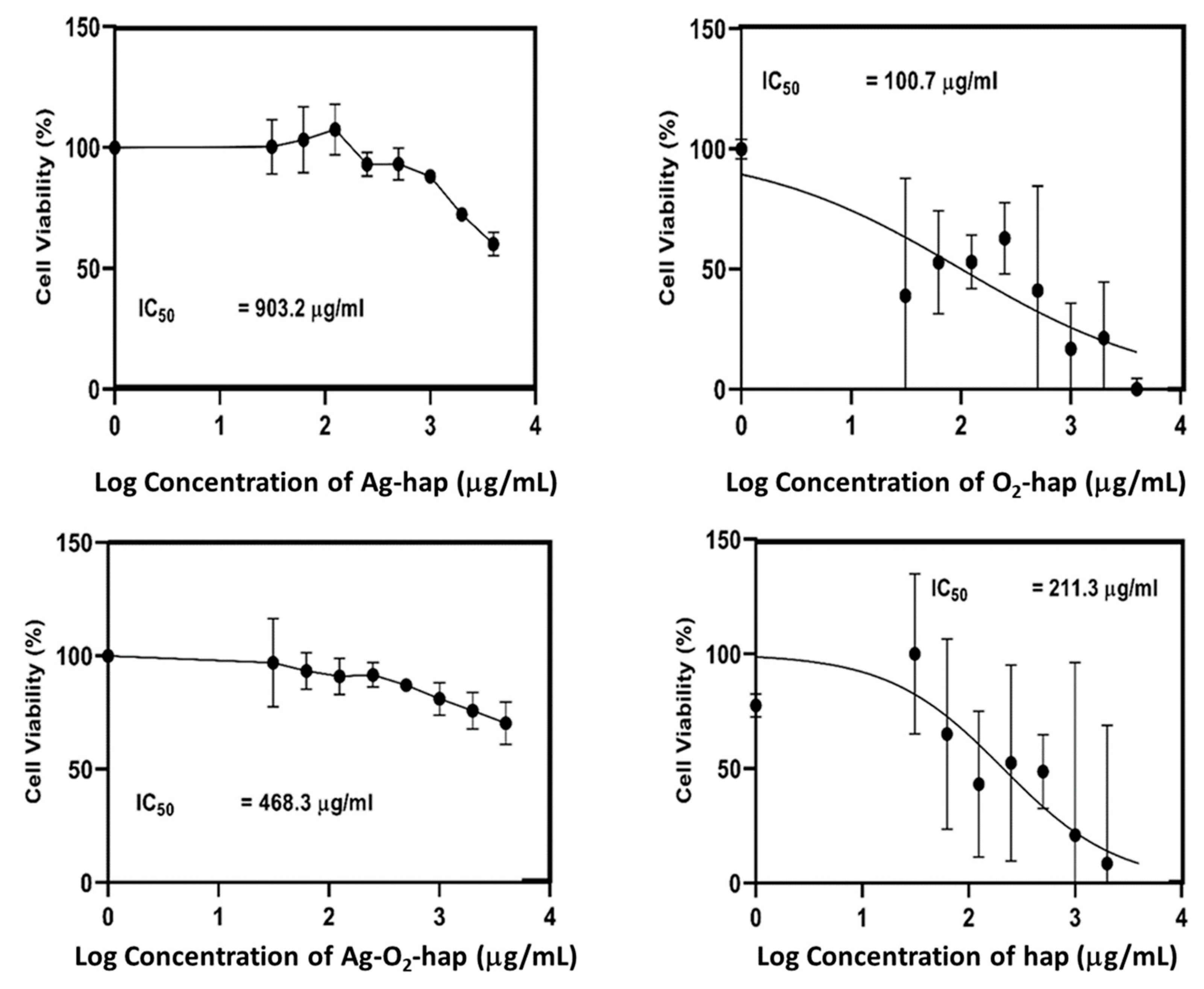
3.2. In Vitro Assessments
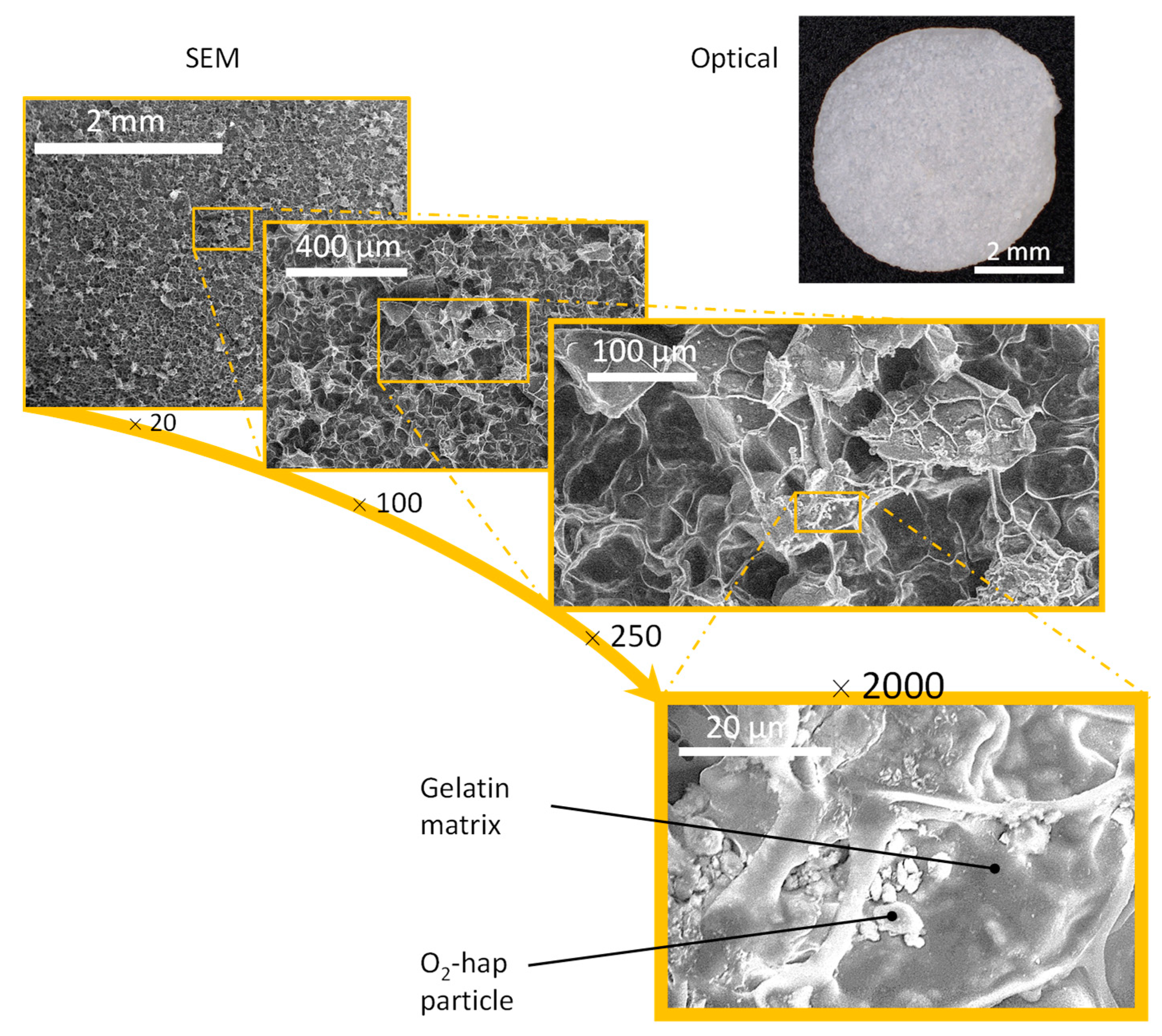
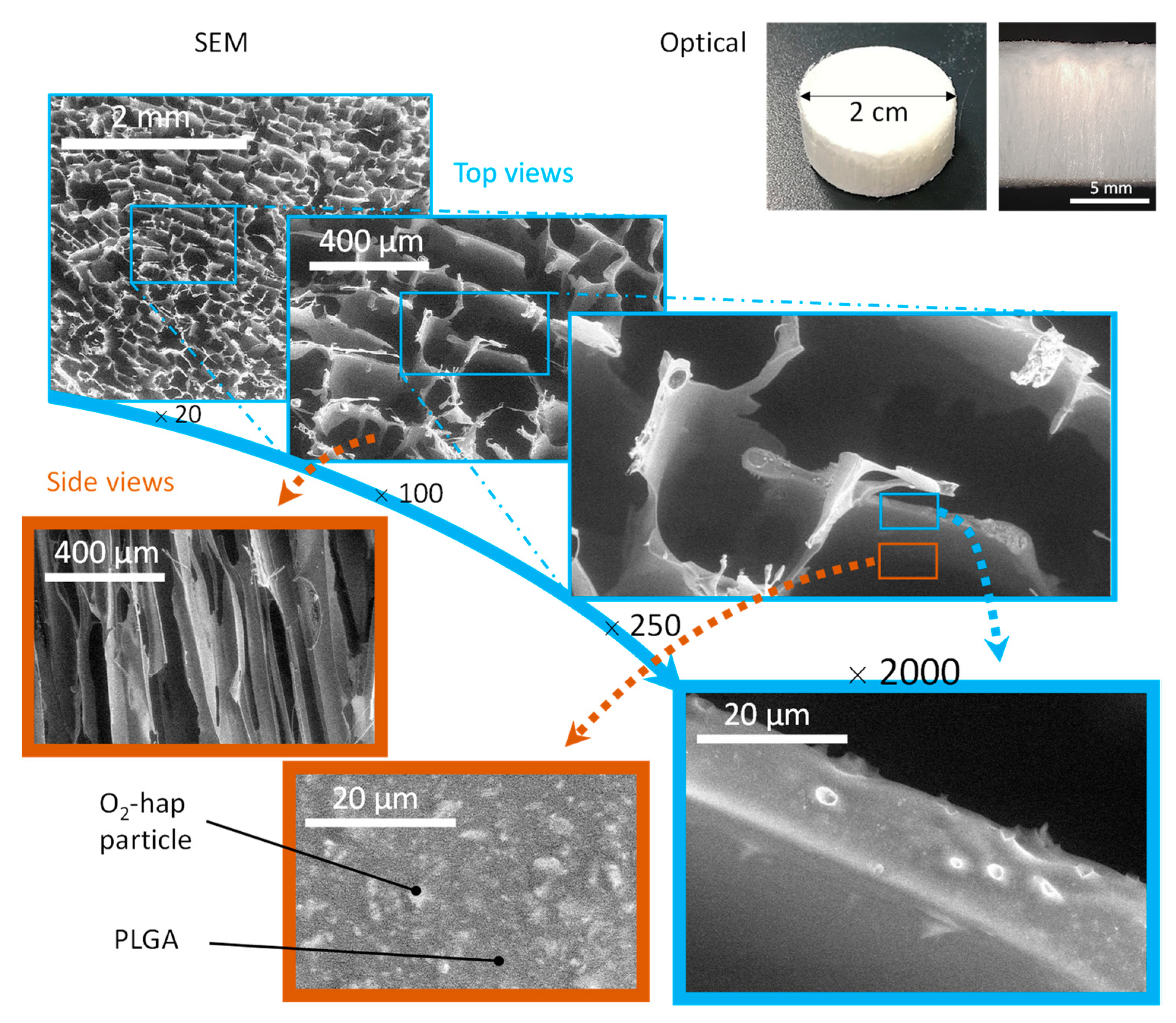
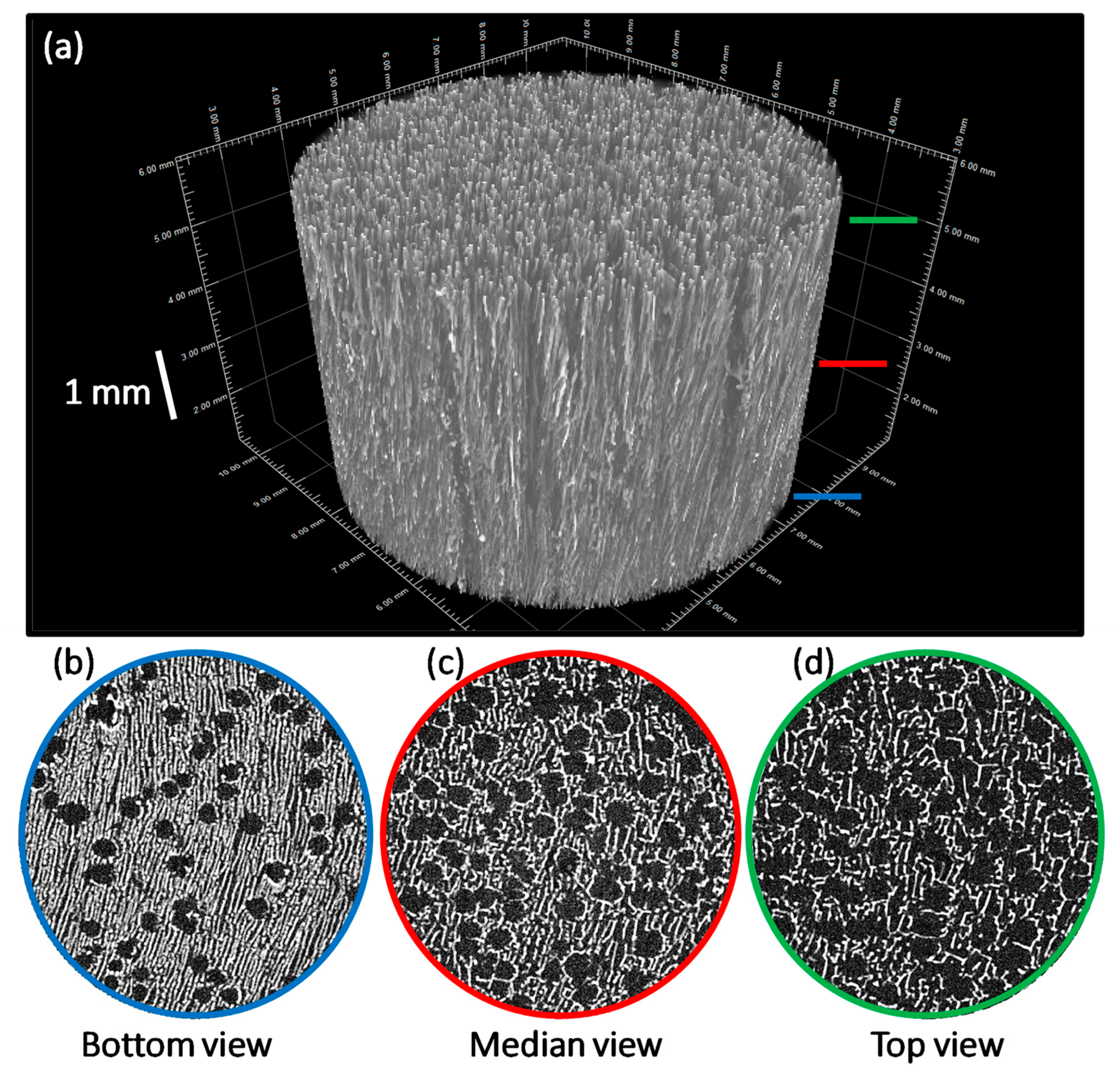
3.3. Processing into 2D Membranes and 3D Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, H.A. Bone infection. East. Mediterr. Health J. 2003, 9, 208–214. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Choi, Y. Innate immune response to oral bacteria and the immune evasive characteristics of periodontal pathogens. J. Periodontal Implant. Sci. 2013, 43, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef]
- Benjamin, P. Why big pharma has abandoned antibiotics. Nature 2020, 586, 50–52. [Google Scholar]
- Kalelkar, P.P.; Riddick, M.; García, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2021, 7, 39–54. [Google Scholar] [CrossRef]
- Choimet, M.; Tourrette, T.; Marsan, O.; Rassu, G.; Drouet, C. Bio-inspired apatite particles limit skin penetration of drugs for dermatology applications. Acta Biomater. 2020, 111, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Morales, J.; Iafisco, M.; Delgado-Lopez, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Rey, C.; Combes, C.; Cazalbou, S.; Sarda, S.; Grossin, D. Nanocrystalline apatites: A versatile functionalizable platform for biomedical applications for bone engineering… and beyond. Key Eng. Mater. 2016, 696, 14–22. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2016, 4, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Cazalbou, S.; Bertrand, G.; Drouet, C. Tetracycline-loaded biomimetic apatite: An adsorption study. J. Phys. Chem. B 2015, 119, 3014–3024. [Google Scholar] [CrossRef]
- Martinez, T.; Sarda, S.; Dupret-Bories, A.; Charvillat, C.; Projetti, F.; Drouet, C. Toward a doxorubicin-loaded bioinspired bone cement for the localized treatment of osteosarcoma. Future Oncol. 2021, 17, 3511–3528. [Google Scholar] [CrossRef]
