Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges
Abstract
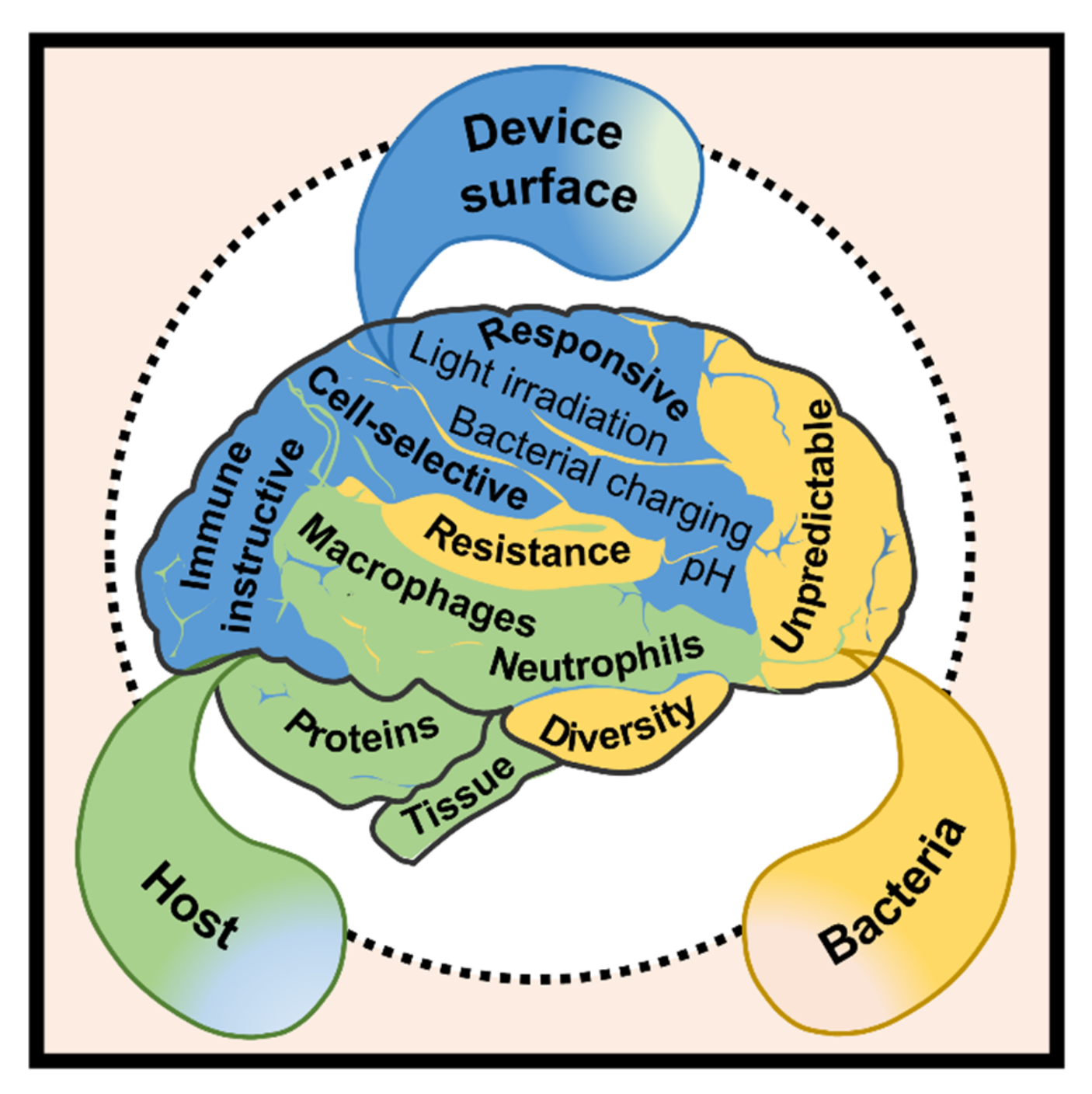
:1. Introduction
2. Clinical Features of Device-Associated Infections
2.1. Site-Specific Incidence
2.2. The Unpredictable Onset
2.3. Diversity of Relevant Pathogens
2.4. Prevalence of Antibiotic Resistance
3. Innovative Designs to Mitigate Device-Associated Infections
3.1. Prolonged Antibacterial Efficacy
3.2. Response to pH Shifts
3.3. Response to Bacterial Charging
3.4. Response to Light Irradiation
3.5. Cell-Selective Materials Surfaces
3.6. Immune-Instructive Materials Surfaces
4. Directions to Improve the Quality of Antibacterial Reports
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.E.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef]
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009-a World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.; Wiese, J.; Amory, J.K.; Bernstein, M.L.; Patel, U.D.; Zemencuk, J.K.; Bernstein, S.J.; Lipsky, B.A.; Hofer, T.P. Are physicians aware of which of their patients have indwelling urinary catheters. Am. J. Med. 2000, 109, 476–480. [Google Scholar] [CrossRef]
- Sloan, M.; Premkumar, A.; Sheth, N.P. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J. Bone Jt. Surg. Am. 2018, 100, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of primary TKA and THA in Germany from 2016 through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Matharu, G.S.; Culliford, D.J.; Blom, A.W.; Judge, A. Projections for primary hip and knee replacement surgery up to the year 2060: An analysis based on data from the national joint registry for England, Wales, Northern Ireland and the Isle of Man. Ann. R. Coll. Surg. Engl. 2021, 104, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Emerging infections program healthcare-associated infections and antimicrobial use prevalence survey team. multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices-an update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Chang, C.H.; Lee, S.H.; Lin, Y.C.; Wang, Y.C.; Chang, C.J.; Hsieh, P.H. Increased periprosthetic hip and knee infection projected from 2014 to 2035 in Taiwan. J. Infect. Public Health 2020, 13, 1768–1773. [Google Scholar] [CrossRef]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected economic burden of periprosthetic joint infection of the hip and knee in the United States. J. Arthroplast. 2021, 36, 1484–1489. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.; Wang, H.; Ren, L.; Yang, K. Study on mechanical behavior of Cu-bearing antibacterial titanium alloy implant. J. Mech. Behav. Biomed. Mater. 2022, 125, 104926. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Xie, J.; Qu, X.; Liu, C. Lysozyme amyloid fibril-integrated PEG injectable hydrogel adhesive with improved antiswelling and antibacterial capabilities. Biomacromolecules 2022, 23, 1376–1391. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, J.; Fu, Z.; Meng, S.; Dai, L.; Cai, K. Polydopamine-Mediated Interfacial Functionalization of Implants for Accelerating Infected Bone Repair through Light-Activatable Antibiosis and Carbon Monoxide Gas Regulated Macrophage Polarization. Adv. Funct. Mater. 2022, 2200374. [Google Scholar] [CrossRef]
- Mou, X.; Zhang, H.; Qiu, H.; Zhang, W.; Wang, Y.; Xiong, K.; Huang, N.; Santos, H.A.; Yang, Z. Mussel-inspired and bioclickable peptide engineered surface to combat thrombosis and infection. Research 2022, 2022, 9780879. [Google Scholar] [CrossRef]
- Cao, H.; Dauben, T.J.; Helbing, C.; Jia, Z.; Zhang, Y.; Huang, M.; Müller, L.; Gu, S.; Zhang, X.; Qin, H.; et al. The antimicrobial effect of calcium-doped titanium is activated by fibrinogen adsorption. Mater. Horiz. 2022. [Google Scholar] [CrossRef]
- Ye, J.; Li, B.; Li, M.; Zheng, Y.; Wu, S.; Han, Y. Formation of a ZnO nanorods-patterned coating with strong bactericidal capability and quantitative evaluation of the contribution of nanorods-derived puncture and ROS-derived killing. Bioact Mater 2022, 11, 181–191. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, Z.; Yang, Y.; Shi, J.; Lv, J.; Fang, Y.; Shen, Z.; Lv, Z.; Li, P.; Yao, X.; et al. A multifunctional antibacterial coating on bone implants for osteosarcoma therapy and enhanced osteointegration. A multifunctional antibacterial coating on bone implants for osteosarcoma therapy and enhanced osteointegration. Chem. Eng. J. 2022, 428, 131155. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired integration of naturally occurring molecules towards universal and smart antibacterial coatings. Adv. Funct. Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Li, W.; Hua, G.; Cai, J.; Zhou, Y.; Zhou, X.; Wang, M.; Wang, X.; Fu, B.; Ren, L. Multi-stimulus responsive multilayer coating for treatment of device-associated infections. J. Funct. Biomater. 2022, 13, 24. [Google Scholar] [CrossRef]
- Shiue, S.; Syu, F.; Lin, H. Two types of bacteriophage-modified alginate hydrogels as antibacterial coatings for implants. Two types of bacteriophage-modified alginate hydrogels as antibacterial coatings for implants. J. Taiwan Inst. Chem. Eng. 2022, 134, 104353. [Google Scholar] [CrossRef]
- Tredget, E.E.; Shankowsky, H.A.; Groenveld, A.; Burrell, R. A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. J. Burn Care Rehabil. 1998, 19, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Reul, M.; Nijs, S. The use of gentamicin-coated nails in complex open tibia fracture and revision cases: A retrospective analysis of a single centre case series and review of the literature. Injury 2015, 46, 2433–2437. [Google Scholar] [CrossRef]
- Takakura, Y.; Tanaka, Y.; Kumai, T.; Sugimoto, K.; Ohgushi, H. Ankle arthroplasty using three generations of metal and ceramic prostheses. Clin. Orthop. Relat. Res. 2004, 424, 130–136. [Google Scholar] [CrossRef]
- El-Sayed, D.; Nouvong, A. Infection protocols for implants. Clin. Podiat.r Med. Surg. 2019, 36, 627–649. [Google Scholar] [CrossRef]
- Merola, M.; Affatato, S. Materials for hip prostheses: A review of wear and loading considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Henderson, R.A.; Austin, M.S. Management of periprosthetic joint infection: The more we learn, the less we know. J. Arthroplast. 2017, 32, 2056–2059. [Google Scholar] [CrossRef]
- Mihalko, W.M.; Haider, H.; Kurtz, S.; Marcolongo, M.; Urish, K. New materials for hip and knee joint replacement: What’s hip and what’s in kneed? J. Orthop. Res. 2020, 38, 1436–1444. [Google Scholar] [CrossRef]
- Perry, D.; Frame, J.D. The history and development of breast implants. Ann. R. Coll. Surg. Engl. 2020, 10, 478–482. [Google Scholar] [CrossRef]
- Hall, B.R.; Billue, K.L.; Sanders, S.E.; Meyer, B.R.; Johnson, P.J. Salmonella infection of breast implant associated with traveler’s diarrhea: A case report. JPRAS Open 2018, 18, 59–64. [Google Scholar] [CrossRef]
- Franchelli, S.; Pesce, M.; Savaia, S.; Marchese, A.; Barbieri, R.; Baldelli, I.; De Maria, A. Clinical and microbiological characterization of late breast implant infections after reconstructive breast cancer surgery. Surg. Infect. 2015, 16, 636–644. [Google Scholar] [CrossRef]
- Chakfé, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.W.J.M.; Koncar, I.; Lindholt, J.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical practice guidelines on the management of vascular graft and endograft infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef] [Green Version]
- Viola, G.M.; Darouiche, R.O. Cardiovascular implantable device infections. Curr. Infect. Dis. Rep. 2011, 13, 333–342. [Google Scholar] [CrossRef]
- Zheng, Q.; Tang, Q.; Wang, Z.L.; Li, Z. Self-powered cardiovascular electronic devices and systems. Nat. Rev. Cardiol. 2021, 18, 7–21. [Google Scholar] [CrossRef]
- Zerbo, S.; Perrone, G.; Bilotta, C.; Adelfio, V.; Malta, G.; Di Pasquale, P.; Maresi, E.; Argo, A. Cardiovascular implantable electronic device infection and new insights about correlation between pro-inflammatory markers and heart failure: A systematic literature review and meta-analysis. Front. Cardiovasc. Med. 2021, 8, 602275. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Chan, E.J.; Cantillon, D.J.; Doonan, A.L.; Hu, T.; Schmitt, S.; Fraser, T.G.; Kim, A.; Gordon, S.M.; Wilkoff, B.L. Cardiac implantable electronic device infections: Presentation, management, and patient outcomes. Heart Rhythm. 2010, 7, 1043–1047. [Google Scholar] [CrossRef]
- Korkerdsup, T.; Ngarmukos, T.; Sungkanuparph, S.; Phuphuakrat, A. Cardiac implantable electronic device infection in the cardiac referral center in Thailand: Incidence, microbiology, risk factors, and outcomes. J. Arrhythm. 2018, 34, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Stöver, T.; Lenarz, T. Biomaterials in cochlear implants. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc10. [Google Scholar]
