Polycaprolactone-Based 3D-Printed Scaffolds as Potential Implant Materials for Tendon-Defect Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bulk Material
2.2. Manufacturing of Test Specimens
2.3. Surface Hydrophilization with Oxygen-Plasma Treatment
2.4. Coating with Fibronectin and Collagen I
2.5. Cell-Culture Tests
2.6. Statistics
3. Results
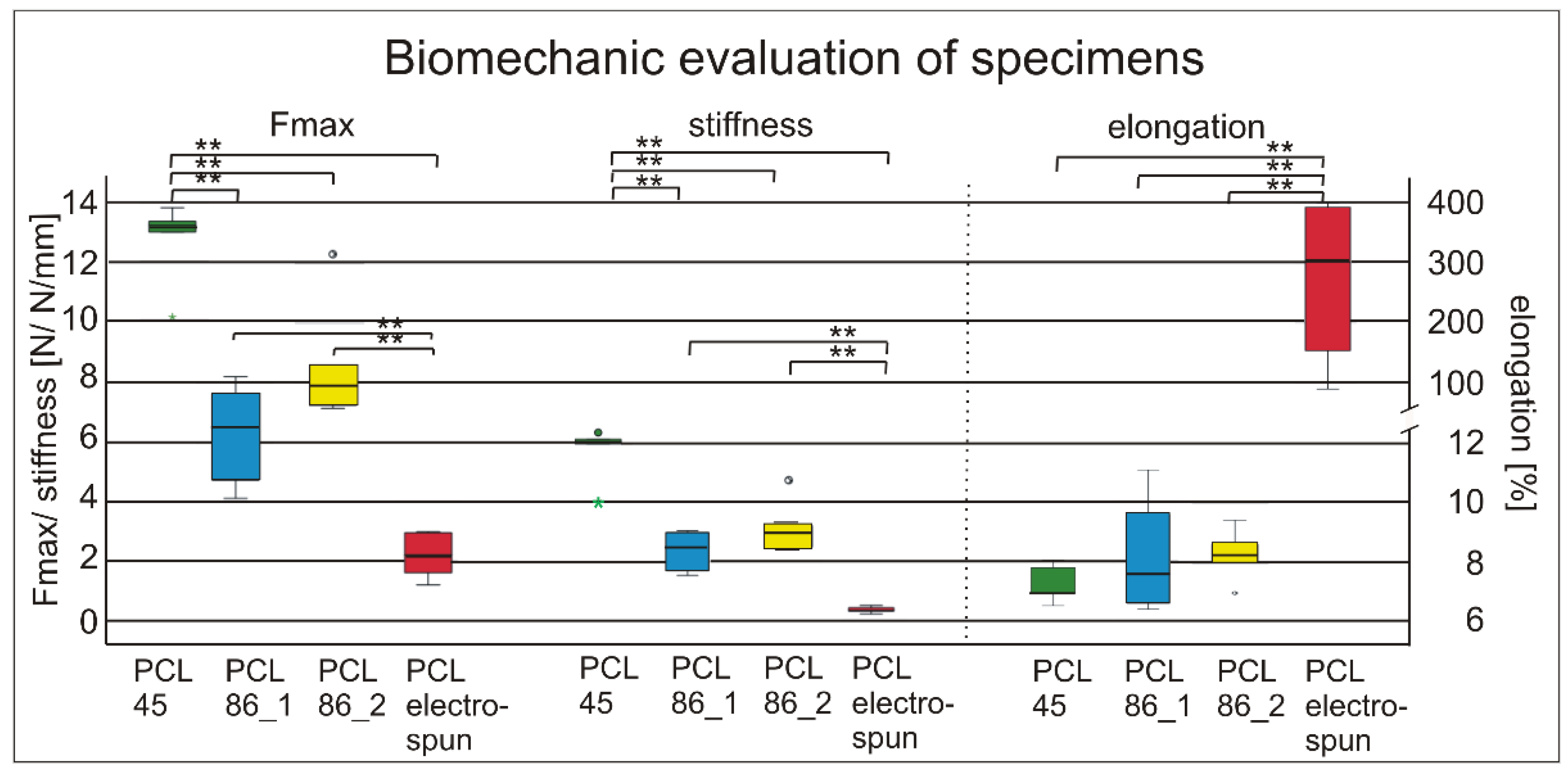
3.1. Mechanical Examination of Specimens
3.2. Influence of Design Variant and Plasma Treatment on Hydrophilicity of Specimens
3.3. Influence of 3D-Printed Specimens on Cellular Growth
3.4. Influence of Design Variant and Plasma Treatment of Specimens on Viability of Hbm-MSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angrisani, A.; Willbold, E.; Kampmann, A.; Derksen, A.; Reifenrath, J. Histology of tendon and enthesis—Suitable techniques for specific research questions. Eur. Cell Mater. 2022, 24, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Zumstein, M.-A.; Lädermann, A.; Raniga, S.; Schär, M. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. 2017, 103, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.N.; Wu, X.L.; Lam, P.H.; Murrell, G.A.C. Factors predicting rotator cuff retears: An analysis of 1000 consecutive rotator cuff repairs. Am. J. Sports Med. 2014, 42, 1134–1142. [Google Scholar] [CrossRef]
- Seppel, G.; Plath, J.E.; Völk, C.; Seiberl, W.; Buchmann, S.; Waldt, S.; Imhoff, A.B.; Braun, S. Long-term Results After Arthroscopic Repair of Isolated Subscapularis Tears. Am. J. Sports Med. 2016, 45, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 34, 123–140. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, N. Preparation and characterization of poly(ε-caprolactone) scaffolds modified with cell-loaded fibrin gel. Int. J. Biol. Macromol. 2018, 125, 683–689. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2019, 10, 381–388. [Google Scholar] [CrossRef]
- Hou, Y.; Zhou, B.; Ni, M.; Wang, M.; Ding, L.; Li, Y.; Liu, Y.; Zhang, W.; Li, G.; Wang, J.; et al. Nonwoven-based gelatin/polycaprolactone membrane loaded with ERK inhibitor U0126 for treatment of tendon defects. Stem Cell Res. Ther. 2022, 13, 5. [Google Scholar] [CrossRef]
- Beason, D.P.; Connizzo, B.K.; Dourte, L.M.; Mauck, R.L.; Soslowsky, L.J.; Steinberg, D.R.; Bernstein, J. Fiber-aligned polymer scaffolds for rotator cuff repair in a rat model. J. Shoulder Elb. Surg. 2012, 21, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Dudhia, J.; Dakin, S.G.; Snelling, S.J.B.; De Godoy, R.; Mouthuy, P.-A.; Smith, R.K.W.; Morrey, M.; Carr, A.J. Histopathological and immunohistochemical evaluation of cellular response to a woven and electrospun polydioxanone (PDO) and polycaprolactone (PCL) patch for tendon repair. Sci. Rep. 2020, 10, 4754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.; Zhang, X.; Yang, L.; Zhang, J. Research Progress of Biodegradable Polymers in Repairing Achilles Tendon Injury. Front. Mater. 2022, 9, 815930. [Google Scholar] [CrossRef]