- Sarda, S.; Iafisco, M.; Pascaud-Mathieu, P.; Adamiano, A.; Montesi, M.; Panseri, S.; Marsan, O.; Thouron, C.; Dupret-Bories, A.; Tampieri, A. Interaction of folic acid with nanocrystalline apatites and extension to methotrexate (antifolate) in view of anticancer applications. Langmuir 2018, 34, 12036–12048. [Google Scholar] [CrossRef]
- Wu, V.M.; Uskoković, V. Is there a relationship between solubility and resorbability of different calcium phosphate phases in vitro? Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2016, 1860, 2157–2168. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, I.; Delgado-López, J.M.; Durán-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gómez-Morales, J. pH-responsive delivery of doxorubicin from citrate—Apatite nanocrystals with tailored carbonate content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef]
- Pascaud, P.; Errassifi, F.; Brouillet, F.; Sarda, S.; Barroug, A.; Legrouri, A.; Rey, C. Adsorption on apatitic calcium phosphates for drug delivery: Interaction with bisphosphonate molecules. J. Mater. Sci. Mater. Med. 2014, 25, 2373–2381. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.G.; Mueller, M.; Vandecandelaere, N.; Trick, I.; Burger-Kentischer, A.; Maucher, T.; Drouet, C. Enzyme-functionalized biomimetic apatites: Concept and perspectives in view of innovative medical approaches. J. Mater. Sci. -Mater. Med. 2014, 25, 595–606. [Google Scholar] [CrossRef]
- Jacquart, S.; Belime, A.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Tadier, S.; Auzély-Velty, R.; Girod-Fullana, S.; Bareille, R.; Roques, C.; et al. Development of an injectable composite for bone regeneration. IRBM 2013, 34, 176–179. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Meurice, E.; Leriche, A.; Hornez, J.C.; Bouchart, F.; Rguiti, E.; Boilet, L.; Descamps, M.; Cambier, F. Functionalisation of porous hydroxyapatite for bone substitutes. J. Eur. Ceram. Soc. 2012, 32, 2673–2678. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nemati, R.; Nosoudi, N. Preparation and characterisation of porous calcium phosphate bone cement as antibiotic carrier. Adv. Appl. Ceram. 2009, 108, 231–240. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Ratier, A.; Freche, M.; Lacout, J.L.; Rodriguez, F. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int. J. Pharm. 2004, 274, 261–268. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Predoi, D. Vibrational investigations of silver-dovep hydroxyapatite with antibacterial properties. J. Spectrosc. 2013, 2013, 471061. [Google Scholar] [CrossRef]
- Schneider, O.D.; Loher, S.; Brunner, T.J.; Schmidlin, P.; Stark, W.J. Flexible, silver containing nanocomposites for the repair of bone defects: Antimicrobial effect against E. coli infection and comparison to tetracycline containing scaffolds. J. Mater. Chem. 2008, 18, 2679–2684. [Google Scholar] [CrossRef]
- Sanpo, N.; Tan, M.L.; Cheang, P.; Khor, K.A. Antibacterial property of cold-sprayed HA-Ag/PEEK coating. J. Therm. Spray Technol. 2008, 18, 10–15. [Google Scholar] [CrossRef]
- Chen, W.; Oh, S.; Ong, A.P.; Oh, N.; Liu, Y.; Courtney, H.S.; Appleford, M.; Ong, J.L. Antibacterial and osteogenic properties of silver containing hydroxyapatite coatings produced using a sol gel process. J. Biomed. Mater. Res. Part A 2007, 82, 899–906. [Google Scholar] [CrossRef]
- Yang, L.; Ning, X.; Xiao, Q.; Chen, K.; Zhou, H. Development and characterization of porous silver-incorporated hydroxyapatite ceramic for separation and elimination of microorganisms. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxidative Med. Cell. Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvarnapathaki, S.; Nguyen, M.A.; Goulopoulos, A.A.; Lantigua, D.; Camci-Unal, G. Engineering calcium peroxide based oxygen generating scaffolds for tissue survival. Biomater. Sci. 2021, 9, 2519–2532. [Google Scholar] [CrossRef]
