Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants
Abstract
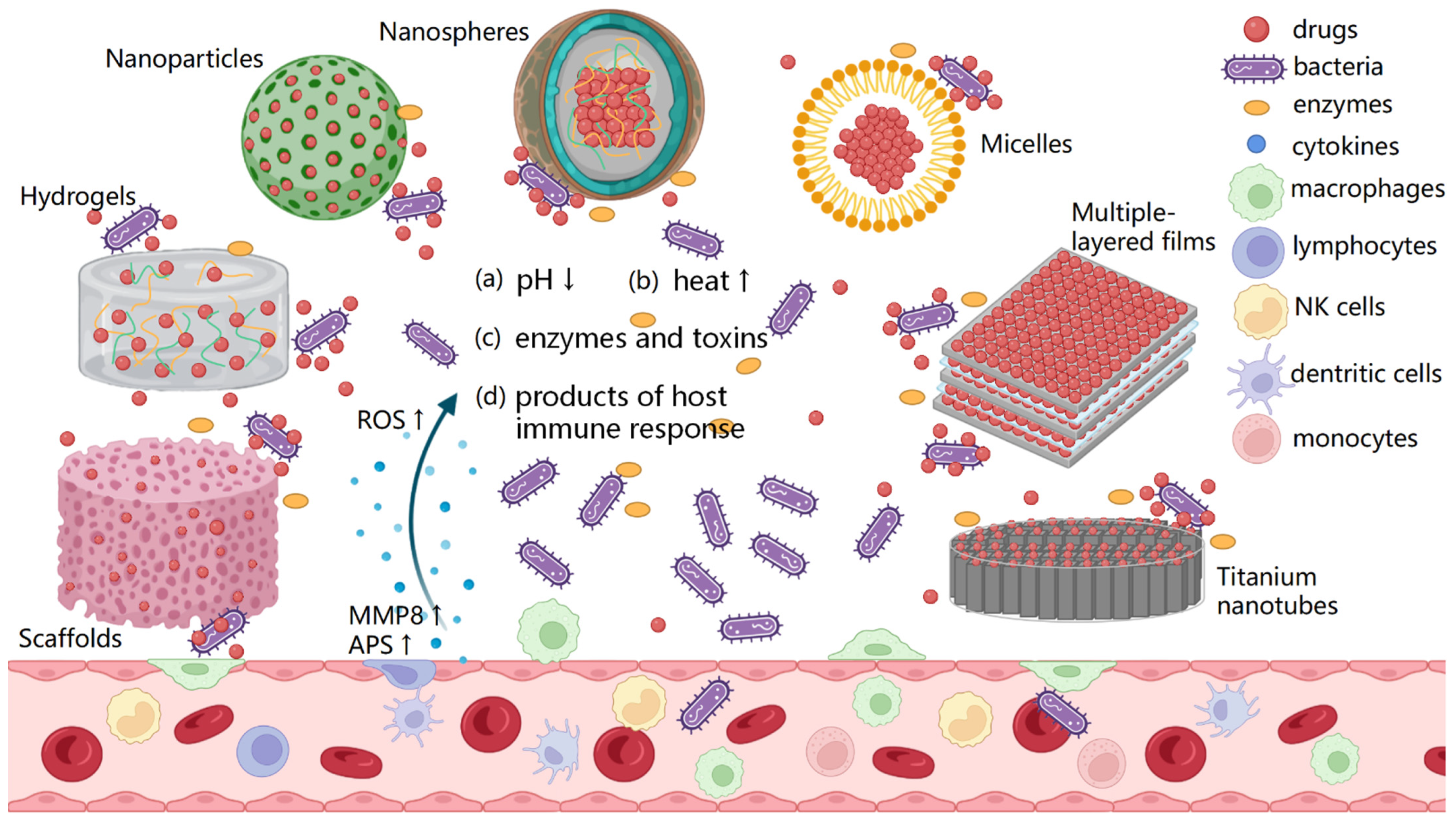
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection Processes
2.2. Inclusion and Exclusion Criteria
- 1
- Primary studies regarding autonomous bacteria-responsive DDSs.
- 2
- Studies aiming to eliminate bacteria via releasing antibacterial drugs.
- 3
- Studies reporting the detailed data of anti-bacterial assays in vitro or in vivo, such as bacterial inhibition rate (BIR), zones of bacterial inhibition (ZOI) and morphological characterization of bacteria (MCB).
- 1
- Studies that performed controlled drug release by additional artificial activation.
- 2
- The DDSs were not designed for antibacterial purposes.
- 3
- Studies missing detailed data of anti-bacterial assays.