- Cengiz, I.F.; Pereira, H.; De Girolamo, L.; Cucchiarini, M.; Espregueira-Mendes, J.; Reis, R.L.; Oliveira, J.M. Orthopaedic regenerative tissue engineering en route to the holy grail: Disequilibrium between the demand and the supply in the operating room. J. Exp. Orthop. 2018, 5, 14. [Google Scholar] [CrossRef]
- Willbold, E.; Wellmann, M.; Welke, B.; Angrisani, N.; Gniesmer, S.; Kampmann, A.; Hoffmann, A.; De Cassan, D.; Menzel, H.; Hoheisel, A.L.; et al. Possibilities and limitations of electrospun chitosan-coated polycaprolactone grafts for rotator cuff tear repair. J. Tissue Eng. Regen. Med. 2019, 14, 186–197. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Chen, Y.-W.; Wang, K.; Shie, M.-Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Reifenrath, J.; Wellmann, M.; Kempfert, M.; Angrisani, N.; Welke, B.; Gniesmer, S.; Kampmann, A.; Menzel, H.; Willbold, E. TGF–β3 Loaded Electrospun Polycaprolacton Fibre Scaffolds for Rotator Cuff Tear Repair: An in Vivo Study in Rats. Int. J. Mol. Sci. 2020, 21, 1046. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Cho, D.-W. Solid Free-form Fabrication Technology and Its Application to Bone Tissue Engineering. Int. J. Stem Cells 2010, 3, 85–95. [Google Scholar] [CrossRef]
- Steffens, D.; Rezende, R.A.; Santi, B.; Pereira, F.D.A.D.S.; Neto, P.I.; da Silva, J.V.L.; Pranke, P. 3D-Printed PCL Scaffolds for the Cultivation of Mesenchymal Stem Cells. J. Appl. Biomater. Funct. Mater. 2016, 14, 19–25. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Safinia, L.; Datan, N.; Höhse, M.; Mantalaris, A.; Bismarck, A. Towards a methodology for the effective surface modification of porous polymer scaffolds. Biomaterials 2005, 26, 7537–7547. [Google Scholar] [CrossRef] [PubMed]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—a review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Teske, M. Nass- und Plasmachemische Oberflächenmodifizierung Biodegradierbarer, Polymerer Implantatwerkstoffe unter Immobilisierung von Wirkstoffen zur Optimierung der Zelle-Implantat-Interaktion. Ph.D. Thesis, Rostock University Medical Center, Rostock, Germany, 2013. [Google Scholar]
- Von Der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K. Funktionalisierte, Degradierbare Polymerbeschichtungen zur Lokalen Freisetzung von Wirkstoffen—Entwicklung und Charakterisierung von Drug-Eluting Stents für Verschiedene Medizinische Indikationen. Habilitation Thesis, Rostock University Medical Center, Rostock, Germany, 2008. [Google Scholar] [CrossRef]
- Cassan, D.; Becker, A.; Glasmacher, B.; Roger, Y.; Hoffmann, A.; Gengenbach, T.; Easton, C.; Hänsch, R.; Menzel, H. Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications. J. Appl. Polym. Sci. 2019, 137, 48650. [Google Scholar] [CrossRef]