- Lodhi, F.; Coelho, D.H. Non-tuberculous mycobacterial cochlear implant infection: An emerging pathogen. Cochlear Implant. Int. 2015, 16, 237–240. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, A.; Bhatia, K.; Lahiri, A.K.; Singh, S. Salvaging cochlear implant after wound infection: Well worth a try. Cochlear Implant. Int. 2017, 18, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, K.O.; Golub, J.S.; Roland, J.T.; Samy, R.N. Recurrent cochlear implant infection treated with exteriorization and partial mastoid obliteration. Cochlear Implant. Int. 2016, 17, 58–61. [Google Scholar] [CrossRef]
- Vaid, N.; Vaid, S.; Manikoth, M. Case report-Biofilm infection of a cochlear implant. Cochlear Implant. Int. 2013, 14, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Biomaterials selection for neuroprosthetics. Curr. Opin. Biomed. Eng. 2018, 6, 99–109. [Google Scholar] [CrossRef]
- Shenai, M.B.; Falconer, R.; Rogers, S. A cupriavidus pauculus infection in a patient with a deep brain stimulation implant. Cureus 2019, 11, e6104. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Gordon, C.R.; Bergey, G.K.; Sacks, J.M.; Anderson, W.S. Implant site infection and bone flap osteomyelitis associated with the neuropace responsive neurostimulation system. World Neurosurg. 2016, 88, 687.e1–687.e6. [Google Scholar] [CrossRef]
- Lawrence, E.L.; Turner, I.G. Materials for urinary catheters: A review of their history and development in the UK. Med. Eng. Phys. 2005, 27, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wann, S.R.; Lin, S.L.; Kunin, C.M.; Kung, M.H.; Lin, C.H.; Hsu, C.W.; Liu, C.P.; Lee, S.S.; Liu, Y.C.; et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect. Control Hosp. Epidemiol. 2004, 25, 974–978. [Google Scholar] [CrossRef]
- Lo, E.; Nicolle, L.E.; Coffin, S.E.; Gould, C.; Maragakis, L.L.; Meddings, J.; Pegues, D.A.; Pettis, A.M.; Saint, S.; Yokoe, D.S. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 2014, 35, 464–479. [Google Scholar] [CrossRef] [Green Version]
- Luzum, M.; Sebolt, J.; Chopra, V. Catheter-associated urinary tract infection, clostridioides difficile colitis, central line-associated bloodstream infection, and methicillin-resistant staphylococcus aureus. Med. Clin. North Am. 2020, 104, 663–679. [Google Scholar] [CrossRef]
- Li, F.; Song, M.; Xu, L.; Deng, B.; Zhu, S.; Li, X. Risk factors for catheter-associated urinary tract infection among hospitalized patients: A systematic review and meta-analysis of observational studies. J. Adv. Nurs. 2019, 75, 517–527. [Google Scholar] [CrossRef]
- Shuman, E.K.; Chenoweth, C.E. Urinary catheter-associated infections. Infect. Dis. Clin. North Am. 2018, 32, 885–897. [Google Scholar] [CrossRef]
- Del Bigio, M.R. Biological reactions to cerebrospinal fluid shunt devices: A review of the cellular pathology. Neurosurgery 1998, 42, 319–326. [Google Scholar] [CrossRef]
- Canadian Nosocomial Infection Surveillance Program. Device-associated infections in Canadian acute-care hospitals from 2009 to 2018. Can. Commun. Dis. Rep. 2020, 46, 387–397. [Google Scholar] [CrossRef]
- Shibamura-Fujiogi, M.; Ormsby, J.; Breibart, M.; Warf, B.; Priebe, G.P.; Soriano, S.G.; Sandora, T.J.; Yuki, K. Risk factors for pediatric surgical site infection following neurosurgical procedures for hydrocephalus: A retrospective single-center cohort study. BMC Anesthesiol. 2021, 21, 124. [Google Scholar] [CrossRef]
- Benachinmardi, K.K.; Ravikumar, R.; Indiradevi, B. Role of biofilm in cerebrospinal fluid shunt infections: A study at tertiary neurocare center from South India. J. Neurosci. Rural. Pract. 2017, 8, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Méndez, R.; Richards, H.K.; Seeley, H.M.; Pickard, J.D.; Joannides, A.J. UKSR collaborators, Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004–2013). J. Neurol. Neurosurg. Psychiatry 2019, 90, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.M.; Kulkarni, S.S. A review on biomaterials in orthopedic bone plate application. International J. Curr. Eng. Technol. 2015, 5, 2587–2591. [Google Scholar]
- Toro-Aguilera, Á.; Zuriarrain, S.W.; Masdeu, M.G.; Sayol, R.R.; Billi, A.M.; Carrera, I.; de Caso, J. Risk factors for infection in fixation of distal tibia fractures. Injury 2021, 52 (Suppl. 4), S104–S108. [Google Scholar] [CrossRef]
- Guillaume, B. Dental implants: A review. Morphologie 2016, 100, 189–198. [Google Scholar] [CrossRef]
- Neely, A.L.; Maalhagh-Fard, A. Successful management of early peri-implant infection and bone loss using a multidisciplinary treatment approach. Clin. Adv. Periodontics 2018, 8, 5–10. [Google Scholar] [CrossRef]
- Patton, D.; Kiewiet, N.; Brage, M. Infected total ankle arthroplasty: Risk factors and treatment options. Foot Ankle Int. 2015, 36, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Gbejuade, H.O.; Lovering, A.M.; Webb, J.C. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015, 86, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Bayston, R.; Lari, J. A study of the sources of infection in colonised shunts. Dev. Med. Child Neurol. 1974, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Reynolds-Campbell, G.; Nicholson, A.; Thoms-Rodriguez, C.A. Oral bacterial infections: Diagnosis and management. Dent. Clin. N. Am. 2017, 61, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; de Mesy Bentley, K.L.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Bain, C.J.; Odili, J. Late infection of an alloplastic chin implant masquerading as squamous cell carcinoma. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, e151–e152. [Google Scholar] [CrossRef]
- Chang, J.; Lee, G.W. Late hematogenous bacterial infections of breast implants: Two case reports of unique bacterial infections. Ann. Plast. Surg. 2011, 67, 14–16. [Google Scholar] [CrossRef]
- Beidas, O.E.; Rabb, C.H.; Sawan, K.T.; Tan, B.K. The pseudomeningocoele that wasn’t: Case report of an adult who presented with a late infection of an implant. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1228–1231. [Google Scholar] [CrossRef]
- Vichitvejpaisal, P.; Dalvin, L.A.; Lally, S.E.; Shields, C.L. Delayed implant infection with Cutibacterium acnes (Propionibacterium acnes) 30 years after silicone sheet orbital floor implant. Orbit 2020, 39, 139–142. [Google Scholar] [CrossRef]
- Coden, D.J.; Hornblass, A. Propionibacterium acnes orbital abscess. Arch. Ophthalmol. 1990, 108, 481. [Google Scholar] [CrossRef]
- Hannouille, J.; Belgrado, J.P.; Vankerchove, S.; Vandermeeren, L. Breast implant infection with pasteurella canis: First case-report. JPRAS Open 2019, 21, 86–88. [Google Scholar] [CrossRef]
- Oses, M.; Ordás, C.M.; Feliz, C.; Del Val, J.; Ayerbe, J.; García-Ruiz, P.J. Disease-modifying anti-rheumatic drugs as a risk factor for delayed DBS implant infection. Parkinsonism Relat. Disord. 2018, 55, 143–144. [Google Scholar] [CrossRef]
- Young, P.; Riga, A.; Brunelli, J. Nocardia nova infection of tibia tenodesis implant after anterior cruciate ligament reconstruction in an immunocompetent patient. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e19.00167. [Google Scholar] [CrossRef]
- Paziuk, T.; Levicoff, E.; Tan, T.; Good, R. Periprosthetic joint infection with listeria monocytogenes: A case report. JBJS Case Connect 2020, 10, e1900489. [Google Scholar] [CrossRef]
- Madden, G.R.; Poulter, M.D.; Crawford, M.P.; Wilson, D.S.; Donowitz, G.R. Case report: Anaerobiospirillum prosthetic joint infection in a heart transplant recipient. BMC Musculoskelet. Disord. 2019, 20, 301. [Google Scholar] [CrossRef]
- Haimes, M.A.; Nelms, N.J. Total knee bartonella henselae infection: An unusual manifestation of cat scratch disease: A case report. JBJS Case Connect 2019, 9, e0081. [Google Scholar] [CrossRef]
- Posti, J.P.; Piitulainen, J.M.; Hupa, L.; Fagerlund, S.; Frantzén, J.; Aitasalo, K.M.J.; Vuorinen, V.; Serlo, W.; Syrjänen, S.; Vallittu, P.K. A glass fiber-reinforced composite—bioactive glass cranioplasty implant: A case study of an early development stage implant removed due to a late infection. J. Mech. Behav. Biomed. Mater. 2016, 55, 191–200. [Google Scholar] [CrossRef]
- Wahl, P.; Sprecher, C.M.; Brüning, C.; Meier, C.; Milz, S.; Gautier, E.; Moriarty, T.F. Successful bony integration of a porous tantalum implant despite longlasting and ongoing infection: Histologic workup of an explanted shoulder prosthesis. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2924–2931. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Dowd, S.E.; Sun, Y.; Secor, P.R.; Rhoads, D.D.; Wolcott, B.M.; James, G.A.; Wolcott, R.D. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, K.J.; Ahmad, N.; Alspaugh, J.A.; Drew, W.L. Sherris Medical Microbiology, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 381–735. [Google Scholar]