3. Results
3.1. Physical Stimuli-Responsive Systems
3.1.1. pH-Responsive Systems
3.1.2. Temperature-Responsive Systems
3.1.3. Contact-Responsive Systems
3.2. Virulence-Factor-Responsive Systems
3.2.1. Protease-Triggered Systems
3.2.2. Hyaluronidase (HAS)-Triggered Systems
3.2.3. Lipase-Triggered Systems
3.2.4. Gelatinase-Triggered Systems
3.3. Dual Responsive Systems
3.4. Host-Immune-Response-Responsive Systems
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, E.; Shen, K.; Lin, F.; Lin, W.; Zhang, T.; Zhang, Y.; Lin, F.; Yang, Y.; Lin, C. Improved osteogenic activity and inhibited bacterial biofilm formation on andrographolide-loaded titania nanotubes. Ann. Transl. Med. 2020, 8, 987. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.L.; Drake, D.R.; Dawson, D.V.; Blanchette, D.R.; Brogden, K.A.; Wertz, P.W. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob. Agents Chemother. 2012, 56, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Zhou, Z.; Seta, J.; Markel, D.C.; Song, W.; Yurgelevic, S.M.; Yu, X.W.; Ren, W. Release of vancomycin and tobramycin from polymethylmethacrylate cements impregnated with calcium polyphosphate hydrogel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2827–2840. [Google Scholar] [CrossRef] [Green Version]
- Jose, B.; Antoci, V., Jr.; Zeiger, A.R.; Wickstrom, E.; Hickok, N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Gerits, E.; Kucharikova, S.; Van Dijck, P.; Erdtmann, M.; Krona, A.; Lovenklev, M.; Frohlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 2191–2198. [Google Scholar] [CrossRef]
- Li, D.; Tang, G.; Yao, H.; Zhu, Y.; Shi, C.; Fu, Q.; Yang, F.; Wang, X. Formulation of pH-responsive PEGylated nanoparticles with high drug loading capacity and programmable drug release for enhanced antibacterial activity. Bioact. Mater. 2022, 16, 47–56. [Google Scholar] [CrossRef]
- Fu, M.; Gan, Y.; Jiang, F.; Lv, X.; Tan, N.; Zhao, X.; Yang, Y.Y.; Yuan, P.; Ding, X. Interpenetrating Polymer Network Hydrogels Formed Using Antibiotics as a Dynamic Crosslinker for Treatment of Infected Wounds. Adv. Healthc. Mater. 2022, 11, 2200902. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired Integration of Naturally Occurring Molecules towards Universal and Smart Antibacterial Coatings. Adv. Funct. Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Cámara-Torres, M.; Duarte, S.; Sinha, R.; Egizabal, A.; Álvarez, N.; Bastianini, M.; Sisani, M.; Scopece, P.; Scatto, M.; Bonetto, A.; et al. 3D additive manufactured composite scaffolds with antibiotic-loaded lamellar fillers for bone infection prevention and tissue regeneration. Bioact. Mater. 2021, 6, 1073–1082. [Google Scholar] [CrossRef]
- Guo, R.; Li, K.; Tian, B.; Wang, C.; Chen, X.; Jiang, X.; He, H.; Hong, W. Elaboration on the architecture of pH-sensitive surface charge-adaptive micelles with enhanced penetration and bactericidal activity in biofilms. J. Nanobiotechnol. 2021, 19, 232. [Google Scholar] [CrossRef]
- Ramesh, S.; Kovelakuntla, V.; Meyer, A.S. Three-dimensional printing of stimuli-responsive hydrogel with antibacterial activity. Bioprinting 2021, 24, e00106. [Google Scholar] [CrossRef]
- Sang, S.; Guo, G.; Yu, J.; Zhang, X. Antibacterial application of gentamicin-silk protein coating with smart release function on titanium, polyethylene, and Al2O3 materials. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 124, 112069. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Xu, F.; Wei, W.; Yang, C.; Wang, D.; Shi, X. Electrochemical synthesis of chitosan/silver nanoparticles multilayer hydrogel coating with pH-dependent controlled release capability and antibacterial property. Colloids Surfaces. B Biointerfaces 2021, 202, 111711. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Mao, X.; Hu, S.; Shang, K.; Yin, J. Acid- and Thiol-Cleavable Multifunctional Codelivery Hydrogel: Fabrication and Investigation of Antimicrobial and Anticancer Properties. ACS Appl. Bio. Mater. 2021, 4, 1515–1523. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.S.; Govender, T. Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 1093. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Chen, X.; Jin, S.; Chen, W.; Meng, Y.; Liu, Y.; Guo, Y.; Jiang, W.; Xu, X.; et al. Chemical grafting of antibiotics into multilayer films through Schiff base reaction for self-defensive response to bacterial infections. Chem. Eng. J. 2020, 382, 122973. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Zhu, Y.; Chen, Y.; Gao, M.; Gao, H.; Liu, L.; Wang, L.; Mao, C.; Wang, Y. On-demand storage and release of antimicrobial peptides using Pandora’s box-like nanotubes gated with a bacterial infection-responsive polymer. Theranostics 2020, 10, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Deng, Y.; Song, L.; Ma, W.; Qian, Y.; Lin, C.; Yuan, Z.; Lu, L.; Chen, M.; Yang, X.; et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surf. B Biointerfaces 2019, 177, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiong, Y.-H.; Zhang, X.-Y.; Wang, R.; Xing, Y.; Duan, S.; Chen, D.; Tian, W.; Xu, F.-J. Self-Adaptive Antibacterial Porous Implants with Sustainable Responses for Infected Bone Defect Therapy. Adv. Funct. Mater. 2019, 29, 1807915. [Google Scholar] [CrossRef]
