Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Preparation of Ulvan–Containing Liposomes
2.4. Preparation of FA-Loaded Liposomes
2.5. Scanning Electron Microscopy
2.6. FTIR Spectroscopy
2.7. Particle Size Analysis
2.8. Zeta Potential Determination
2.9. Differential Scanning Calorimetry
2.10. Determination of Entrapment Efficiency
2.11. Evaluation of Antibacterial Activity
3. Results and Discussion
3.1. Thermodynamic Evaluation of Lipid-Ulvan Interactions
3.2. Preparation and Characterization of Ulvan–Containing Liposomes
3.3. Preparation and Characterization of Fusidic Acid-Loaded Liposomes
3.4. Evaluation of the Antibacterial Activity of the Liposome Nanocarriers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, C.Y.; Yang, S.C.; Sung, C.T.; Weng, Y.H.; Fang, J.Y. Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int. J. Nanomed. 2017, 12, 8227–8238. [Google Scholar] [CrossRef] [Green Version]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Alhariri, M.; Azghani, A.; Omri, A. Liposomal antibiotics for the treatment of infectious diseases. Expert Opin. Drug Deliv. 2013, 10, 1515–1532. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafee, N. Nanocarriers against bacterial biofilms: Current status and future perspectives. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Elsevier: London, UK, 2015; pp. 167–189. [Google Scholar]
- Rukavina, Z.; Vanić, Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachetelli, S.; Khalil, H.; Chen, T.; Beaulac, C.; Senechal, S.; Lagace, J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2000, 1463, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Lemarchand, C.; Gref, R.; Couvreur, P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004, 58, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, S. Polysaccharides as biomaterials. In Polymeric Biomaterials; Dumitriu, S., Ed.; Marcel Dekker: New York, NY, USA, 2001; pp. 1–61. [Google Scholar]
- Na, K.; Park, K.-H.; Kim, S.W.; Bae, Y.H. Self-assembled hydrogel nanoparticles from curdlan derivatives: Characterization, anti-cancer drug release and interaction with hepatoma cell line (HepG2). J. Control. Release 2000, 69, 225–236. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Oldenkamp, H.F.; Vela Ramirez, J.E.; Peppas, N.A. Re-evaluating the importance of carbohydrates as regenerative biomaterials. Regen. Biomater. 2019, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Woddle, M.C.; Lasic, D.D. Sterically stabilized liposomes. Biochim. Biophys. Acta 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Allen, T.M. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 1994, 13, 285–309. [Google Scholar] [CrossRef]