- Männik, J.; Driessen, R.; Galajda, P.; Keymer, J.E.; Dekker, C. Bacterial growth and motility in sub-micron constrictions. Proc. Natl. Acad. Sci. USA 2009, 106, 14861–14866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pianetti, A.; Battistelli, M.; Citterio, B.; Parlani, C.; Falcieri, E.; Bruscolini, F. Morphological changes of Aeromonas hydrophila in response to osmotic stress. Micron 2009, 40, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Alfredo, N.; Santos-Coquillat, A.; Martínez-Campos, E.; Dorronsoro, A.; Cortajarena, A.L.; Del Campo, A.; Rodríguez-Hernández, J. Highly efficient antibacterial surfaces based on bacterial/cell size selective microporous supports. ACS Appl. Mater. Interfaces 2017, 9, 44270–44280. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Cristina, M.; Martins, L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Lister, J. On a new method of treating compound fracture, abscess, etc.: With observations on the conditions of suppuration. Lancet 1867, 89, 326–329. [Google Scholar] [CrossRef]
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Bryson, D.J.; Morris, D.L.J.; Shivji, F.S.; Rollins, K.R.; Snape, S.; Ollivere, B.J. Antibiotic prophylaxis in orthopaedic surgery: Difficult decisions in an era of evolving antibiotic resistance. Bone Joint J. 2016, 98, 1014–1019. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Vasiliadis, A.V.; Poutoglidou, F.; Chatziravdeli, V.; Metaxiotis, D.; Beletsiotis, A. Acute periprosthetic hip joint infection caused by multidrug-resistant acinetobacter baumannii: Is debridement, antibiotics, irrigation, and implant retention a viable treatment option? Cureus 2021, 13, e13090. [Google Scholar] [CrossRef]
- Okada, A.; Shoda, M.; Tabata, H.; Kobayashi, H.; Shoin, W.; Okano, T.; Yoshie, K.; Kato, K.; Motoki, H.; Kuwahara, K. Simultaneous infection of abandoned leads and newly implanted leadless cardiac pacemaker: Why did this occur? J. Cardiol. Cases 2020, 23, 35–37. [Google Scholar] [CrossRef]
- Jhaveri, V.V.; Singhal, D.; Riedel, S.; Rowley, C.F.; Nathavitharana, R.R. Surgical cure of clarithromycin resistant Mycobacterium chelonae breast implant infection: A case report and review of the literature. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 21, 100183. [Google Scholar] [CrossRef]
- El-Zein, R.S.; Stelzer, M.; Hatanelas, J.; Goodlive, T.W.; Amin, A.K. A ghost left behind after transvenous lead extraction: A finding to be feared. Am. J. Case Rep. 2020, 21, e924243. [Google Scholar] [CrossRef]
- Palacios, L.; de Nova, A.A.; Pardo, M.G. Conservative multimodal management of osteosynthesis material in surgical wounds with polymicrobial superinfection, including methicillin-resistant Staphylococcus aureus, Clinical case. Rev. Española Cirugía Ortopédica Traumatol. (Engl. Ed.) 2020, 64, 125–129. [Google Scholar] [CrossRef]
- Hwang, S.O.; Chang, L.S. Salvage of an exposed cranial prosthetic implant using a transposition flap with an indwelling antibiotic irrigation system. Arch. Craniofac. Surg. 2020, 21, 73–76. [Google Scholar] [CrossRef]
- Fukushima, S.; Komune, N.; Kamizono, K.; Matsumoto, N.; Takaiwa, K.; Nakagawa, T.; Kadota, H. Use of negative pressure wound therapy to treat a cochlear implant infection around the auricle: A case report. J. Wound Care 2020, 29, 568–571. [Google Scholar] [CrossRef]
- Bajaj, T.; Karapetians, A.; Karapetians, N.; Duong, H.; Heidari, A. Methicillin resistant Staphylococcus aureus infective endocarditis presenting as neutrophilic meningoencephalitis. AME Case Rep. 2020, 4, 4. [Google Scholar] [CrossRef]
- Hisanaga, K.; Kadota, H.; Fukushima, S.; Inatomi, Y.; Shimamoto, R.; Kamizono, K.; Hanada, M.; Yoshida, S. Toxic shock syndrome caused by staphylococcal infection after breast implant surgery: A case report and literature review. Ann. Plast. Surg. 2019, 83, 359–362. [Google Scholar] [CrossRef]
- Meleca, J.B.; Bryson, P.C. Delayed laryngeal implant infection and laryngocutaneous fistula after medialization laryngoplasty. Am. J. Otolaryngol. 2019, 40, 462–464. [Google Scholar] [CrossRef]
- Siebenbürger, G.; Grabein, B.; Schenck, T.; Kammerlander, C.; Böcker, W.; Zeckey, C. Eradication of acinetobacter baumannii/enterobacter cloacae complex in an open proximal tibial fracture and closed drop foot correction with a multidisciplinary approach using the taylor spatial frame®: A case report. Eur. J. Med. Res. 2019, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, M.; Yoshida, D.; Nagatomo, D.; Suematsu, N.; Kubota, T.; Okabe, M.; Yamamoto, Y. Successful percutaneous retrieval of a micra transcatheter pacing system at 8 weeks after implantation. J. Arrhythm. 2018, 34, 653–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonacker, J.; Darowski, M.; Haar, P.; Westphal, T.; Bergschmidt, P. Periprosthetic tibial fracture with nonunion and ascending prosthetic joint infection: A case report of an individual treatment strategy. J. Orthop. Case Rep. 2018, 8, 3–8. [Google Scholar] [PubMed]
- Rico-Nieto, A.; Moreno-Ramos, F.; Fernández-Baillo, N. Lumbar arthrodesis infection by multi-resistant Klebsiella pneumoniae, successfully treated with implant retention and ceftazidime/avibactam. Rev. Española Cirugía Ortopédica Traumatol. (Engl. Ed.) 2018, 62, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, G.N.; Deam, A.G. Simultaneous suction debulking of lead vegetation prior to percutaneous lead extraction. J. Cardiol. Cases. 2018, 18, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian, S.; Malhotra, R.; Pande, A.; Gautam, D.; Xess, I.; Dhawan, B. Staged reimplantation of a total hip prosthesis after co-infection with candida tropicalis and staphylococcus haemolyticus: A case report. Mycopathologia 2018, 183, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, P.; Topiwalla, T.T.; Ganesan, G. Drug-resistant coagulase-negative staphylococcal endophthalmitis following dexamethasone intravitreal implant. Indian J. Ophthalmol. 2017, 65, 634–636. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Dworak, M.; Dababneh, A.S.; Palraj, R.V.; Roskos, M.C.; Chapman, S.C. Acute infection of viabahn stent graft in the popliteal artery. J. Vasc. Surg. Cases Innov. Tech. 2017, 3, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Takizawa, T.; Tsutsumimoto, T.; Yui, M.; Misawa, H. Surgical site infections caused by methicillin-resistant staphylococcus epidermidis after spinal instrumentation surgery. Spine 2017, 42, 525–530. [Google Scholar] [CrossRef]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [Green Version]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control Release 2008, 130, 202–215. [Google Scholar] [CrossRef]
- Freischmidt, H.; Armbruster, J.; Reiter, G.; Grützner, P.A.; Helbig, L.; Guehring, T. Individualized techniques of implant coating with an antibiotic-loaded, hydroxyapatite/calcium sulphate bone graft substitute. Ther. Clin. Risk Manag. 2020, 16, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Cao, H.; Qin, H.; Li, Y.; Jandt, K.D. The action-networks of nanosilver: Bridging the gap between material and biology. Adv. Healthc. Mater. 2021, 26, e2100619. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Nadeem, S.F.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Moula Ali, A.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial resistance: More than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 2020, 46, 578–599. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef]
- Wu, X.X.; Zhang, Y.; Hu, T.; Li, W.X.; Li, Z.L.; Hu, H.J.; Zhu, S.R.; Chen, W.Z.; Zhou, C.S.; Jiang, G.B. Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. 2021, 167, 1211–1220. [Google Scholar] [CrossRef]
- Mukai, M.; Uchida, K.; Sugo, K.; Nakasu, M.; Nakajima, T.; Takata, K.; Takaso, M.; Urabe, K. Long-term antibacterial activity of vancomycin from calcium phosphate cement in vivo. Biomed. Mater. Eng. 2022, 33, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mei, S.; Kong, X.; Liu, X.; Gao, B.; Chen, B.; Wu, J. Long-term antibacterial activity of a composite coating on titanium for dental implant application. J. Biomater. Appl. 2021, 35, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, H.; Tsutsumi, Y.; Shimabukuro, M.; Manaka, T.; Chen, P.; Ashida, M.; Ishikawa, K.; Katayama, H.; Hanaw, T. Investigation of the long-term antibacterial properties of titanium by two-step micro-arc oxidation treatment. Coatings 2021, 11, 798. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-outside Ag nanoparticles-loaded polylactic acid electrospun fiber for long-term antibacterial and bone regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [CrossRef]
- Tao, S.; Yang, X.; Liao, L.; Yang, J.; Liang, K.; Zeng, S.; Zhou, J.; Zhang, M.; Li, J. A novel anticaries agent, honokiol-loaded poly(amido amine) dendrimer, for simultaneous long-term antibacterial treatment and remineralization of demineralized enamel. Dent. Mater. 2021, 37, 1337–1349. [Google Scholar] [CrossRef]