- Pedahzur, R.; Lev, O.; Fattal, B.; Shuval, H.I. The interaction of silver ions and hydrogen-peroxide in the inactivation of Escherichia Coli—A prelimlinary evaluation of a new long-lasting residual drinking-water desinfectant. Water Sci. Technol. 1995, 31, 123–129. [Google Scholar] [CrossRef]
- Feuerstein, O.; Moreinos, D.; Steinberg, D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J. Antimicrob. Chemother. 2006, 57, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Vandecandelaere, N.; Bosc, F.; Rey, C.; Drouet, C. Peroxide-doped apatites: Preparation and effect of synthesis parameters. Powder Technol. 2014, 255, 3–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surf. B Biointerfaces 2018, 163, 394–401. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Porous structure of bioceramics carbonated hydroxyapatite-based honeycomb scaffold for bone tissue engineering. Mater. Today Commun. 2021, 26, 102135. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Drouet, C.; Rey, C. Composé Nanocristallin de Structure Apatitique à Propriétés Antimicrobiennes et Son utilisation Pour la Fabrication d’un Matériau à Propriétés Antimicrobiennes. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2012. [Google Scholar]
- Rey, C.; Lian, J.; Grynpas, M.; Shapiro, F.; Zylberberg, L.; Glimcher, M.J. Non-apatitic environments in bone mineral: FT-IR detection, biological properties and changes in several disease states. Connect. Tissue Res. 1989, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-S.; Hwangbo, S.; Kim, J.-T. Silver-doped calcium phosphate nanopowders prepared by electrostatic spraying. J. Nanoparticle Res. 2008, 10, 1337–1341. [Google Scholar] [CrossRef]