- de Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of sterilization by electron beam, gamma radiation and X-rays on electrospun poly-(ε-caprolactone) fiber mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Fricke, D.; Becker, A.; Jütte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller Matrix Measurement of Electrospun Fiber Scaffolds for Tissue Engineering. Polymers 2019, 11, 2062. [Google Scholar] [CrossRef]
- Ijavi, M.; Style, R.W.; Emmanouilidis, L.; Kumar, A.; Meier, S.M.; Torzynski, A.L.; Allain, F.H.T.; Barral, Y.; Steinmetz, M.O.; Dufresne, E.R. Surface tensiometry of phase separated protein and polymer droplets by the sessile drop method. Soft Matter 2020, 17, 1655–1662. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, S.; Kuss, M.; Kong, Y.; Shi, W.; Streubel, P.N.; Li, T.; Duan, B. 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact. Mater. 2020, 5, 636–643. [Google Scholar] [CrossRef]
- Zhang, W.; Ullah, I.; Shi, L.; Zhang, Y.; Ou, H.; Zhou, J.; Ullah, M.W.; Zhang, X.; Li, W. Fabrication and characterization of porous polycaprolactone scaffold via extrusion-based cryogenic 3D printing for tissue engineering. Mater. Des. 2019, 180, 107946. [Google Scholar] [CrossRef]
- Fuchs, A.; Youssef, A.; Seher, A.; Hochleitner, G.; Dalton, P.D.; Hartmann, S.; Brands, R.C.; Müller-Richter, U.D.A.; Linz, C. Medical-grade polycaprolactone scaffolds made by melt electrospinning writing for oral bone regeneration—A pilot study in vitro. BMC Oral Health 2019, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Großhaus, C.; Bakirci, E.; Berthel, M.; Hrynevich, A.; Kade, J.C.; Hochleitner, G.; Groll, J.; Dalton, P.D. Melt Electrospinning of Nanofibers from Medical-Grade Poly(ε-Caprolactone) with a Modified Nozzle. Small 2020, 16, e2003471. [Google Scholar] [CrossRef] [PubMed]
- Cannella, V.; Altomare, R.; Chiaramonte, G.; Di Bella, S.; Mira, F.; Russotto, L.; Pisano, P.; Guercio, A. Cytotoxicity Evaluation of Endodontic Pins on L929 Cell Line. BioMed Res. Int. 2019, 2019, 2314–6133. [Google Scholar] [CrossRef] [PubMed]
- Jablonská, E.; Kubásek, J.; Vojtěch, D.; Ruml, T.; Lipov, J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci. Rep. 2021, 11, 6628. [Google Scholar] [CrossRef]
- Fischer, J.; Pröfrock, D.; Hort, N.; Willumeit, R.; Feyerabend, F. Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B 2011, 176, 830–834. [Google Scholar] [CrossRef]
- Sgarioto, M.; Adhikari, R.; Gunatillake, P.A.; Moore, T.; Patterson, J.; Nagel, M.-D.; Malherbe, F. High Modulus Biodegradable Polyurethanes for Vascular Stents: Evaluation of Accelerated in vitro Degradation and Cell Viability of Degradation Products. Front. Bioeng. Biotechnol. 2015, 3, 52. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Miroshnichenko, S.; Timofeeva, V.; Permyakova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-Coated Polycaprolactone Nanofibers with Covalently Bonded Platelet-Rich Plasma Enhance Adhesion and Growth of Human Fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef]