- Yerushalmi, N.; Margalit, R. Hyaluronic acid-modified bioadhesive liposomes as local drug depots: Effects of cellular and fluid dynamics on liposome retention at target sites. Arch. Biochem. Biophys. 1998, 349, 21–26. [Google Scholar] [CrossRef]
- Fan, Y.; Sahdev, P.; Ochyl, L.J.; Akerberg, J.J.; Moon, J.J. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 2015, 208, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cansell, M.; Parisel, C.; Jozefonvicz, J.; Letouneur, D. Liposomes coated with chemically modified dextrans interact with human endothelial cells. J. Biomed. Mater. Res. 1999, 44, 140–148. [Google Scholar] [CrossRef]
- Letourneur, D.; Parisel, C.; Prigent-Richard, S.; Cansell, M. Interactions of functionalized dextran-coated liposomes with vascular smooth muscle cells. J. Control. Release 2000, 65, 83–91. [Google Scholar] [CrossRef]
- Venkatesan, N.; Vyas, S.P. Polysaccharide coated liposomes for oral immunization—Development and characterization. Int. J. Pharm. 2000, 203, 169–177. [Google Scholar] [CrossRef]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef]
- Jung, I.-W.; Han, H.K. Effective mucoadhesive liposomal delivery system for risedronate: Preparation and in vitro/in vivo characterization. Int. J. Nanomed. 2014, 9, 2299–2306. [Google Scholar] [CrossRef] [Green Version]
- Jøraholmen, M.W.; Vanić, Ž.; Tho, I.; Škalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Mishchenko, E.; Eide Flaten, G.; Ericson Sollid, J.U.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-based nanomedicine to fight genital Candida infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Alshamsan, A.; Sfouq Aleanizy, F.; Badran, M.; Yahya Alqahtania, F.; Alfassam, H.; Almalik, A.; Alosaimy, S. Exploring anti-MRSA activity of chitosan-coated liposomal dicloxacillin. J. Microbiol. Methods 2019, 156, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Fujimoto, K. Control in mineralization by the polysaccharide-coated liposome via the counter-diffusion of ions. Chem. Mater. 2011, 23, 4701–4708. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and function properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Sassi, J.F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physicochemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Chiellini, F.; Morelli, A. Ulvan: A versatile platform of biomaterials from renewable resources. In Biomaterials-Physics and Chemistry; Pignatello, R., Ed.; InTech: London, UK, 2011; pp. 75–98. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.-K. Sulfated polysaccharides as bioactive agents from marine algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Duarte, A.R.C.; Mano, J.F.; Sousa, R.A.; Reis, R.L. PDLLA enriched with ulvan particles as a novel 3D porous scaffold targeted for bone engineering. J. Supercrit. Fluids 2012, 65, 32–38. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. Processing of degradable ulvan 3D porous structures for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101A, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.A.; Alves, A.; Nunes, C.; Coimbra, M.A.; Pires, R.A.; Reis, R.L. Carboxymethylation of ulvan and chitosan and their use as polymeric components of bone cements. Acta Biomater. 2013, 9, 9086–9097. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Samal, S.K.; Bartoli, C.; Morelli, A.; Smet, P.F.; Dubruel, P.; Chiellini, F. Biofunctionalization of ulvan scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Chiellini, F. Ulvan as a new type of biomaterial from renewable resources: Functionalization and hydrogel preparation. Macromol. Chem. Phys. 2010, 211, 821–832. [Google Scholar] [CrossRef]
- Morelli, A.; Betti, M.; Puppi, D.; Chiellini, F. Design, preparation and characterization of ulvan based thermosensitive hydrogels. Carbohydr. Polym. 2016, 136, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.-A.; Sapalidis, A.; Kikionis, S.; Aggelidou, E.; Demiri, E.; Kritis, A.; Ioannou, E.; Roussis, V. Hybrid sponge-like scaffolds based on ulvan and gelatin: Design, characterization and evaluation of their potential use in bone tissue engineering. Materials 2020, 13, 1763. [Google Scholar] [CrossRef]
- Toskas, G.; Heinemann, S.; Heinemann, C.; Cherif, C.; Hund, R.-D.; Roussis, V.; Hanke, T. Ulvan and ulvan/chitosan polyelectrolyte nanofibrous membranes as a potential substrate material for the cultivation of osteoblasts. Carbohydr. Polym. 2012, 89, 1002. [Google Scholar] [CrossRef]