- de Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Lowik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J.J.P. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Liu, Y.; Tian, S.; Zheng, C.; Liu, C.; Zhai, Y.; An, Y.; Busscher, H.J.; Shi, L.; et al. Phosphorylcholine-Based Polymer Encapsulated Chitosan Nanoparticles Enhance the Penetration of Antimicrobials in a Staphylococcal Biofilm. ACS Macro Lett. 2019, 8, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, Z.; Liu, C.; Hu, Q.; Cai, X.; Xiao, J.; Cheng, Y. A pH-responsive hydrogel with potent antibacterial activity against both aerobic and anaerobic pathogens. Biomater. Sci. 2019, 7, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Karakeçili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110098. [Google Scholar] [CrossRef]
- Maji, R.; Omolo, C.A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. PH-Responsive Lipid-Dendrimer Hybrid Nanoparticles: An Approach to Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A.U. Enhancement in site-specific delivery of carvacrol against methicillin resistant staphylococcus aureus induced skin infections using enzyme responsive nanoparticles: A proof of concept study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Li, Y.; Yan, J.; Xiong, P.; Li, Q.; Cheng, Y.; Zheng, Y. Construction of Self-defensive Antibacterial and Osteogenic AgNPs/Gentamicin Coatings with Chitosan as Nanovalves for Controlled release. Sci. Rep. 2018, 8, 13432. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Liu, X.; Mao, C.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Zheng, Y.; Wu, S. Infection-prevention on Ti implants by controlled drug release from folic acid/ZnO quantum dots sealed titania nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Placente, D.; Benedini, L.A.; Baldini, M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Multi-drug delivery system based on lipid membrane mimetic coated nano-hydroxyapatite formulations. Int. J. Pharm. 2018, 548, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- Dubovoy, V.; Ganti, A.; Zhang, T.; Al-Tameemi, H.; Cerezo, J.D.; Boyd, J.M.; Asefa, T. One-Pot Hydrothermal Synthesis of Benzalkonium-Templated Mesostructured Silica Antibacterial Agents. J. Am. Chem. Soc. 2018, 140, 13534–13537. [Google Scholar] [CrossRef] [PubMed]
- Mhule, D.; Kalhapure, R.S.; Jadhav, M.; Omolo, C.A.; Rambharose, S.; Mocktar, C.; Singh, S.; Waddad, A.Y.; Ndesendo, V.M.K.; Govender, T. Synthesis of an oleic acid based pH-responsive lipid and its application in nanodelivery of vancomycin. Int. J. Pharm. 2018, 550, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Soltani, B.; Nabipour, H.; Nasab, N.A. Efficient Storage of Gentamicin in Nanoscale Zeolitic Imidazolate Framework-8 Nanocarrier for pH-Responsive Drug Release. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1090–1097. [Google Scholar] [CrossRef]
- Yu, X.; Pan, Q.; Zheng, Z.; Chen, Y.; Chen, Y.; Weng, S.; Huang, L. pH-responsive and porous vancomycin-loaded PLGA microspheres: Evidence of controlled and sustained release for localized inflammation inhibition in vitro. RSC Adv. 2018, 8, 37424–37432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xing, M.; Li, B. Capsule-Integrated Polypeptide Multilayer Films for Effective pH-Responsive Multiple Drug Co-Delivery. ACS Appl. Mater. Interfaces 2018, 10, 44267–44278. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, M.; Cheng, Y.; Zheng, Y. Novel pH-responsive tobramycin-embedded micelles in nanostructured multilayer-coatings of chitosan/heparin with efficient and sustained antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 693–705. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Zhu, Y.; Cui, Z.D.; Yang, X.J.; Pan, H.; Yeung, K.W.K.; Wu, S. Metal Ion Coordination Polymer-Capped pH-Triggered Drug Release System on Titania Nanotubes for Enhancing Self-antibacterial Capability of Ti Implants. ACS Biomater. Sci. Eng. 2017, 3, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Pamfil, D.; Vasile, C.; Tartau, L.; Verestiuc, L.; Poiata, A. pH-Responsive 2-hydroxyethyl methacrylate/citraconic anhydride-modified collagen hydrogels as ciprofloxacin carriers for wound dressings. J. Bioact. Compat. Polym. 2017, 32, 355–381. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.; Liu, X.; Yeung, K.W.K.; Wu, S. Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Colloids Surf. B Biointerfaces 2017, 151, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ye, H.; Liu, Y.; Xu, L.; Wu, Z.; Hu, X.; Ma, J.; Pathak, J.L.; Liu, J.; Wu, G. pH dependent silver nanoparticles releasing titanium implant: A novel therapeutic approach to control peri-implant infection. Colloids Surf. B Biointerfaces 2017, 158, 127–136. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef]