- Jia, J.; Duan, S.; Zhou, X.; Sun, L.; Qin, C.; Li, M.; Ge, F. Long-term antibacterial film nanocomposite incorporated with patchouli essential oil prepared by supercritical co 2 cyclic impregnation for wound dressing. Molecules 2021, 26, 5005. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kitagawa, R.; Tsuboi, R.; Hirose, N.; Thongthai, P.; Sakai, H.; Ueda, M.; Ono, S.; Sasaki, J.; Ooya, T.; et al. Development of endodontic sealers containing antimicrobial-loaded polymer particles with long-term antibacterial effects. Dent. Mater. 2021, 37, 1248–1259. [Google Scholar] [CrossRef]
- Wan, R.; Chu, S.; Wang, X.; Lei, L.; Tang, H.; Hu, G.; Dong, L.; Li, D.; Gu, H. Study on the osteogenesis of rat mesenchymal stem cells and the long-term antibacterial activity of Staphylococcus epidermidis on the surface of silver-rich TiN/Ag modified titanium alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3008–3021. [Google Scholar] [CrossRef]
- Liang, T.; Wang, Y.; Zeng, L.; Liu, Y.; Qiao, L.; Zhang, S.; Zhao, R.; Li, G.; Zhang, R.; Xiang, J.; et al. Copper-doped 3D porous coating developed on Ti-6Al-4V alloys and its in vitro long-term antibacterial ability. Appl. Surf. Sci. 2020, 509, 144717. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, M.; Gu, W.; Shen, Z.; Ma, X.; Lu, F.; Yang, X.; Zheng, Y.; Gou, Z. Zinc-/copper-substituted dicalcium silicate cement: Advanced biomaterials with enhanced osteogenesis and long-term antibacterial properties. J. Mater. Chem. B. 2020, 8, 1060–1070. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X.; Cai, J.; Ye, D.; Wu, Y.; Liu, P. Dual controlled release nanomicelle-in-nanofiber system for long-term antibacterial medical dressings. J. Biomater. Sci. Polym. Ed. 2019, 30, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.M.; Masri, N.A.; Malek, N.A.N.N.; Razak, S.I.A.; Saidin, S. Long-term antibacterial and stable chlorhexidine-polydopamine coating on stainless steel 316L. Prog. Org. Coat. 2018, 122, 147–153. [Google Scholar] [CrossRef]
- Shivaram, A.; Bose, S.; Bandyopadhyay, A. Understanding long-term silver release from surface modified porous titanium implants. Acta Biomater. 2017, 58, 550–560. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable nanocomposite based on pegylated and silver nanoparticles loaded graphene oxide for long-term antibacterial activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef]
- Wang, G.; Feng, H.; Jin, W.; Gao, A.; Peng, X.; Li, W.; Wu, H.; Li, Z.; Chu, P.K. Long-term antibacterial characteristics and cytocompatibility of titania nanotubes loaded with Au nanoparticles without photocatalytic effects. Appl. Surf. Sci. 2017, 414, 230–237. [Google Scholar] [CrossRef]
- Uhm, S.; Kwon, J.; Song, D.; Lee, E.; Jeong, W.; Oh, S.; Kim, K.; Choi, E.H.; Kim, K. Long-Term antibacterial performance and bioactivity of plasma-engineered Ag-NPs/TiO2. J. Biomed. Nanotechnol. 2016, 12, 1890–1906. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Zhu, C.; Cheng, T.; Wang, Q.; Peng, X.; Cheng, M.; Wang, J.; Jin, G.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014, 35, 9114–9125. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Xu, L.Q.; Wang, R.; Kang, E.; Lau, T.; Olszyna, D.P.; Chiong, E. Surface modification of silicone for biomedical applications requiring long-term antibacterial, antifouling, and hemocompatible properties. Langmuir 2012, 28, 16408–16422. [Google Scholar] [CrossRef]
- Urabe, K.; Naruse, K.; Hattori, H.; Hirano, M.; Uchida, K.; Onuma, K.; Park, H.J.; Itoman, M. In vitro comparison of elution characteristics of vancomycin from calcium phosphate cement and polymethylmethacrylate. J. Orthop. Sci. 2009, 14, 784–793. [Google Scholar] [CrossRef]
- Uchida, K.; Sugo, K.; Nakajima, T.; Nakawaki, M.; Takano, S.; Nagura, N.; Takaso, M.; Urabe, K. In vivo release of vancomycin from calcium phosphate cement. Biomed. Res. Int. 2018, 2018, 4560647. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Shivaram, A.; Tarafder, S.; Sahasrabudhe, H.; Banerjee, D.; Bose, S. In vivo response of laser processed porous titanium implants for load-bearing implants. Ann. Biomed. Eng. 2017, 45, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, W.; Meng, F.; Guo, J.; Wang, D.; Qian, S.; Jiang, X.; Liu, X.; Chu, P.K. Osteogenesis catalyzed by titanium-supported silver nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, C.; Yang, K. Contact killing of Cu-bearing stainless steel based on charge transfer caused by the microdomain potential difference. ACS Appl. Mater. Interfaces 2020, 12, 361–372. [Google Scholar] [CrossRef]
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, E1. [Google Scholar] [CrossRef] [Green Version]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Sawyer, R.G.; Spengler, M.D.; Adams, R.B.; Pruett, T.L. The peritoneal environment during infection. The effect of monomicrobial and polymicrobial bacteria on pO2 and pH. Ann. Surg. 1991, 213, 253–260. [Google Scholar] [CrossRef]
- Stassen, W.N.; McCullough, A.J.; Bacon, B.R.; Gutnik, S.H.; Wadiwala, I.M.; McLaren, C.; Kalhan, S.C.; Tavill, A.S. Immediate diagnostic criteria for bacterial infection of ascitic fluid. Evaluation of ascitic fluid polymorphonuclear leukocyte count, pH, and lactate concentration, alone and in combination. Gastroenterology 1986, 90, 1247–1254. [Google Scholar] [CrossRef]
- Del Campo, A.; Echeverría, C.; Martín, M.S.; Cuervo-Rodríguez, R.; Fernández-García, M.; Muñoz-Bonilla, A. Porous microstructured surfaces with ph-triggered antibacterial properties. Macromol. Biosci. 2019, 19, 1900127. [Google Scholar] [CrossRef]
- Wei, T.; Yu, Q.; Zhan, W.; Chen, H. A smart antibacterial surface for the on-demand killing and releasing of bacteria. Adv. Healthc. Mater. 2016, 5, 449–456. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.; Long, S.; Zhang, G.; Wang, X. Smart and in-situ formation electrospun fibrous membrane for the control of antimicrobial efficacy. Smart Mater. Med. 2021, 2, 87–95. [Google Scholar] [CrossRef]
- Pinho, E.; Machado, S.; Soares, G. Smart hydrogel for the ph-selective drug delivery of antimicrobial compounds. Macromol. Symp. 2019, 385, 1800182. [Google Scholar] [CrossRef]
- Ramos, M.L.P.; González, J.A.; Fabian, L.; Pérez, C.J.; Villanueva, M.E.; Copello, G.J. Sustainable and smart keratin hydrogel with pH-sensitive swelling and enhanced mechanical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 619–626. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Dall Orto, V.C.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid. Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Tao, B.; Deng, Y.; Song, L.; Ma, W.; Qian, Y.; Lin, C.; Yuan, Z.; Lu, L.; Chen, M.; Yang, X.; et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surf. B Biointerfaces 2019, 177, 242–252. [Google Scholar] [CrossRef]
- Phoungtawee, P.; Seidi, F.; Treetong, A.; Warin, C.; Klamchuen, A.; Crespy, D. Polymers with hemiaminal ether linkages for ph-responsive antibacterial materials. ACS Macro. Lett. 2021, 10, 365–369. [Google Scholar] [CrossRef]
- De Silva, C.C.; Israni, N.; Zanwar, A.; Jagtap, A.; Leophairatana, P.; Koberstein, J.T.; Modak, S.M. “Smart” polymer enhances the efficacy of topical antimicrobial agents. Burns 2019, 45, 1418–1429. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Quartinello, F.; Tallian, C.; Auer, J.; Schön, H.; Vielnascher, R.; Weinberger, S.; Wieland, K.; Weihs, A.M.; Herrero-Rollett, A.; Lendl, B.; et al. Smart textiles in wound care: Functionalization of cotton/PET blends with antimicrobial nanocapsules. J. Mater. Chem. B. 2019, 7, 6592–6603. [Google Scholar] [CrossRef]
- Kaila, V.R.I.; Wikström, M. Architecture of bacterial respiratory chains. Nat. Rev. Microbiol. 2021, 19, 319–330. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Harris, H.W.; El-Naggar, M.Y.; Bretschger, O.; Ward, M.J.; Romine, M.F.; Obraztsova, A.Y.; Nealson, K.H. Electrokinesis is a microbial behavior that requires extracellular electron transport. Proc. Natl. Acad. Sci. USA 2010, 107, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Light, S.H.; Méheust, R.; Ferrell, J.L.; Cho, J.; Deng, D.; Agostoni, M.; Iavarone, A.T.; Banfield, J.F.; D’Orazio, S.E.F.; Portnoy, D.A. Extracellular electron transfer powers flavinylated extracellular reductases in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 26892–26899. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Pankratova, G.; Hederstedt, L.; Gorton, L. Extracellular electron transfer features of Gram-positive bacteria. Anal. Chim. Acta 2019, 1076, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Qiao, Y.; Liu, X.; Lu, T.; Cui, T.; Meng, F.; Chu, P.K. Electron storage mediated dark antibacterial action of bound silver nanoparticles: Smaller is not always better. Acta. Biomater. 2013, 9, 5100–5110. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, Y.; Meng, F.; Liu, X. Spacing-dependent antimicrobial efficacy of immobilized silver nanoparticles. J. Phys. Chem. Lett. 2014, 5, 743–748. [Google Scholar] [CrossRef]