- Yildirim, E.D.; Besunder, R.; Pappas, D.; Allen, F.; Güçeri, S.; Sun, W. Accelerated differentiation of osteoblast cells on polycaprolactone scaffolds driven by a combined effect of protein coating and plasma modification. Biofabrication 2010, 2, 014109. [Google Scholar] [CrossRef]
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.A.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Ikada, Y. Effect of Preadsorbed Proteins on Cell Adhesion to Polymer Surfaces. J. Colloid Interface Sci. 1993, 155, 334–339. [Google Scholar] [CrossRef]
- Kosik-Kozioł, A.; Graham, E.; Jaroszewicz, J.; Chlanda, A.; Kumar, P.T.S.; Ivanovski, S.; Swieszkowski, W.; Vaquette, C.; Kumar, S. Surface Modification of 3D Printed Polycaprolactone Constructs via a Solvent Treatment: Impact on Physical and Osteogenic Properties. ACS Biomater. Sci. Eng. 2018, 5, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yang, H.J.; Woo, D.G.; Na Yang, H.; Na, K.; Park, K.-H. Chondrogenic differentiation of mesenchymal stem cells embedded in a scaffold by long-term release of TGF-β3 complexed with chondroitin sulfate. J. Biomed. Mater. Res. Part A 2009, 92, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shi, G.; Bei, J.; Wang, S.; Cao, Y.; Shang, Q.; Yang, G.; Wang, W. Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 2002, 62, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, A.; Barradas, A.; de Boer, J.; Moroni, L.; Van Blitterswijk, C.; Habibovic, P. Combining technologies to create bioactive hybrid scaffolds for bone tissue engineering. Biomatter 2013, 3, e23705. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, R.; Poggioli, F.; Martínez-Díaz, S.; Piera-Trilla, M.; Torres-Claramunt, R.; Tío, L.; Monllau, J.C. Fibronectin-coating enhances attachment and proliferation of mesenchymal stem cells on a polyurethane meniscal scaffold. Regen. Ther. 2021, 18, 480–486. [Google Scholar] [CrossRef]
- Leite, M.L.; Soares, D.G.; Anovazzi, G.; Soares, I.P.M.; Hebling, J.; Costa, C.A.S. Development of fibronectin-loaded nanofiber scaffolds for guided pulp tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 1244–1258. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.X.; Chen, J.L.; Duan, L.; Jia, Z.F.; Huang, J.H.; Li, W.C.; Liu, J.Q.; Xiong, J.Y.; et al. A 3D-Printed PLCL Scaffold Coated with Collagen Type I and Its Biocompatibility. BioMed Res. Int. 2018, 2018, 5147156. [Google Scholar] [CrossRef]