- Sang, Q.; Williams, G.R.; Wu, H.; Liu, K.; Li, H.; Zhu, L.-M. Electrospun gelatin/sodium bicarbonate and poly(lactide-co-epsilon-caprolactone)/sodium bicarbonate nanofibers as drug delivery systems. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 81, 359–365. [Google Scholar] [CrossRef]
- Onat, B.; Bütün, V.; Banerjee, S.; Erel-Goktepe, I. Bacterial anti-adhesive and pH-induced antibacterial agent releasing ultra-thin films of zwitterionic copolymer micelles. Acta Biomater. 2016, 40, 293–309. [Google Scholar] [CrossRef]
- Fullriede, H.; Abendroth, P.; Ehlert, N.; Doll, K.; Schäske, J.; Winkel, A.; Stumpp, S.N.; Stiesch, M.; Behrens, P. PH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. BioNanoMaterials 2016, 17, 59–72. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Singh, S.; Renukuntla, J.; Govender, T. pH-responsive chitosan nanoparticles from a novel twin-chain anionic amphiphile for controlled and targeted delivery of vancomycin. Colloids Surf. B Biointerfaces 2017, 158, 650–657. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Gokul, P.; Raichur, A.M. Stimuli-responsive weak polyelectrolyte multilayer films: A thin film platform for self triggered multi-drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 622–628. [Google Scholar] [CrossRef]
- Zhang, Z.; Nong, J.; Zhong, Y. Antibacterial, anti-inflammatory and neuroprotective layer-by-layer coatings for neural implants. J. Neural Eng. 2015, 12, 046015. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, I.; Jariwala, F.; Attygalle, A.B.; Wu, Y.; Libera, M.R.; Sukhishvili, S.A. Self-defensive layer-by-layer films with bacteria-triggered antibiotic release. ACS Nano 2014, 8, 7733–7745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nix, C.A.; Ercan, U.K.; Gerstenhaber, J.A.; Joshi, S.G.; Zhong, Y. Calcium binding-mediated sustained release of minocycline from hydrophilic multilayer coatings targeting infection and inflammation. PLoS ONE 2014, 9, e84360. [Google Scholar] [CrossRef]
- Pichavant, L.; Amador, G.; Jacqueline, C.; Brouillaud, B.; Héroguez, V.; Durrieu, M.C. pH-controlled delivery of gentamicin sulfate from orthopedic devices preventing nosocomial infections. J. Control. Release 2012, 162, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Schulte, A.; Müller, M.; Park, M.; Jo, S.; Schönherr, H. Drug Release from Thermo-Responsive Polymer Brush Coatings to Control Bacterial Colonization and Biofilm Growth on Titanium Implants. Adv. Healthc. Mater. 2021, 10, e2100069. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Wang, D.; Peng, F.; Zhao, X.; Liang, C.; Li, H.; Wang, H. Thermosensitive -hydrogel-coated titania nanotubes with controlled drug release and immunoregulatory characteristics for orthopedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111878. [Google Scholar] [CrossRef]
- Liang, J.; Wang, H.; Libera, M. Biomaterial surfaces self-defensive against bacteria by contact transfer of antimicrobials. Biomaterials 2019, 204, 25–35. [Google Scholar] [CrossRef]
- Bourgat, Y.; Mikolai, C.; Stiesch, M.; Klahn, P.; Menzel, H. Enzyme-Responsive Nanoparticles and Coatings Made from Alginate/Peptide Ciprofloxacin Conjugates as Drug Release System. Antibiotics 2021, 10, 653. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef]
- Liao, X.; Yu, X.; Yu, H.; Huang, J.; Zhang, B.; Xiao, J. Development of an anti-infective coating on the surface of intraosseous implants responsive to enzymes and bacteria. J. Nanobiotechnol. 2021, 19, 241. [Google Scholar] [CrossRef]
- Yu, X.; Liao, X.; Chen, H. Antibiotic-Loaded MMT/PLL-Based Coating on the Surface of Endosseous Implants to Suppress Bacterial Infections. Int. J. Nanomed. 2021, 16, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, K.; Xing, X.; Zhang, J.; Zhang, M.R.; Ma, X.; Shi, R.; Zhang, L. Smart Titanium Coating Composed of Antibiotic Conjugated Peptides as an Infection-Responsive Antibacterial Agent. Macromol. Biosci. 2021, 21, e2000194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, G.; Sha, X.; Li, L.; Zhang, K.; Liu, D.; Hao, Y.; Cui, X.; Wang, L.; Wang, H. An intelligent vancomycin release system for preventing surgical site infections of bone tissues. Biomater. Sci. 2020, 8, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, H.; Sun, L.; Jin, Y.; Ding, X.; Li, L.; Ji, J.; Chen, H. Construction of High Drug Loading and Enzymatic Degradable Multilayer Films for Self-Defense Drug Release and Long-Term Biofilm Inhibition. Biomacromolecules 2018, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Dong, K.; Yan, Z.; Ding, C.; Chen, Z.; Ren, J.; Qu, X. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Shi, R.; Ye, J.; Li, W.; Zhang, J.; Li, J.; Wu, C.; Xue, J.; Zhang, L. Infection-responsive electrospun nanofiber mat for antibacterial guided tissue regeneration membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Filipović, N.; Veselinović, L.; Ražić, S.; Jeremić, S.; Filipič, M.; Žegura, B.; Tomić, S.; Čolić, M.; Stevanović, M. Poly (ε-caprolactone) microspheres for prolonged release of selenium nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 776–789. [Google Scholar] [CrossRef]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-Targeting Nanoparticles with Microenvironment-Responsive Antibiotic Release To Eliminate Intracellular Staphylococcus aureus and Associated Infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef]
- Li, Y.-m.; Liu, S.-y. Enzyme-triggered Transition from Polymeric Vesicles to Core Cross-linked Micelles for Selective Release of Antimicrobial Agents. Acta Polym. Sin. 2017, 7, 1178–1190. [Google Scholar] [CrossRef]
- Xiong, M.H.; Li, Y.J.; Bao, Y.; Yang, X.Z.; Hu, B.; Wang, J. Bacteria-responsive multifunctional nanogel for targeted antibiotic delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Wang, Y.C.; Sun, B.; Wang, J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J. Am. Chem. Soc. 2012, 134, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.B.; Zhang, D.; Liu, F.H.; Qiao, Z.Y.; Wang, H. An “On-Site Transformation” Strategy for Treatment of Bacterial Infection. Adv. Mater. 2017, 29, 1703461. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Xu, J.-H.; Qi, G.-B.; Zhao, X.; Yu, F.; Wang, H. Core-Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery. ACS Nano 2014, 8, 4975–4983. [Google Scholar] [CrossRef]
- Tonkin, R.L.; Klöckner, A.; Najer, A.; Simoes da Silva, C.J.; Echalier, C.; Dionne, M.S.; Edwards, A.M.; Stevens, M.M. Bacterial Toxin-Triggered Release of Antibiotics from Capsosomes Protects a Fly Model from Lethal Methicillin-Resistant Staphylococcus aureus (MRSA) Infection. Adv. Healthc. Mater. 2022, 11, e2200036. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Zhou, S.; Xu, J.H.; Jiang, W.; Tan, L.H.; Fu, J.J. Nanovalves-Based Bacteria-Triggered, Self-Defensive Antibacterial Coating: Using Combination Therapy, Dual Stimuli-Responsiveness, and Multiple Release Modes for Treatment of Implant-Associated Infections. Chem. Mater. 2017, 29, 8325–8337. [Google Scholar] [CrossRef]
- Chen, M.; Wei, J.; Xie, S.; Tao, X.; Zhang, Z.; Ran, P.; Li, X. Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 2019, 11, 1410–1422. [Google Scholar] [CrossRef]
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial Micelles with Vancomycin-Mediated Targeting and pH/Lipase-Triggered Release of Antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 36814–36823. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Stanton, M.M.; Park, B.W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Sun, H.; Lei, W.; Tang, Y.; Hong, S.; Yang, H.; Tay, F.R.; Huang, C. MMP-8-Responsive Polyethylene Glycol Hydrogel for Intraoral Drug Delivery. J. Dent. Res. 2019, 98, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.; Gómez-Cerezo, N.; García-Fernández, A.; Aznar, E.; Vivancos, J.L.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Mesoporous Bioactive Glasses Equipped with Stimuli-Responsive Molecular Gates for Controlled Delivery of Levofloxacin against Bacteria. Chemistry 2018, 24, 18944–18951. [Google Scholar] [CrossRef] [Green Version]
- Stavrakis, A.I.; Zhu, S.; Hegde, V.; Loftin, A.H.; Ashbaugh, A.G.; Niska, J.A.; Miller, L.S.; Segura, T.; Bernthal, N.M. In vivo efficacy of a smart antimicrobial implant coating. J. Bone Jt. Surg.—Am. Vol. 2016, 98, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Yan, R.; Chen, S.; Hao, X.; Dou, W.; Liu, H.; Guo, Z.; Kilula, D.; Seok, I. Narrow pH response multilayer films with controlled release of ibuprofen on magnesium alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111414. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, J.M.; Overstreet, D.J.; Vernon, B.L.; McLemore, R.Y.; Nagy, T.; Moore, R.C.; Badha, V.S.; Childers, E.P.; Nguyen, M.B.; Gentry, D.D.; et al. In vivo evaluation of temperature-responsive antimicrobial-loaded PNIPAAm hydrogels for prevention of surgical site infection. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 103–114. [Google Scholar] [CrossRef]
- McKay, C.S.; Finn, M.G. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [Green Version]
- Kuckling, D. Stimuli-Responsive Gels. Gels 2018, 4, 60. [Google Scholar] [CrossRef] [Green Version]
- Alem, H.; Duwez, A.S.; Lussis, P.; Lipnik, P.; Jonas, A.M.; Demoustier-Champagne, S. Microstructure and thermo-responsive behavior of poly(N-isopropylacrylamide) brushes grafted in nano-pores of track-etched membranes. J. Membr. Sci. 2008, 308, 75–86. [Google Scholar] [CrossRef]