- Wang, M.; Cao, H.; Meng, F.; Zhao, X.; Ping, Y.; Lü, X.; Liu, X. Schottky barrier dependent antimicrobial efficacy of silver nanoparticles. Mater. Lett. 2016, 179, 1–4. [Google Scholar] [CrossRef]
- Cao, H.; Meng, F.; Liu, X. Antimicrobial activity of tantalum oxide coatings decorated with Ag nanoparticles. J. Vac. Sci. Technol. A 2016, 34, 04C102. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Qiu, C.; Iatsunskyi, I.; Coy, E.; Moya, S.; Wang, Z.; Wu, W.; Zhao, X.; Wang, G. Electron transfer correlated antibacterial activity of biocompatible graphene Nanosheets-TiO2 coatings. Carbon 2020, 166, 350–360. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, D.; Guo, G.; Yeung, K.W.K.; Zhang, X.; Liu, X. Band gap engineering of titania film through cobalt regulation for oxidative damage of bacterial respiration and viability. ACS Appl. Mater. Interfaces 2017, 9, 27475–27490. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, T.; Liu, J.; Zhang, X.; Long, F.; Liu, L. Bilayer microstructure of antibacterial TiO2 coating on Ti6Al4V fabricated via micro-arc oxidation in W-containing electrolytes. Surf. Coat. Technol. 2021, 413, 127094. [Google Scholar] [CrossRef]
- Ray, P.C.; Khan, S.A.; Singh, A.K.; Senapati, D.; Fan, Z. Nanomaterials for targeted detection and photothermal killing of bacteria. Chem. Soc. Rev. 2012, 41, 3193–3209. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wei, T.; Zhao, J.; Jiang, S.; Yang, P.; Yu, Q.; Chen, H. Regenerable smart antibacterial surfaces: Full removal of killed bacteria via a sequential degradable layer. J. Mater. Chem. B. 2018, 6, 3946–3955. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, T.; Qu, Y.; Zhou, Y.; Zheng, Y.; Huang, C.; Zhang, Y.; Yu, Q.; Chen, H. Smart, photothermally activated, antibacterial surfaces with thermally triggered bacteria-releasing properties. ACS Appl. Mater. Interfaces 2020, 12, 21283–21291. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2020, 6, 12–25. [Google Scholar] [CrossRef]
- Kováčová, M.; Kleinová, A.; Vajďák, J.; Humpolíček, P.; Kubát, P.; Bodík, M.; Marković, Z.; Špitálský, Z. Photodynamic-active smart biocompatible material for an antibacterial surface coating. J. Photochem. Photobiol. B. 2020, 211, 112012. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Zheng, Y.; Wang, X.; Wu, S. In situ disinfection through photo inspired radical oxygen species storage and thermal-triggered release from black phosphorous with strengthened chemical stability. Small 2018, 14, 1703197. [Google Scholar] [CrossRef]
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mater. 2018, 30, e1801808. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, B.; He, Y.; Liu, J.; Lin, C.; Shen, X.; Ding, Y.; Yu, Y.; Mu, C.; Liu, P.; et al. Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials 2019, 217, 119290. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, X.; Yin, W.; Ma, D.; Xie, C.; Zheng, L.; Dong, X.; Mei, L.; Yu, J.; Wang, C.; et al. Functionalized MoS2 nanovehicle with near-infrared laser-mediated nitric oxide release and photothermal activities for advanced bacteria-infected wound therapy. Small 2018, 14, e1802290. [Google Scholar] [CrossRef]
- Liu, L.; Pan, X.; Liu, S.; Hu, Y.; Ma, D. Near-infrared light-triggered nitric oxide release combined with low-temperature photothermal therapy for synergetic antibacterial and antifungal. Smart Mater. Med. 2021, 2, 302–313. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Stansbury, J.W.; Zhu, X.; Wang, X.; Nie, J. Smart antibacterial surface made by photopolymerization. ACS Appl. Mater. Interfaces 2016, 8, 28047–28054. [Google Scholar] [CrossRef]
- Děkanovský, L.; Elashnikov, R.; Kubiková, M.; Vokatá, B.; Švorčík, V.; Lyutakov, O. Dual-action flexible antimicrobial material: Switchable self-cleaning, antifouling, and smart drug release. Adv. Funct. Mater. 2019, 29, 1901880. [Google Scholar] [CrossRef]
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Miller, L.S.; Segura, T.; Bernthal, N.M. In vivo efficacy of a “smart” antimicrobial implant coating. J. Bone Joint Surg. Am. 2016, 98, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Liu, X. Plasma sprayed ceramic coatings for osseointegration. Int. J. Appl. Ceram. Technol. 2013, 10, 1–10. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: “the race for the surface” experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J.; Kuijer, R. Microbial biofilm growth vs. tissue integration on biomaterials with different wettabilities and a polymer-brush coating. J. Biomed. Mater. Res. A 2010, 94, 533–538. [Google Scholar] [PubMed]
- Subbiahdoss, G.; Pidhatika, B.; Coullerez, G.; Charnley, M.; Kuijer, R.; van der Mei, H.C.; Textor, M.; Busscher, H.J. Bacterial biofilm formation versus mammalian cell growth on titanium-based mono- and bi-functional coating. Eur. Cells Mater. 2010, 19, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Subbiahdoss, G.; Fernandez, I.C.; Domingues, J.F.; Kuijer, R.; van der Mei, H.C.; Busscher, H.J. In vitro interactions between bacteria, osteoblast-like cells and macrophages in the pathogenesis of biomaterial-associated infections. PLoS ONE 2011, 6, e24827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Tanoira, R.; Han, X.; Soininen, A.; Aarnisalo, A.A.; Tiainen, V.M.; Eklund, K.K.; Esteban, J.; Kinnari, T.J. Competitive colonization of prosthetic surfaces by staphylococcus aureus and human cells. J. Biomed. Mater. Res. A 2017, 105, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, M.; Perez-Jorge, C.; Lozano, D.; Portal-Nuñez, S.; Perez-Tanoira, R.; Conde, A.; Arenas, M.A.; Hernandez-Lopez, J.M.; de Damborenea, J.J.; Gomez-Barrena, E.; et al. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model: An in vitro approach to the “race for the surface” theory. Bone Joint Res. 2017, 6, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Mangum, L.H.; Wenke, J.C. Revisiting the “race for the surface” in a pre-clinical model of implant infection. Eur. Cells Mater. 2020, 39, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Gottenbos, B.; Klatter, F.; Van Der Mei, H.C.; Busscher, H.J.; Nieuwenhuis, P. Late hematogenous infection of subcutaneous implants in rats. Clin. Diagn. Lab. Immunol. 2001, 8, 980–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The lack of differentiation between biofilm reducing and inhibitory effects as a current problem in biofilm methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding., C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef]
- Shi, Q.; Luo, X.; Huang, Z.; Midgley, A.C.; Wang, B.; Liu, R.; Zhi, D.; Wei, T.; Zhou, X.; Qiao, M.; et al. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019, 86, 465–479. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Liu, H.; Ren, L.; Wang, Q.; Zhang, Y. Effects of surface roughening on antibacterial and osteogenic properties of Ti-Cu alloys with different Cu contents. J. Mater. Sci. Technol. 2021, 88, 158–167. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Zeng, Q.; Wu, Y.; Hu, Y.; Duan, S.; Tang, Z.; Xu, F. Dual-functional implants with antibacterial and osteointegration-promoting performances. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M.; Minneboo, M.; Modaresifar, K.; van Hengel, I.A.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Osteogenic and antibacterial surfaces on additively manufactured porous Ti-6Al-4V implants: Combining silver nanoparticles with hydrothermally synthesized HA nanocrystals. Mater. Sci. Eng. C 2021, 120, 111745. [Google Scholar] [CrossRef]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart hydrogel-based DVDMS/bFGF nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef]
- Wei, S.; Chang, L.; Huang, C.; Chang, H. Dual-functional gold nanoparticles with antimicrobial and proangiogenic activities improve the healing of multidrug-resistant bacteria-infected wounds in diabetic mice. Biomater. Sci. 2019, 7, 4482–4490. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Yuan, K.; Yang, S.; Tang, T. Dual-functional hybrid quaternized chitosan/Mg/alginate dressing with antibacterial and angiogenic potential for diabetic wound healing. J. Orthop. Translat. 2021, 30, 6–15. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Deshpande, K.B. Numerical modeling of micro-galvanic corrosion. Electrochim. Acta 2011, 56, 1737–1745. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Jin, G.; Cheng, M.; Wang, J.; Jiang, Y.; An, Z.; Zhang, X.; Liu, X. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl. Mater. Interfaces 2015, 7, 10785–10794. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653–664. [Google Scholar]
- Cao, H.; Tang, K.; Liu, X. Bifunctional galvanics mediated selective toxicity on titanium. Mater. Horiz. 2018, 5, 264–267. [Google Scholar] [CrossRef]