- Rameshbabu, N.; Sampath Kumar, T.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Prasad Rao, K. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2007, 80, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Badrour, L.; Sadel, A.; Zahir, M.; Kimakh, L.; El Hajbi, A. Synthesis and physical and chemical characterization of Ca10−xAgx(PO4)6(OH)2−xx apatites. Ann. De Chim. Sci. Des Matériaux 1998, 23, 61–64. [Google Scholar] [CrossRef]
- Lim, P.N.; Tay, B.Y.; Chan, C.M.; Thian, E.S. Synthesis and characterization of silver/silicon-cosubstituted nanohydroxyapatite. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.C.; Li, X.; Wang, J.; Qu, S.; Weng, J.; Zhang, X. Characterization of peroxide ions in hydroxyapatite lattice. J. Biomed. Mater. Res. 2000, 52, 157–163. [Google Scholar] [CrossRef]
- Carrouel, F.; Viennot, S.; Santamaria, J.; Veber, P.; Bourgeois, D. Quantitative molecular detection of 19 major pathogens in the interdental biofilm of periodontally healthy young adults. Front. Microbiol. 2016, 7, 840. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M. Antimicrobial peptides and periodontal disease. J. Clin. Periodontol. 2011, 38, 126–141. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ducheyne, P.; Shapiro, I.M. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. J. Orthop. Res. 1999, 17, 340–345. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Johnson, H.J.; Northup, S.J.; Seagraves, P.A.; Garvin, P.J.; Wallin, R.F. Biocompatibility test procedures for materials evaluation in vitro. II. Objective methods of toxicity assessment. J. Biomed. Mater. Res. 1985, 19, 489–508. [Google Scholar] [CrossRef]
- Schardosim, M.; Soulié, J.; Poquillon, D.; Cazalbou, S.; Duployer, B.; Tenailleau, C.; Rey, C.; Hübler, R.; Combes, C. Freeze-casting for PLGA/carbonated apatite composite scaffolds: Structure and properties. Mater. Sci. Eng. C 2017, 77, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J.; Maquet, V.; Day, R.M.; Jérôme, R. Preparation and characterisation of poly(lactide-co-glycolide) (PLGA) and PLGA/Bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2005, 25, 23–31. [Google Scholar] [CrossRef]