- Barber, F.A.; Herbert, M.A.; Boothby, M.H. Ultimate Tensile Failure Loads of a Human Dermal Allograft Rotator Cuff Augmentation. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 20–24. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, Y.; Wang, Q.; Xie, C.; Ren, Y.; Clark, R.L. Reinforcement of electrospun membranes using nanoscale Al2O3 whiskers for improved tissue scaffolds. J. Biomed. Mater. Res. Part A 2012, 100A, 903–910. [Google Scholar] [CrossRef] [PubMed]






| Distributor | Purity | Molecular Weight (g/mol) (Manufacturer Information) | Molecular Weight (g/mol) (Own Measurements) | Further Name |
|---|---|---|---|---|
| Sigma, Darmstadt, Germany | technical | 45.000 | 34.000 | PCL45 |
| ITV, Denkendorf, Germany | medical | - | 86.000 | PCL86 |
| Group | Time (min) | Pressure (mbar) | Energy (W) |
|---|---|---|---|
| a | 1 | 0.1 | 30 |
| b | 1 | 0.3 | 30 |
| c | 2 | 0.1 | 30 |
| d | 3 | 0.1 | 30 |
| e | 3 | 0.3 | 40 |
| f | 8 | 0.1 | 40 |
| g | 10 | 0.1 | 30 |
| h | 10 | 0.1 | 40 |
| i | 10 | 0.1 | 50 |
| k | 10 | 0.1 | 40 |
| Scientific Question | Cell Type | Cell Viability Test | Microscopy |
|---|---|---|---|
| Influence of design variants PCL86_1 vs. PCL86_2 | L929 | 24 h (n = 4) 3 d (n = 4) 7 d (n = 4) | - |
| Influence of design variants PCL86_1 vs. PCL86_2 and plasma treatments a/b/c/e/f/h | hbm-MSC | 24 h (n = 6) 3 d (n = 5) 7 d (n= 4) | 24 h (n = 1) 3 d (n = 1) 7 d (n = 4) |
| Influence of fibronectin and collagen I coating on plasma-treated (f) PCL86_2 variant | hbm-MSC | 24 h (n = 6) 3 d (n = 5) 7 d (n = 4) | 24 h (n = 1) 3 d (n = 1) 7 d (n = 4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempfert, M.; Willbold, E.; Loewner, S.; Blume, C.; Pitts, J.; Menzel, H.; Roger, Y.; Hoffmann, A.; Angrisani, N.; Reifenrath, J. Polycaprolactone-Based 3D-Printed Scaffolds as Potential Implant Materials for Tendon-Defect Repair. J. Funct. Biomater. 2022, 13, 160. https://doi.org/10.3390/jfb13040160
Kempfert M, Willbold E, Loewner S, Blume C, Pitts J, Menzel H, Roger Y, Hoffmann A, Angrisani N, Reifenrath J. Polycaprolactone-Based 3D-Printed Scaffolds as Potential Implant Materials for Tendon-Defect Repair. Journal of Functional Biomaterials. 2022; 13(4):160. https://doi.org/10.3390/jfb13040160
Chicago/Turabian StyleKempfert, Merle, Elmar Willbold, Sebastian Loewner, Cornelia Blume, Johannes Pitts, Henning Menzel, Yvonne Roger, Andrea Hoffmann, Nina Angrisani, and Janin Reifenrath. 2022. "Polycaprolactone-Based 3D-Printed Scaffolds as Potential Implant Materials for Tendon-Defect Repair" Journal of Functional Biomaterials 13, no. 4: 160. https://doi.org/10.3390/jfb13040160
APA StyleKempfert, M., Willbold, E., Loewner, S., Blume, C., Pitts, J., Menzel, H., Roger, Y., Hoffmann, A., Angrisani, N., & Reifenrath, J. (2022). Polycaprolactone-Based 3D-Printed Scaffolds as Potential Implant Materials for Tendon-Defect Repair. Journal of Functional Biomaterials, 13(4), 160. https://doi.org/10.3390/jfb13040160