- Hazell, G.; May, P.W.; Taylor, P.; Nobbs, A.H.; Welch, C.C.; Su, B. Studies of black silicon and black diamond as materials for antibacterial surfaces. Biomater. Sci. 2018, 6, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Chatterjee, K. Bactericidal anisotropic nanostructures on titanium fabricated by maskless dry etching. ACS Appl. Nano Mater. 2022, 5, 4447–4461. [Google Scholar] [CrossRef]
- Tang, K.; Wang, L.; Geng, H.; Qiu, J.; Cao, H.; Liu, X. Molybdenum disulfide (MoS2) nanosheets vertically coated on titanium for disinfection in the dark. Arab. J. Chem. 2020, 13, 1612–1623. [Google Scholar] [CrossRef]
- Luo, Q.; Cao, H.; Wang, L.; Ma, X.; Liu, X. ZnO@ZnS nanorod-array coated titanium: Good to fibroblasts but bad to bacteria. J. Colloids Interface Sci. 2020, 579, 50–60. [Google Scholar] [CrossRef]
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine nanorods. ACS Nano 2017, 11, 11250–11263. [Google Scholar] [CrossRef]
- Lüdecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf. B Biointerfaces 2016, 145, 617–625. [Google Scholar] [CrossRef]
- Dauben, T.J.; Dewald, C.; Firkowska-Boden, I.; Helbing, C.; Peisker, H.; Roth, M.; Bossert, J.; Jandt, K.D. Quantifying the relationship between surfaces’ nano-contact point density and adhesion force of Candida albicans. Colloids Surf. B Biointerfaces. 2020, 194, 111177. [Google Scholar] [CrossRef]
- Hawi, S.; Goel, S.; Kumar, V.; Pearce, O.; Ayre, W.N.; Ivanova, E.P. Critical review of nanopillar-based mechanobactericidal systems. ACS Appl. Nano Mater. 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale topography on black titanium imparts multi-biofunctional properties for orthopedic applications. Sci. Rep. 2017, 7, 41118. [Google Scholar] [CrossRef] [Green Version]
- Ganjian, M.; Modaresifar, K.; Zhang, H.; Hagedoorn, P.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Reactive ion etching for fabrication of biofunctional titanium nanostructures. Sci. Rep. 2019, 9, 18815. [Google Scholar] [CrossRef] [Green Version]
- Modaresifar, K.; Ganjian, M.; Angeloni, L.; Minneboo, M.; Ghatkesar, M.K.; Hagedoorn, P.; Fratila-Apachitei, L.E.; Zadpoor, A.A. On the use of black Ti as a bone substituting biomaterial: Behind the scenes of dual-functionality. Small 2021, 17, e2100706. [Google Scholar] [CrossRef]
- Zimmerli, W.; Lew, P.D.; Waldvogel, F.A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J. Clin. Invest. 1984, 73, 1191–1200. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Pathogenesis of implant-associated infection: The role of the host. Semin. Immunopathol. 2011, 33, 295–306. [Google Scholar] [CrossRef]
- Zimmerli, W.; Waldvogel, F.A.; Vaudaux, P.; Nydegger, U.E. Pathogenesis of foreign body infection: Description and characteristics of an animal model. J. Infect. Dis. 1982, 146, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Southwood, R.T.; Rice, J.L.; McDonald, P.J.; Hakendorf, P.H.; Rozenbilds, M.A. Infection in experimental hip arthroplasties. J. Bone Joint Surg. Br. 1985, 67, 229–231. [Google Scholar] [CrossRef]
- Yavari, S.A.; Castenmiller, S.M.; van Strijp, J.A.G.; Croes, M. Combating implant infections: Shifting focus from bacteria to host. Adv. Mater. 2020, 32, e2002962. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.; Nijnik, A.; Philpott, D. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Fang, F. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef]
- André, A.C.; Laborde, M.; Marteyn, B.S. The battle for oxygen during bacterial and fungal infections. Trends. Microbiol. 2022, 30, 643–653. [Google Scholar] [CrossRef]
- Nadzam, G.S.; De La Cruz, C.; Greco, R.S.; Haimovich, B. Neutrophil adhesion to vascular prosthetic surfaces triggers nonapoptotic cell death. Ann. Surg. 2000, 231, 587–599. [Google Scholar] [CrossRef]
- Chang, S.; Popowich, Y.; Greco, R.S.; Haimovich, B. Neutrophil survival on biomaterials is determined by surface topography. J. Vasc. Surg. 2003, 37, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef] [Green Version]
- Erpenbeck, L.; Gruhn, A.L.; Kudryasheva, G.; Günay, G.; Meyer, D.; Busse, J.; Neubert, E.; Schön, M.P.; Rehfeldt, F.; Kruss, S. Effect of adhesion and substrate elasticity on neutrophil extracellular trap formation. Front. Immunol. 2019, 10, 2320. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, S.S.; Heine, R.P.; Simmons, R.L. Defensins impair phagocytic killing by neutrophils in biomaterial-related infection. Infect. Immun. 1999, 67, 1640–1645. [Google Scholar] [CrossRef]
- Eriksson, C.; Nygren, H. Adhesion receptors of polymorphonuclear granulocytes on titanium in contact with whole blood. J. Lab. Clin. Med. 2001, 137, 56–63. [Google Scholar] [CrossRef]
- Vitkov, L.; Krautgartner, W.; Obermayer, A.; Stoiber, W.; Hannig, M.; Klappacher, M.; Hartl, D. The initial inflammatory response to bioactive implants is characterized by NETosis. PLoS ONE 2015, 10, e0121359. [Google Scholar] [CrossRef] [Green Version]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.D.; Krupka, T.; Anderson, J.M. iNOS-mediated generation of reactive oxygen and nitrogen species by biomaterial-adherent neutrophils. J. Biomed. Mater. Res A 2007, 80, 381–390. [Google Scholar] [CrossRef]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef]
- Makaremi, S.; Luu, H.; Boyle, J.P.; Zhu, Y.; Cerson, C.; Bowdish, D.M.E.; Moran-Mirabal, J.M. The topography of silica films modulates primary macrophage morphology and function. Adv. Mater. Interfaces 2019, 6, 1900677. [Google Scholar] [CrossRef]
- Singh, S.; Awuah, D.; Rostam, H.M.; Emes, R.D.; Kandola, N.K.; Onion, D.; Htwe, S.S.; Rajchagool, B.; Cha, B.; Kim, D.; et al. Unbiased analysis of the impact of micropatterned biomaterials on macrophage behavior provides insights beyond predefined polarization states. ACS Biomater. Sci. Eng. 2017, 3, 969–978. [Google Scholar] [CrossRef]
- Vassey, M.J.; Figueredo, G.P.; Scurr, D.J.; Vasilevich, A.S.; Vermeulen, S.; Carlier, A.; Luckett, J.; Beijer, N.R.M.; Williams, P.; Winkler, D.A.; et al. Immune modulation by design: Using topography to control human monocyte attachment and macrophage differentiation. Adv Sci 2020, 7, 1903392. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J. Biomed. Mater. Res. A 2012, 100, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Previtera, M.L.; Sengupta, A. Substrate stiffness regulates proinflammatory mediator production through tlr4 activity in macrophages. PLoS ONE 2015, 10, e0145813. [Google Scholar] [CrossRef]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Lietaert, K.; van Kessel, K.P.M.; Pouran, B.; van der Wal, B.C.H.; Vogely, H.C.; Van Hecke, W.; Fluit, A.C.; et al. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Liz, R.; Simard, J.; Leonardi, L.B.A.; Girard, D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. Int. Immunopharmacol. 2015, 28, 616–625. [Google Scholar] [CrossRef]
- Huang, M.; Ye, K.; Hu, T.; Liu, K.; You, M.; Wang, L.; Qin, H. Silver nanoparticles attenuate the antimicrobial activity of the innate immune system by inhibiting neutrophil-mediated phagocytosis and reactive oxygen species production. Int. J. Nanomed. 2021, 16, 1345–1360. [Google Scholar] [CrossRef]
- Haase, H.; Fahmi, A.; Mahltig, B. Impact of silver nanoparticles and silver ions on innate immune cells. J. Biomed. Nanotechnol. 2014, 10, 1146–1156. [Google Scholar] [CrossRef]
- Nishanth, R.P.; Jyotsna, R.G.; Schlager, J.J.; Hussain, S.M.; Reddanna, P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway. Nanotoxicology 2011, 5, 502–516. [Google Scholar] [CrossRef]
- Kim, B.; Lee, W. Regulatory role of zinc in immune cell signaling. Mol. Cells 2021, 44, 335–341. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Guo, G.; Tan, J.; Wang, Q.; Tang, J.; Liu, W.; Shen, H.; Li, J.; Zhang, X. Enhanced anti-infective efficacy of zno nanoreservoirs through a combination of intrinsic anti-biofilm activity and reinforced innate defense. ACS Appl. Mater. Interfaces 2017, 9, 33609–33623. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Li, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Qian, Y.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. A surface-engineered polyetheretherketone biomaterial implant with direct and immunoregulatory antibacterial activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2019, 208, 8–20. [Google Scholar] [CrossRef]
- Lemire, J.; Harrison, J.; Turner, R. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Bussière, F.I.; Gueux, E.; Rock, E.; Girardeau, J.; Tridon, A.; Mazur, A.; Rayssiguier, Y. Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br. J. Nutr. 2002, 87, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Hann, J.; Bueb, J.-L.; Tolle, F.; Bréchard, S. Calcium signaling and regulation of neutrophil functions: Still a long way to go. J. Leukoc. Biol. 2020, 107, 285–297. [Google Scholar] [CrossRef]
- Cao, H.; Qin, H.; Zhao, Y.; Jin, G.; Lu, T.; Meng, F.; Zhang, X.; Liu, X. Nano-thick calcium oxide armed titanium: Boosts bone cells against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2016, 6, 21761. [Google Scholar] [CrossRef]