- Schaer, T.P.; Stewart, S.; Hsu, B.B.; Klibanov, A.M. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials 2012, 33, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Richards, R.G. Staphylococci and implant surfaces: A review. Injury 2006, 37 (Suppl. S2), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.G.; Richards, R.G. Staphylococcus aureus adhesion to different treated titanium surfaces. J. Mater. Sci. Mater. Med. 2004, 15, 311–314. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; van Heuvel, M.; Misset, O. Bacterial lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef]
- Chawla, J.S.; Amiji, M.M. Biodegradable poly(epsilon -caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002, 249, 127–138. [Google Scholar] [CrossRef]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef]
- Wijagkanalan, W.; Kawakami, S.; Higuchi, Y.; Yamashita, F.; Hashida, M. Intratracheally instilled mannosylated cationic liposome/NFkappaB decoy complexes for effective prevention of LPS-induced lung inflammation. J. Control. Release 2011, 149, 42–50. [Google Scholar] [CrossRef]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Ali, L.; Goraya, M.U.; Arafat, Y.; Ajmal, M.; Chen, J.L.; Yu, D. Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches. Int. J. Mol. Sci. 2017, 18, 960. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, J.; Chen, S.; Oyama, N.; Nishiguchi, K.; Azab, E.A.; Tanaka, E.; Kariyama, R.; Sonomoto, K. Revised model for Enterococcus faecalis fsr quorum-sensing system: The small open reading frame fsrD encodes the gelatinase biosynthesis-activating pheromone propeptide corresponding to staphylococcal agrd. J. Bacteriol. 2006, 188, 8321–8326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, L.; Wang, C.; Lei, X.; Du, X.; Guo, Q.; Zhou, S.; Cui, P.; Hong, T.; Jiang, P.; Wang, J.; et al. Gelatinase-responsive release of an antibacterial photodynamic peptide against Staphylococcus aureus. Biomater. Sci. 2021, 9, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, W.; Zhou, X.; Yin, Z.; He, C. Thermo-and pH dual-responsive mesoporous silica nanoparticles for controlled drug release. J. Control. Release Off. J. Control. Release Soc. 2015, 213, e69–e70. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.W.; Labow, R.S.; Santerre, J.P. Enzyme induced biodegradation of polycarbonate-polyurethanes: Dose dependence effect of cholesterol esterase. Biomaterials 2003, 24, 2003–2011. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Hernandez-Rios, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernandez, M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.J.; Overall, C.M.; McCulloch, C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000 2003, 31, 77–104. [Google Scholar] [CrossRef]
- Alkekhia, D.; LaRose, C.; Shukla, A. β-Lactamase-Responsive Hydrogel Drug Delivery Platform for Bacteria-Triggered Cargo Release. ACS Appl. Mater. Interfaces 2022, 14, 27538–27550. [Google Scholar] [CrossRef]
- Ghimire, A.; Song, J. Anti-Periprosthetic Infection Strategies: From Implant Surface Topographical Engineering to Smart Drug-Releasing Coatings. ACS Appl. Mater. Interfaces 2021, 13, 20921–20937. [Google Scholar] [CrossRef]




| Study | Drug(s) | Trigger(s) | Type | Structures | Bacteria | Outcome(s) |
|---|---|---|---|---|---|---|
| Li D. et al., 2022 [10] | VAN | pH switches | Nanoparticles | VAN@PEG-VAN | S. aureus | ZOI, MCB (in vitro), BIR (in vivo) |
| Fu M. et al., 2022 [11] | GTM | pH switches | Hydrogels | GTM@P(AA-co-HEMA) | E. coli, S. aureus | DLC, ZOI, BIR (in vivo) |
| Yang, L. et al., 2022 [12] | TOB | pH switches | Films | TOB@Protocatechualdehyde-aminoglycosides | E. coli, P. aeruginosa, S. epidermidis, S. aureus | ZOI, MCB (in vitro), BIR (in vivo) |
| Cámara-Torres M. et al., 2021 [13] | CFX, GTM | pH switches, ion exchange | Scaffolds | PEOT/PBT-MgAl-CFX, PEOT/PBT-Zrp-GTM | S. epidermidis, P. aeruginosa | ZOI |
| Guo, R. et al., 2021 [14] | Triclosan | pH switches | Micelles | PLA-PEG-PAE | E. coli, S. aureus | BIR (in vitro), MCB (in vitro) |
| Ramesh, S. et al., 2021 [15] | ZnONPs | pH switches | Hydrogels | ZnONPs@CS-GP | E. coli, S. aureus | BIR (in vitro), DLC |
| Sang, S. et al., 2021 [16] | GTM | pH switches | Films | GTM-Silk protein | E. coli, S. aureus | ZOI, DLC, BIR (in vitro), MCB (in vitro) |
| Yan K. et al., 2021 [17] | AgNPs | pH switches | Hydrogels | CS-AgNPs | E. coli, S. aureus | ZOI, MCB (in vitro) |
| Zha, J. et al., 2021 [18] | Curcumin | pH switches | Hydrogels | Curcumin@POEGMA-PEI | MRSA | BIR (in vitro) |
| Hassan D. et al., 2020 [19] | VAN | pH switches | Quatsomes | VAN-StBAclm | MRSA | DLC, BIR (in vitro and in vivo), MCB (in vitro) |
| Li, M. et al., 2020 [20] | GTM | pH switches | Films | GTM@(al-ALG/PEI)10 | E. coli, S. aureus | ZOI, DLC, BIR (in vitro), MCB (in vitro) |