- Chou, Y.-F.; Dunn, J.C.Y.; Wu, B.M. In vitro response of MC3T3-E1 preosteoblasts within three-dimensional apatite-coated PLGA scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Inj. Int. J. Care Inj. 2005, 36, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Deville, S. Freeze-casting of porous biomaterials: Structure, properties and opportunities. Materials 2010, 3, 1913–1927. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Maquet, V. Bioresorbable and bioactive polymer/Bioglass® composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Preparation, characterization, and in vitro degradation of bioresorbable and bioactive composites based on Bioglass-filled polylactide foams. J. Biomed. Mater. Res. A 2003, 66, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, D.; Su, C.; Cong, W.; Mo, X.; Hou, G.; Wang, C. Functionalized biomimetic composite nanfibrous scaffolds with antibacterial and hemostatic efficacy for facilitating wound healing. J. Biomed. Nanotechnol. 2019, 15, 1267–1279. [Google Scholar] [CrossRef]


| Sample Ref | % H2O2 Initial (vol.%) | Maturation Time (Days) | Final Contents (mol Per Unit Cell Formula *) | Selected for Biological Tests | |
|---|---|---|---|---|---|
| Ag+ | O22- | ||||
| O2-hap-10-1 | 10 | 1 | 0 | 0.39 | X (denoted “O2-hap”) |
| O2-hap-25-1 | 25 | 1 | 0 | 0.43 | |
| O2-hap-50-1 | 50 | 1 | 0 | 0.47 | |
| O2-hap-10-3 | 10 | 3 | 0 | 0.42 | |
| O2-hap-25-3 | 25 | 3 | 0 | 0.47 | |
| O2-hap-50-3 | 50 | 3 | 0 | 0.51 | |
| O2-hap-10-7 | 10 | 7 | 0 | 0.46 | |
| O2-hap-25-7 | 25 | 7 | 0 | 0.53 | |
| O2-hap-50-7 | 50 | 7 | 0 | 0.59 | |
| Ag-hap-0-1 | 0 | 1 | 0.11 | 0 | X (denoted “Ag-hap”) |