- Hou, Y.; Witte, F.; Li, J.; Guan, S. The increased ratio of Mg2+/Ca2+ from degrading magnesium alloys directs macrophage fate for functionalized growth of endothelial cells. Smart Mater. Med. 2022, 3, 188–198. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Jandt, K.D. The action-network of nanomaterials in On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol. 2019, 14, 629–635. [Google Scholar]
- Damiati, L.A.; Tsimbouri, M.P.; Hernandez, V.; Jayawarna, V.; Ginty, M.; Childs, P.; Xiao, Y.; Burgess, K.; Wells, J.; Sprott, M.R.; et al. Materials-driven fibronectin assembly on nanoscale topography enhances mesenchymal stem cell adhesion, protecting cells from bacterial virulence factors and preventing biofilm formation. Biomaterials 2022, 280, 121263. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Chen, H.; Zhao, R.; Deng, X.; Chen, G.; Yang, X.; Xiao, Z.; Aurora, A.; Iulia, B.A.; Zhang, K.; et al. Construction of a magnesium hydroxide/graphene oxide/hydroxyapatite composite coating on Mg–Ca–Zn–Ag alloy to inhibit bacterial infection and promote bone regeneration. Bioact. Mater. 2022, 18, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yi, Y.; Song, L.; Chen, Y.; Tian, L.; Zhao, J.; Ren, L. Biocompatible mechano-bactericidal nanopatterned surfaces with salt-responsive bacterial release. Acta Biomater. 2022, 141, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sun, T.; Su, W.; Jing, X.; Ye, B.; Su, Y.; Zeng, L.; Qu, Y.; Yang, X.; Wu, Y.; et al. Bioinspired multifunctional black phosphorus hydrogel with antibacterial and antioxidant properties: A stepwise countermeasure for diabetic skin wound healing. Adv. Healthc. Mater. 2022, 11, 2102791. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, S.; Coy, E.; Li, S.; Załęski, K.; Zhang, Y.; Pan, H.; Wang, G. NIR-Responsive TiO2 Biometasurfaces: Toward in situ photodynamic antibacterial therapy for biomedical implants. Adv. Mater. 2022, 34, 2106314. [Google Scholar] [CrossRef]
- Qu, X.; Wang, M.; Wang, M.; Tang, H.; Zhang, S.; Yang, H.; Yuan, W.; Wang, Y.; Yang, J.; Yue, B. Multi-mode antibacterial strategies enabled by gene-transfection and immunomodulatory nanoparticles in 3D-printed scaffolds for synergistic exogenous and endogenous treatment of infections. Adv. Mater. 2022, 34, 2200096. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Teng, W.; Zhou, X.; Ye, Y.; Zhou, H.; Sun, H.; Wang, F.; Liu, A.; Lin, P.; et al. An orthobiologics-free strategy for synergistic photocatalytic antibacterial and osseointegration. Biomaterials 2021, 274, 120853. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef]
- Sun, B.B.; Maranville, J.C.; Peters, J.E.; Stacey, D.; Staley, J.R.; Blackshaw, J.; Burgess, S.; Jiang, T.; Paige, E.; Surendran, P.; et al. Genomic atlas of the human plasma proteome. Nature 2018, 558, 73–79. [Google Scholar] [CrossRef]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Ebara, S.; Kamimura, M.; Kinoshita, T.; Misawa, H.; Shimogata, M.; Tozuka, M.; Takaoka, K. Pro-inflammatory and anti-inflammatory cytokine increases after spinal instrumentation surgery. J. Spinal Disord. Tech. 2002, 15, 294–300. [Google Scholar] [CrossRef] [PubMed]







| Active Ingredients | Devices | Phase | Locations | First Posted |
|---|---|---|---|---|
| Silver coating | Intravenous catheters | Not applicable | United States | 25 August 2009 |
| Antibiotics (minocycline and rifampin) | Antibacterial envelope for a cardiac implantable electronic device | Not applicable | United States | 7 January 2010 |
| Silver-based coating | Urinary catheter | Not applicable | United States | 10 September 2012 |
| Ionic silver | Wound dressings for a cardiac implantable electronic device | Phase 4 | United States | 24 May 2016 |
| Silver-doped hydroxyapatite coating | Orthopedic implants (hip joint prostheses, intramedullary nails, and external fixator implants) | Not applicable | Turkey | 17 November 2017 |
| Gold-silver-palladium coating | Invasive devices (endotracheal tube, central venous catheter, and urinary catheter) | Phase 1, 2 | Brazil | 11 March 2019 |
| Iodine | Barrier dressing for a cardiac implantable electronic device | Not applicable | Canada | 19 October 2020 |
| Antibiotic (gentamycin) | Platform wound device | Phase 4 | United States | 15 February 2021 |
| Device | Materials | Incidence | Reference |
|---|---|---|---|
| Ankle arthroplasty | Metals (titanium alloys), Ceramic, Polyethylene | 2.4–8.9% | [25,26] |
| Hip arthroplasty | Metals (titanium alloys, stainless steel), Ceramics (alumina, zirconia), Polymers (polyethylene, polyetheretherketone), Composites | 0.4–2.4% | [10,27,28] |
| Knee arthroplasty | Metals (titanium alloys, cobalt-chromium alloy), Ceramics (zirconia, titanium nitride), Polymers (polyethylene,) | 1–2% | [10,29] |
| Breast implants | Silicone | 1–10.2% | [30,31,32] |
| Vascular graft/endograft | Polytetrafluoroethylene, Polyethylene Terephthalate, Nitinol | 0.16–6% | [33] |
| Cardiovascular electronic devices | Plastic polymers, Titanium, Teflon, Gold, Copper | 0.9–7% | [34,35,36,37,38] |
| Cochlear implant | Teflon, Platinum-iridium alloy, Silicone, Titanium, Ceramics | 1–8% | [39,40,41,42,43] |
| Brain stimulation implant | Stainless steel, Platinum, Titanium oxide, Iridium oxide | 2–10% | [44,45,46] |
| Urinary catheters * | Natural rubber, Polyisoprene, Polymer ethylene vinyl acetate, Polytetrafluoroethylene, Hydrogel | 0.1–13.7 cases per 1000 catheter-days | [47,48,49,50,51,52] |
| Cerebrospinal fluid shunts | Silicone rubber | 1.9–27% | [53,54,55,56,57] |
| Internal fixation devices | Stainless steel, Cobalt-chromium alloys, Titanium alloys | 7–32% | [58,59] |
| Dental implants | Titanium, Ceramics (zirconia, alumina) | 6–47% | [60,61] |
| Case | Devices | Latent Period (Post Insertion) | Pathogens | Causes | Reference |
|---|---|---|---|---|---|
| 1 | Alloplastic chin implant | 45 years | / | After scratching herself (soft tissue degeneration due to aging) | [67] |
| 2 | Breast implant | Seven years | Achromobacter xylosoxidans (a pathogen that lives in wet soil) | Development of a chronic footsore (hematogenous spread from distant bacterial infection sites) | [68] |
| 3 | Breast implant | 25 years | Streptococcus viridans (a pathogen that lives in the oral cavity) | After extensive dental treatment (hematogenous spread from distant bacterial infection sites) | [68] |
| 4 | Alloplastic implant | 30 years | Staphylococcus epidermidis | Bacterial contamination years before identifying the infection (a symptom-free chronic infection; the pathogen escaped immune clearance and antibiotic treatments) | [69] |
| 5 | Orbital implant | 30 years | Cutibacterium acnes (previously known as Propionibacterium acnes) | Bacterial contamination during the primary implantation (the pathogen can manifest for several decades) | [70] |
| 6 | Orbital implant | 26 years (implant exposure 10 years before the presentation was documented) | Propionibacterium acnes (renamed Cutibacterium acnes) | Bacterial contamination during the primary implantation or implant exposure during scleral patch graft repair | [71] |
| 7 | Breast Implant | Five months | Salmonella serogroup C | Hematogenous seeding due to developing of diarrhea during a holiday travel | [31] |
| 8 | Generator for brain stimulation | Four months | Multispecies including the rare Cupriavidus pauculus species (an environmental Pathogen in “water”) | Penetration of contaminated water during participating in outdoor activities | [45] |
| 9 | Breast implant | Seven months | Pasteurella canis (a pathogen normally lives in the oropharyngeal commensal flora of cats and dogs) | Bacterial contamination from a patient-owned cat | [72] |
| 10 | Battery for brain stimulation | Two cases (Two years or 10 years) | Staphylococcus aureus | Chronic treatment of rheumatoid arthritis with methotrexate | [73] |
| 11 | Tibia Tenodesis Implant | Four and half months | Nocardia nova (a common environmental pathogen, rarely affects immunocompetent hosts) | Contamination of his tibial wound by the outside facility | [74] |
| 12 | Knee arthroplasty | 4 months | Listeria monocytogenes (a facultative intracellular organism; commonly associated with deli meats and unpasteurized cheeses) | Consuming unpasteurized dairy products (an immunocompromised patient) | [75] |
| 13 | Hip arthroplasty | 10 years | Anaerobiospirillum succiniciproducens (lives in the gastrointestinal tract of cats and dogs) | Breeding a dog (an immunocompromised patient) | [76] |
| 14 | Knee arthroplasty | Eight years | Bartonella henselae (a pathogen that induces acute infections but is hard to be diagnosed by culture) | A cat scratch | [77] |
| 15 | Cranioplasty implant | Two years and three months | No bacteria were cultured, but the infection was clinically evident | / | [78] |