| Chen J. et al., 2020 [21] | AMPs | pH switches | Films | TNTs-PMAA-AMP | S. aureus, E. coli, P. aeruginosa, MRSA | BIR (in vitro and in vivo) |
| Tao B. et al., 2019 [22] | GTM | pH switches | Films | TNTs-BMP2-(ADA-GTM/CS)10 | S. aureus, E. coli | BIR (in vitro), MCB (in vitro) |
| Jin, X. et al., 2019 [23] | GTM | pH switches | Scaffolds | Porous hydroxyapatite-GTM | E. coli, S. aureus | DLC, ZOI, BIR (in vitro and in vivo) |
| de Avila E.D. et al., 2019 [24] | TC | pH switches | Films | (PAA/PLL-TC)10 | P. gingivalis | BIR (in vitro) |
| Cao J. et al., 2019 [25] | CHX | pH switches | Nanoparticles | CHX@PMPC-CS | S. aureus | BIR (in vitro); MCB (in vitro) |
| Hu J. et al., 2019 [26] | TOB, ornidazole | pH switches | Hydrogels | TOB-G1-orni | S. aureus, P. aeruginosa, C. sporogenes, B. fragilis | BIR (in vitro); MCB (in vitro) |
| Karakeçili A. et al., 2019 [27] | VAN | pH switches | Nanoparticles | ZIF8/VAN | S. aureus | DLC, BIR (in vitro) |
| Maji R. et al., 2019 [28] | VAN | pH switches | Nanoparticles | VAN@lipid–dendrimer hybrid NPs | MRSA | DLC, BIR (in vitro) |
| Mir M. et al., 2019 [29] | CAR | pH switches | Nanoparticles | CAR@PCL-NPs | MRSA | BIR (in vitro) |
| Zhou W. et al., 2018 [30] | GTM, AgNPs | pH switches | Films | CS-(AgNPs/GTM-SF) | S. aureus | BIR (in vitro) |
| Xiang Y. et al., 2018 [31] | VAN | pH switches | Quantum dots | TNTs-VAN@ZnO-FA | S. aureus | BIR (in vitro); MCB (in vitro) |
| Placente D. et al., 2018 [32] | CFX | pH switches | Nanoparticles | Lipid membrane mimetic coated nano-hydroxyapatite | E. coli, P. aeruginosa, S. aureus | BIR (in vitro), ZOI |
| Cicuéndez M. et al., 2018 [33] | LFX | pH switches | Scaffolds | MGHA-LFX | S. aureus | BIR (in vitro), MCB (in vitro) |
| Dai T. et al., 2018 [34] | AgNPs | pH switches | Hydrogels | Dex-G5-AgNPs | E. coli, P. aeruginosa, S. aureus, S. epidermidis | BIR (in vitro and in vivo), MCB (in vitro) |
| Dubovoy V. et al., 2018 [35] | BAC | pH swtiches | Nanoparticles | BAC-MSNs | S. aureus | BIR (in vitro) |
| Mhule D. et al., 2018 [36] | VAN | pH swtiches | Nanoparticles | VAN@NMEO | MRSA | DLC, BIR (in vitro and in vivo) |
| Soltani B. et al., 2018 [37] | GTM | pH swtiches | Nanoparticles | GTM@nanoscale zeolitic imidazolate frame-work-8 | E. coli, S. aureus | DLC, BIR (in vitro) |
| Yu X. et al., 2018 [38] | VAN | pH swtiches | Microspheres | PLGA–NaHCO3–Van | S. aureus, MRSA | DLC, BIR (in vitro), ZOI |
| Zhang S. et al., 2018 [39] | AgNPs | pH switches | Films | AgNPs@PLL/PG | S. aureus | BIR (in vitro) |
| Zhou, W. et al., 2018 [40] | TOB | pH switches | Films | TOB@ (CHT/HET)2 | S. aureus | ZOI, BIR (in vitro), MCB (in vitro) |
| Wang T. et al., 2017 [41] | VAN, Ag | pH switches | Films | TNT(NH2)-VAN@Zn-BIX, TNT(NH2)-Ag@Zn-BIX | E. coli, S. aureus | ZOI, BIR (in vitro), MCB (in vitro) |
| Pamfil D. et al., 2017 [42] | CFX | pH switches | Hydrogels | CFX @HEMA/C-CA | S. aureus | ZOI |
| Liu Z. et al., 2017 [43] | VAN | pH switches | Nanoparticles | VAN@PVA/PLGA | S. aureus | ZOI, MCB (in vitro) |
| Dong Y. et al., 2017 [44] | AgNPs | pH switches | Films | TNTs-acetal linker-AgNPs | E. coli, S. aureus | BIR (in vitro) |
| Kalhapure R. S. et al., 2017 [45] | VAN | pH switches | Nanoparticles | VAN@(2-(2,4,6trimethoxyphenyl)-1,3-dioxane-5,5-diyl) bis(methylene) distearate | S. aureus, MRSA | DLC, BIR (in vitro and in vivo) |
| Sang Q et al., 2017 [46] | CFX | pH switches | Nanofibers | Gelatin-sodium bicarbonate | E. coli, S. aureus | DLC, BIR (in vitro) |
| Onat B. et al., 2016 [47] | Triclosan | pH switches | Micelles | (Triclosan@βPDMA-b-PDPA)3 | S. aureus, E. coli | BIR (in vitro), ZOI |
| Fullriede H. et al., 2016 [48] | CHX | pH switches | Nanoparticles | CHX@silica nanoparticles-PVP | S. aureus, S. mutans | BIR (in vitro) |
| Kalhapure R. S. et al., 2017 [49] | VAN | pH switches | Nanoparticles | CS@VAN-AGS | MRSA | BIR (in vitro and in vivo) |
| Anandhakumar S. et al., 2016 [50] | CFX | pH switches | Films | (PAH/PMAA-CFX)8 | E. coli | ZOI |
| Zhang Z. et al., 2015 [51] | MNC | pH switches | Films | (DS-Mg2+-MNC)-GA | E. coli, S. aureus | BIR (in vitro) |
| Zhuk I. et al., 2014 [52] | GTM, TOB, polymyxin B | pH switches | Films | TA-GTM/TOB/polymyxin B (PolyB) | S. epidermidis, S. aureus | BIR (in vitro), MCB (in vitro) |
| Zhang Z. et al., 2014 [53] | MNC | pH switches | Films | (DS-Ca2+/MNC-Ca2+/GA-Ca2+)8 | E. coli, S. aureus, MRSA, S. epidermidis | BIR (in vitro), MCB (in vitro) |
| Pichavant, L. et al., 2012 [54] | GTM | pH switches | Nanoparticles | GTM@Functionalized PEO | S. aureus | DLC, BIR (in vitro) |
| Choi H. et al., 2021 [55] | LFX | High temperatures | Films | Ti-PDEGMA-LFX | S. aureus | DLC, MCB (in vitro and in vivo) |
| Li B. et al., 2021 [56] | Glycerin, simvastatin | High temperatures | Hydrogels | TNTs-CS-glycerin-hydroxypropylmethyl | E. coli, S. aureus | BIR (in vivo) |