- Dash, M.; Samal, S.K.; Morelli, A.; Bartoli, C.; Declercq, H.A.; Douglas, T.E.L.; Dubruel, P.; Chiellini, F. Ulvan-chitosan polyelectrolyte complexes as matrices for enzyme induced biomimetic mineralization. Carbohydr. Polym. 2018, 182, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tziveleka, L.A.; Pippa, N.; Georgantea, P.; Ioannou, E.; Demetzos, C.; Roussis, V. Marine sulfated polysaccharides as versatile polyelectrolytes for the development of drug delivery nanoplatforms: Complexation of ulvan with lysozyme. Int. J. Biol. Macromol. 2018, 118, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.A.; Reis, R.L. Processing ulvan into 2D structures: Crosslinked ulvan membranes as new biomaterials for drug delivery applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Toskas, G.; Roussis, V. Electrospun biocomposite nanofibers of ulvan/PCL and ulvan/PEO. J. Appl. Polym. Sci. 2015, 132, 42153. [Google Scholar] [CrossRef]
- Terezaki, A.; Kikionis, S.; Ioannou, E.; Sfiniadakis, I.; Tziveleka, L.-A.; Vitsos, A.; Roussis, V.; Rallis, M. Ulvan/gelatin-based nanofibrous patches as a promising treatment for burn wounds. J. Drug Deliv. Sci. Technol. 2022, 74, 103535. [Google Scholar] [CrossRef]
- Wadhwa, S.; Singh, B.; Sharma, G.; Raza, K.; Prakash Katare, O. Liposomal fusidic acid as a potential delivery system: A new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016, 23, 1204–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimou, M.; Ioannou, E.; Daskalaki, M.G.; Tziveleka, L.A.; Kampranis, S.C.; Roussis, V. Disulfides with anti-inflammatory activity from the brown alga Dictyopteris membranacea. J. Nat. Prod. 2016, 79, 584–589. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Pippa, N.; Hatziantoniou, S.; Mourelatou, E.A.; Amaro-Luis, J.M.; Villalobos-Osorio, D.; Demetzos, C. Preparation and thermal behavior of liposomal nanoparticles incorporating bioactive labdane epimers. Adv. Sci. Lett. 2012, 16, 226–341. [Google Scholar] [CrossRef]