| 16 | Shoulder prosthesis | Three years | Staphylococcus spp. | / | [79] |
| Case | Resistant Pathogens | Implant | Latent Period | Reference |
|---|---|---|---|---|
| 1 | Multidrug-resistant Acinetobacter baumannii | Hip arthroplasty | 12–25 days | [92] |
| 2 | Methicillin-resistant Staphylococcus aureus (MRSA) | Cardiac pacemaker | Nine years | [93] |
| 3 | Clarithromycin-resistant Mycobacterium chelonae | Breast implant | Four days | [94] |
| 4 | MRSA | Transvenous lead | Four years | [95] |
| 5 | MRSA | Ankle fracture fixation | Eight weeks | [96] |
| 6 | MRSA | Cranial implant | Three months | [97] |
| 7 | MRSA | Cochlear implant | Five months | [98] |
| 8 | MRSA | Pacemaker | Two months | [99] |
| 9 | MRSA | Breast Implant | Two days | [100] |
| 10 | MRSA | Laryngeal implant | More than one year | [101] |
| 11 | Carbapenem-resistant Acinetobacter baumannii; Fluoroquinolone-resistant Enterobacter cloacae complex (AmpC overexpression) | Internal fixation for an open proximal tibial fracture | Two months | [102] |
| 12 | MRSA | Pacemaker | Two years | [103] |
| 13 | Multidrug-resistant Staphylococcus epidermidis | Plates and wire cerclages for periprosthetic fractures | Three months | [104] |
| 14 | Carbapenem-resistant Klebsiella pneumoniae | Lumbar instruments, | Seven days | [105] |
| 15 | MRSA | The ventricular lead of an implanted defibrillator | Eight weeks | [106] |
| 16 | Methicillin-resistant Staphylococcus haemolyticus | Hip joint | Two years | [107] |
| 17 | Ofloxacin-resistant staphylococcal endophthalmitis | Intravitreal ozurdex implant | Three days | [108] |
| 18 | MRSA | Stent graft | Three days | [109] |
| 19 | Methicillin-resistant Staphylococcus epidermidis | Spinal instrumentation | 7–88 days | [110] |
| Active Ingredients | Intended Use (Substrates) | Effective Period | Reference |
|---|---|---|---|
| Tigecycline, Copper ions | Treatment for osteomyelitis (Alginate aerogel) | 18 days | [121] |
| Vancomycin | Cement (Calcium phosphate) | 168 days | [122] |
| (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone | Dental implants (Titanium) | 60 days | [123] |
| Silver/Zinc ions | An orthopedic and dental implant (Titanium) | 180 days | [124] |
| Nanosilver | Bone implant (Polylactic acid fiber) | 11 days | [125] |
| Honokiol | Remineralization of demineralized enamel (Poly(amido amine) (PAMAM) (Dendrimer) | 24 days | [126] |
| Patchouli Essential Oil | Wound Dressing (Polyvinyl alcohol and chitosan) | 2 days | [127] |
| Cetylpyridinium chloride | Endodontic sealers (Polyhydroxyethyl methacrylate trimethylolpropanetrimethacrylate) | 48 days | [128] |
| Metallic silver | Hard tissue replacements (Titanium) | 84 days | [129] |
| Copper | Orthopedics (Titanium) | 14 days | [130] |
| Zinc/Copper | Cement (dicalcium silicate) | 3 days | [131] |
| Amoxicillin | Wound dressing (Poly (e-caprolactone)) | 7 days | [132] |
| Chlorhexidine | Medical devices (not clear, 316L) | 3 days | [133] |
| Silver ions | Orthopedic implants (Titanium) | 189 days (silver release) | [134] |
| Nanosilver | Biomedicine (not clear) | 7 days | [135] |
| Nanogold/Titania | Orthopedic implants (Titanium) | 6 days | [136] |
| Nanosilver | Orthopedic implants (Titanium) | 60 days | [137] |
| Silver nanoparticles | Orthopedic implants (Titanium) | 60 days | [138] |
| Poly (poly (ethylene glycol) dimethacrylate) | Peritoneal dialysis catheters (Silicone) | 30 days | [139] |
| Action: Active Ingredient | Light Parameter | Pathogens Tested | Intended Use | Reference |
|---|---|---|---|---|
| Heat: gold | NIR light | E. coli, MRSA | In vitro (not specific) | [177] |
| Heat: tannic acid and iron | NIR light | E. coli, MRSA | Not specific | [178] |
| Heat: titanium dioxide | NIR light | E. coli, S. aureus | Orthopedic/dental implants | [179] |
| Heat: carbon dots | Blue light | S. aureus | Not specific | [180] |
| ROS: black phosphorus | Visible light | E. coli, S. aureus | Implantable materials/device (not specific) | [181] |
| Heat and ROS: fluorescent modified red phosphorus | NIR light | S. aureus | Treatment for joint implants | [182] |
| Heat and Nitric oxide: molybdenum sulfide assembled with a nitric oxide donor | NIR light | Ampicillin-resistant E. coli, heat-resistant E. faecalis, and S. aureus | Wound repair (not specific) | [184] |
| Case | Antibacterial Designs | Bacterial Strain (In Vitro) | Mammalian Cells Line (In Vitro) | In Vivo Tests | Intended Use | Reference |
|---|---|---|---|---|---|---|
| 1 | Cell-selective: Coating titanium nanowires with poly (ethyl acrylate) to organize fibronectin and deliver BMP-2 | P. aeruginosa (ATCC 27853); cultured for 24 h | Primary human mesenchymal stem cell (MSCs); co-culture with bacteria | None | Orthopedic implant | [272] |
| 2 | Cell-selective: Ion release by Magnesium hydroxide | S. aureus (unidentified source); E. coli (unidentified source) | Mouse MC3T3-E1 pre-osteoblasts | Rat femoral condyle defect model; Placed in for 7 days to examine the dis-infective effects. Placed in for 4 weeks to evaluate the osteogenic property | Not specific | [273] |
| 3 | Long-term efficacy: salt-responsive polyzwitterionic brushes on a nanopatterned surface | P. aeruginosa (BNCC 337005); Escherichia coli (ATCC 25922) | Rabbit red blood cells (2 h- incubation); L929 fibroblasts (cultured for 24 h) | Subcutaneous implant model in mice; Placed in for 5 days | Not specific | [274] |
| 4 | Light-responsive (808 nm laser irradiation, 1 W/cm2, 5 min): Photosensitive gelatin methacryloyl incorporated with 4-octyl itaconate bearing black phosphorus | S. aureus (unidentified source); E. coli (unidentified source); The onset of light irradiation is not clear | Human umbilical vein endothelial cells; The effect of light illumination on the cell function was not clear (No data presented) | Rat type I diabetes model (14 days); The onset for light irradiation is not clear | Wound dressing | [275] |
| 5 | Light-responsive (1060 nm laser, 0.3 W/cm2, 0.6 W/cm2, and 0.9 W/cm2): Yb and Er-doped titanium dioxide nano-shovel/quercetin/L-arginine coatings | S. aureus (ATCC 29213);Light illumination (0.6 W/cm2,15 min) | Osteosarcoma cells (Saos-2, light irradiation at 0.9 W/cm2 for 10 min); Human umbilical vein endothelium cells (light irradiation at 0.6 W/cm2 for 10 min); Bone marrow mesenchymal stem cells (light irradiation at 0.6 W/cm2 for 10 min) | Tumor-bearing mouse model (light irradiation at 0.9 W/cm2 for 10 min and performed every other day); Mice tibia infection model (light irradiation at 0.6 W/cm2 for 15 min and performed one day after surgery); Mice tibia osteogenic model (light irradiation at 0.6 W/cm2 for 15 min and performed one day after surgery; samples collected 4 weeks after surgery) | Bone implants | [18] |
| 6 | Light-responsive (808 nm laser irradiation): TiO2/TiO2−x metasurface | E. coli (ATCC 25922); S. aureus (ATCC 43300); illuminated at 0.5 W/cm2 for 10 min | Human gingival fibroblasts; light illumination at 0.5 W/cm2 for 10 min | Subcutaneous model in rats; light illumination at 1.4 W/cm2 for 10 min after surgery | Dental implant | [276] |
| 7 | Immune-instructive: polydopamine functioned and antimicrobial peptide plasmid (LL37 plasmid) loaded porous zeolitic imidazolate framework-8 (ZIF8) in 3D-Printed Scaffolds | MRSA (ATCC 43300); E. coli (ATCC 25922) | MC3T3 cell; The material effects on immune systems are not considered | Murine quadriceps muscle infection model (MRSA injected after scaffold placement) | Not specific | [277] |
| 8 | Light-responsive (808 nm laser irradiation, 2 W/cm2, 10 min): self-assembly of copper sulfide nanoparticle and reduced graphene oxide on anodized titanium | S. aureus (ATCC 29213); E. coli (ATCC 25922); Light irradiation after inoculation | Mice bone marrow stromal cells; The effect of light illumination on the cell function is not clear (No data presented) | Disinfection in rats (7 days); Osteogenic property in rats (8 weeks); The effect of light illumination on osteogenesis was not clear (No data presented) | Not specific | [278] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Qiao, S.; Qin, H.; Jandt, K.D. Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges. J. Funct. Biomater. 2022, 13, 86. https://doi.org/10.3390/jfb13030086
Cao H, Qiao S, Qin H, Jandt KD. Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges. Journal of Functional Biomaterials. 2022; 13(3):86. https://doi.org/10.3390/jfb13030086
Chicago/Turabian StyleCao, Huiliang, Shichong Qiao, Hui Qin, and Klaus D. Jandt. 2022. "Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges" Journal of Functional Biomaterials 13, no. 3: 86. https://doi.org/10.3390/jfb13030086
APA StyleCao, H., Qiao, S., Qin, H., & Jandt, K. D. (2022). Antibacterial Designs for Implantable Medical Devices: Evolutions and Challenges. Journal of Functional Biomaterials, 13(3), 86. https://doi.org/10.3390/jfb13030086