| Ag-O2-hap-10-1 | 10 | 1 | 0.14 | 0.41 | X (denoted “Ag-O2-hap”) |
| Ag-O2-hap-25-1 | 25 | 1 | 0.15 | 0.53 | |
| Ag-O2-hap-50-1 | 50 | 1 | 0.16 | 0.46 | |
| Ag-O2-hap-10-3 | 10 | 3 | 0.15 | 0.46 | |
| Ag-O2-hap-25-3 | 25 | 3 | 0.15 | 0.50 | |
| Ag-O2-hap-50-3 | 50 | 3 | 0.16 | 0.56 | |
| Ag-O2-hap-10-7 | 10 | 7 | 0.16 | 0.52 | |
| Ag-O2-hap-25-7 | 25 | 7 | 0.16 | 0.59 | |
| Ag-O2-hap-50-7 | 50 | 7 | 0.16 | 0.66 | |
| hap-0-1 | 0 | 1 | 0 | 0 | X (denoted “hap”) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ana, I.D.; Lestari, A.; Lagarrigue, P.; Soulie, J.; Anggraeni, R.; Maube-Bosc, F.; Thouron, C.; Duployer, B.; Tenailleau, C.; Drouet, C. Safe-by-Design Antibacterial Peroxide-Substituted Biomimetic Apatites: Proof of Concept in Tropical Dentistry. J. Funct. Biomater. 2022, 13, 144. https://doi.org/10.3390/jfb13030144
Ana ID, Lestari A, Lagarrigue P, Soulie J, Anggraeni R, Maube-Bosc F, Thouron C, Duployer B, Tenailleau C, Drouet C. Safe-by-Design Antibacterial Peroxide-Substituted Biomimetic Apatites: Proof of Concept in Tropical Dentistry. Journal of Functional Biomaterials. 2022; 13(3):144. https://doi.org/10.3390/jfb13030144
Chicago/Turabian StyleAna, Ika Dewi, Any Lestari, Prescillia Lagarrigue, Jérémy Soulie, Rahmi Anggraeni, Françoise Maube-Bosc, Carole Thouron, Benjamin Duployer, Christophe Tenailleau, and Christophe Drouet. 2022. "Safe-by-Design Antibacterial Peroxide-Substituted Biomimetic Apatites: Proof of Concept in Tropical Dentistry" Journal of Functional Biomaterials 13, no. 3: 144. https://doi.org/10.3390/jfb13030144
APA StyleAna, I. D., Lestari, A., Lagarrigue, P., Soulie, J., Anggraeni, R., Maube-Bosc, F., Thouron, C., Duployer, B., Tenailleau, C., & Drouet, C. (2022). Safe-by-Design Antibacterial Peroxide-Substituted Biomimetic Apatites: Proof of Concept in Tropical Dentistry. Journal of Functional Biomaterials, 13(3), 144. https://doi.org/10.3390/jfb13030144