| Liang J. et al., 2019 [57] | Colistin, AMPs | Bacterial contact | Microgels | PAA-colistin, PAA-AMPs | E. coli, S. epidermidis | BIR (in vitro) |
| Bourgat Y. et al., 2021 [58] | CFX | Enzymes (PS) | Nanogels | Alginate-PLL-CFX | S. aureus | BIR (in vitro) |
| Timin A. et al., 2018 [59] | CFS | Enzymes (PS) | Scaffolds | PCL-CFS, PHB-CFS, (PHB-PANi)-CFS | E. coli | ZOI |
| Liao X. et al., 2021 [60] | CHX | Enzymes (PS) | Films | (MTT/PLL-CHX)10 | S. aureus | ZOI, DLC, BIR (in vitro and in vivo), |
| Yu X. et al., 2021 [61] | VAN | Enzymes (PS) | Films | (MTT/PLL-VAN)8 | S. aureus | ZOI, MCB (in vitro), BIR (in vitro and in vivo) |
| Zhang Y. et al., 2021 [62] | VAN | Enzymes (PS) | Films | Ti-SRP1 peptides-VAN | S. aureus | BIR (in vitro) |
| Johnson CT. et al., 2018 [63] | Lysostaphin | Enzymes (PS) | Hydrogels | PEG-4MAL-lysostaphin | S. aureus | BIR(in vitro and in vivo) |
| Li Y. et al., 2020 [64] | VAN | Enzymes (HAS) | Hydrogels | VAN-HA-CS/β-glycerophosphate | S. aureus, S. epidermidis | BIR (in vitro) |
| Wang B. et al., 2018 [65] | GTM | Enzymes (HAS) | Films | (MMT/HA-GTM)10 | S. aureus, E. coli | BIR (in vitro and in vivo); ZOI; MCB (in vitro and in vivo) |
| Ji H. et al., 2016 [66] | AA, MNPs | Enzymes (HAS) | Nanosheet | AA@GMSN-HA-MNPs | S. aureus, E. coli | BIR (in vitro and in vivo), MCB (in vitro) |
| Shi R. et al., 2019 [67] | MNA | Enzymes (LS) | Films | PCL-dopamine-MNA | H. pylori | BIR (in vitro) |
| Filipović N. et al., 2019 [68] | SeNPs | Enzymes (LS) | Microspheres | PCL-SeNPs | S. epidermidis, S. aureus | ZOI, DLC |
| Yang S. et al., 2018 [69] | GTM | Enzymes (LS) | Nanoparticles | GTM@MSNs-lipid-UBI | S. aureus | BIR (in vitro and in vivo) |
| Li Y.M. et al., 2017 [70] | Triclosan, AMP, parasin I, lysozyme | Enzymes (LS) | Micelles | PEG-b-PA/PN@drugs | S. aureus, E. coli | DLC, BIR (in vitro) |
| Xiong M. et al., 2012 [71] | VAN | Enzymes (LS) | Nanogels | Mannosyl-PEG-polyphosphoester-VAN | S. aureus | DLC, BIR (in vitro and in vivo), MCB (in vivo) |
| Xiong M. et al., 2012 [72] | VAN | Enzymes (LS) | Nanogels | PEG-PCL-polyphosphoester-VAN | S. aureus | DLC, BIR (in vitro) |
| Qi GB. et al. 2017 [73] | AMPs | Enzymes (GS) | Nanoparticles | CS-CPC1-AMPs | S. aureus | BIR (in vitro and in vivo), MCB (in vitro and in vivo) |
| Li L.L. et al., 2014 [74] | VAN | Enzymes (GS) | Nanoparticles | SGNPs-VAN @RBC | S. epidermidis, S. aureus | DLC, BIR (in vitro), MCB |
| Tonkin, R. L., et al., 2022 [75] | VAN | Cytoloytic toxin | Capsosomes | VAN@Mesosilica-PAH-(PMAA-PDA/liposome)3 | MRSA | ZOI, survival rate |
| Wang T. et al., 2017 [76] | Ampicillin, CA | pH switches, enzymes (LS) | Films | VAMSC-CA/ampicillin-monopyridine functionalized β-cyclodextrin | E. coli, S. aureus, MRSA | BIR (in vitro), MCB (in vitro) |
| Chen M. et al., 2019 [77] | D-tyrosine, AZM | pH switches, enzymes (LS) | Micelles | DOEAz-tyrosine | P. aeruginosa | BIR (in vitro and in vivo), MCB (in vitro and in vivo) |
| Chen M. et al., 2018 [78] | VAN, CFX | pH switches, enzymes (LS) | Micelles | CFX@VAN-PECL | P. aeruginosa | BIR (in vitro and in vivo), MCB (in vitro and in vivo) |
| Qu J. et al., 2018 [79] | AMX | pH and electric field switches | Hydrogels | CP/OD-AMX | E. coli, S. aureus | BIR (in vitro) |
| Stanton M. M. et al., 2017 [80] | CFX | pH and external magnetic guidance | Biohybrids | MSR1-CFX@MSM | E. coli | BIR (in vitro), MCB (in vitro) |
| Hu C. et al., 2020 [81] | AMIK, naproxen | pH switches, ROS | Hydrogels | (AMIK@ALG-BA)-(naproxen@HA-cholesterol) | S. aureus, P. aeruginosa | DLC, BIR (in vitro and in vivo), MCB (in vitro) |
| Guo J. et al., 2019 [82] | MNC, AMP | MMP-8 | Hydrogels | MNC@(4-arm PEG-diacrylate)-MMP8 sensitive peptide | P. gingivalis | BIR (in vitro) |
| Polo L. et al., 2018 [83] | LFX | APS | Scaffolds | MBG-LFX-ATP | E. coli | BIR (in vitro) |
| Stavrakis A. et al., 2016 [84] | VAN, TGC | ROS | Films | Van@PEG-PPS, TGC@PEG-PPS | S. aureus | BIR (in vivo) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Jiang, X.; Lai, H.; Zhang, X. Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants. J. Funct. Biomater. 2022, 13, 173. https://doi.org/10.3390/jfb13040173
Yang Y, Jiang X, Lai H, Zhang X. Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants. Journal of Functional Biomaterials. 2022; 13(4):173. https://doi.org/10.3390/jfb13040173
Chicago/Turabian StyleYang, Yijie, Xue Jiang, Hongchang Lai, and Xiaomeng Zhang. 2022. "Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants" Journal of Functional Biomaterials 13, no. 4: 173. https://doi.org/10.3390/jfb13040173
APA StyleYang, Y., Jiang, X., Lai, H., & Zhang, X. (2022). Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants. Journal of Functional Biomaterials, 13(4), 173. https://doi.org/10.3390/jfb13040173