- Morigaki, K.; Tanimoto, Y. Evolution and development of model membranes for physicochemical and functional studies of the membrane lateral heterogeneity. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, S.; Onori, G.; Zuzzi, S.; Bordi, F.; Cametti, C.; Sennato, S.; Diociaiiuti, M. Properties of mixed DOTAP-DPPC bilayer membranes as reported by differential scanning calorimetry and dynamic light scattering measurements. J. Phys. Chem. B 2007, 111, 10032–10039. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Kiselev, M.A.; Wartewig, S.; Janich, M.; Lesieur, P.; Kiselev, A.M.; Ollivon, M.; Neubert, R. Does sucrose influence the properties of DMPC vesicles? Chem. Phys. Lipids 2003, 123, 31–44. [Google Scholar] [CrossRef]
- Andersen, T.; Bleher, S.; Flaten, G.E.; Tho, I.; Mattsson, S.; Škalko-Basnet, N. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Mar. Drugs 2015, 13, 222–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.N.; Castanheira, M.; Rhomberg, P.R.; Woosley, L.N.; Pfaller, M.A. Performance of fusidic acid (CEM-102) susceptibility testing reagents: Broth microdilution, disk diffusion, and as Etest methods applied to Staphylococcus aureus. J. Clin. Microbiol. 2010, 48, 972–976. [Google Scholar] [CrossRef] [Green Version]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Interaction of vesicles: Adhesion, fusion and multicompartment systems formation. ChemBioChem 2011, 12, 510–521. [Google Scholar] [CrossRef]
- Kłodzińska, E.; Szumski, M.; Dziubakiewicz, E.; Hrynkiewicz, K.; Skwarek, E.; Janusz, W.; Buszewski, B. Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis 2010, 31, 1590–1596. [Google Scholar] [CrossRef]
- Patil, R.; Torris, A.; Bhat, S.; Patil, S. Mapping fusogenicity of ciprofloxacin-loaded liposomes with bacterial cells. AAPS PharmSciTech 2019, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Vyas, H.P.; Kumbhani, K.R.; Jagani, R.H. Enhanced activity of antibiotics by liposomal drug delivery. ANTJ 2015, 1, 67–77. [Google Scholar] [CrossRef]
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008, 61, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.N.; Song, Y.-H.; Kaszuba, M.; Reboiras, M.D. The interaction of phospholipid liposomes with bacteria and their use in the delivery of bactericides. J. Drug Target. 1997, 5, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.N.; Kaszuba, M.; Reboiras, M.D.; Lyle, I.G.; Hill, K.J.; Song, Y.-H.; Wilmot, S.W.; Creeth, J.E. The targeting of phospholipid liposomes to bacteria. Biochim. Biophys. Acta 1994, 1196, 57–64. [Google Scholar] [CrossRef]


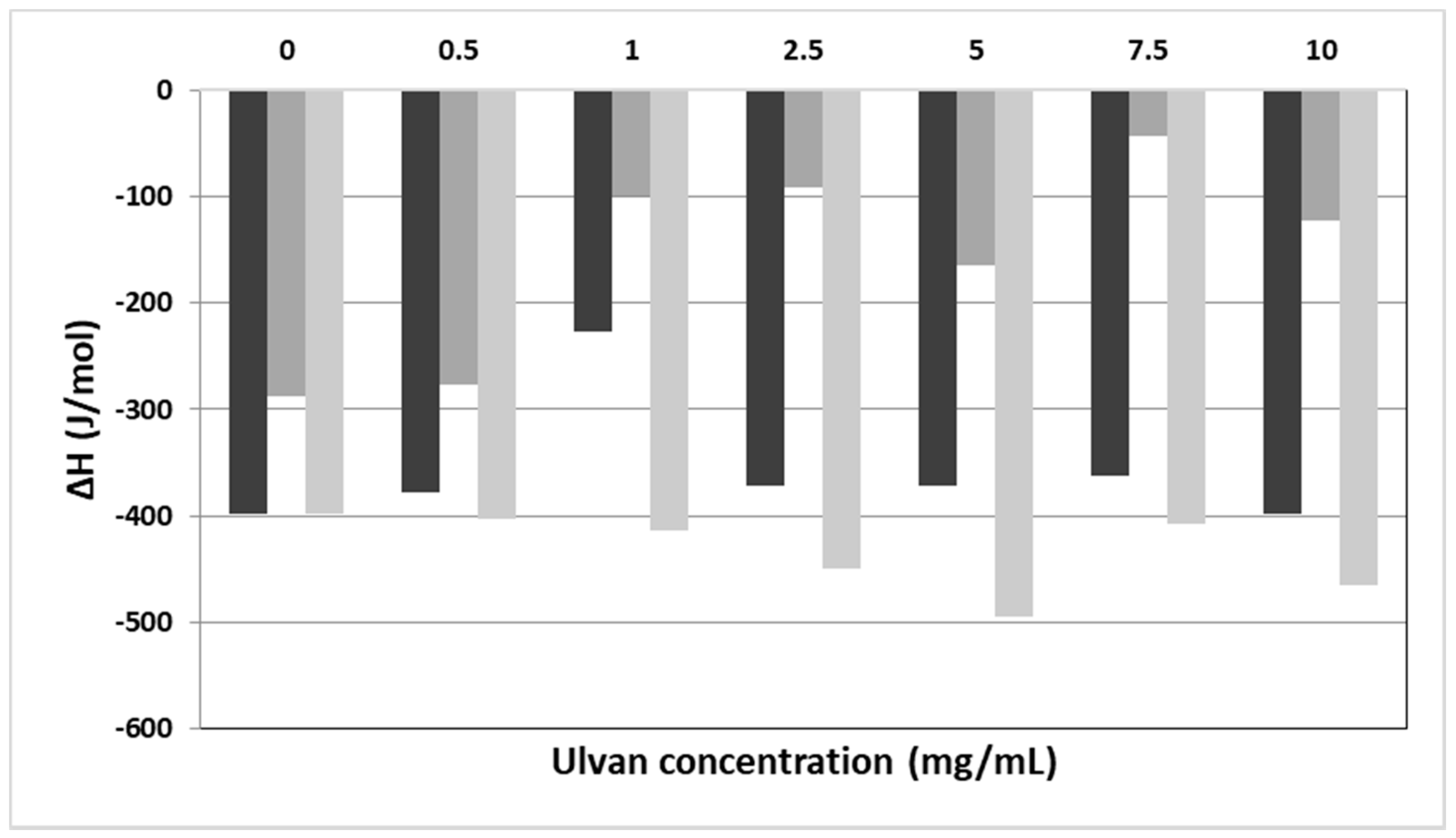
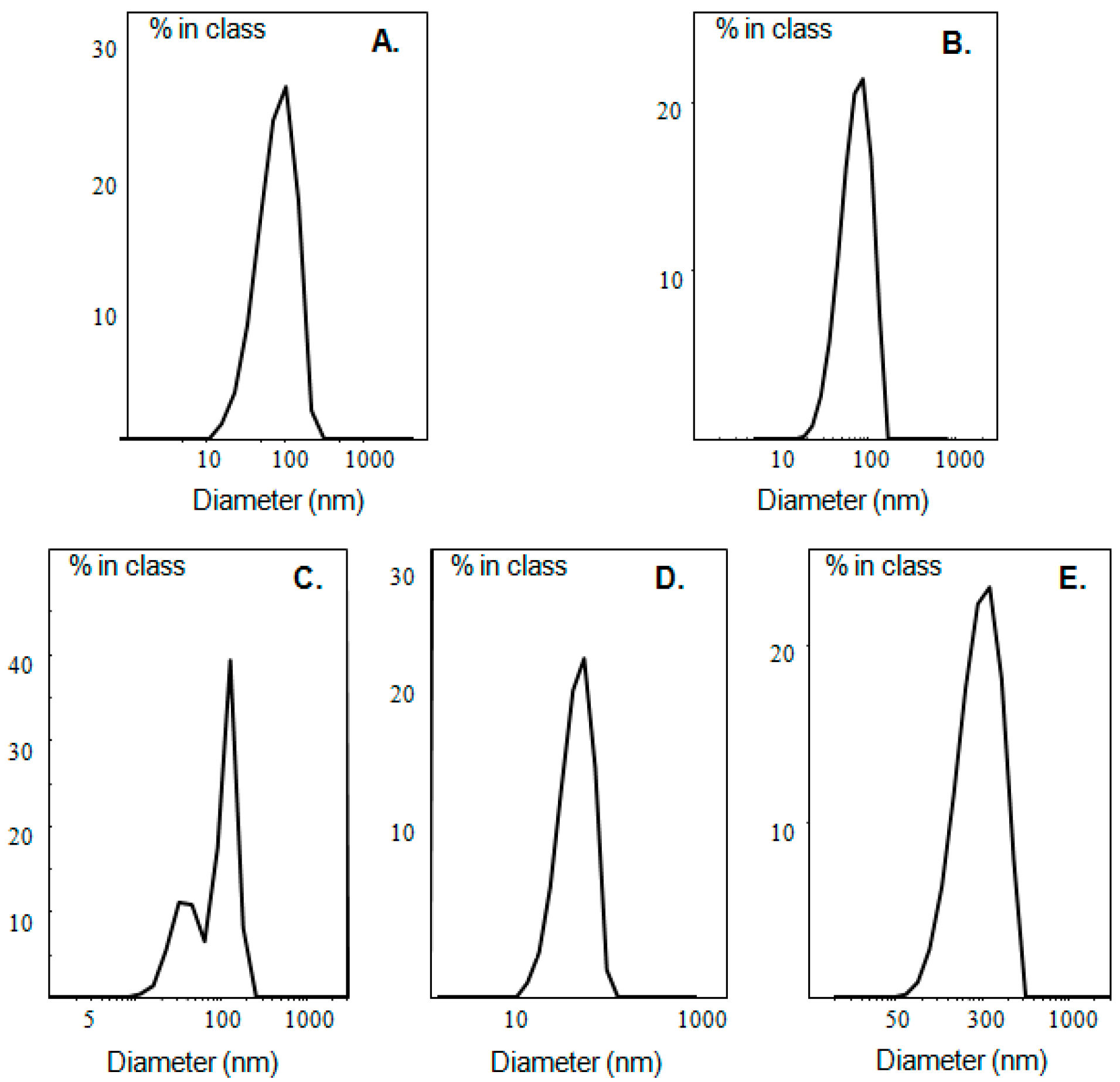
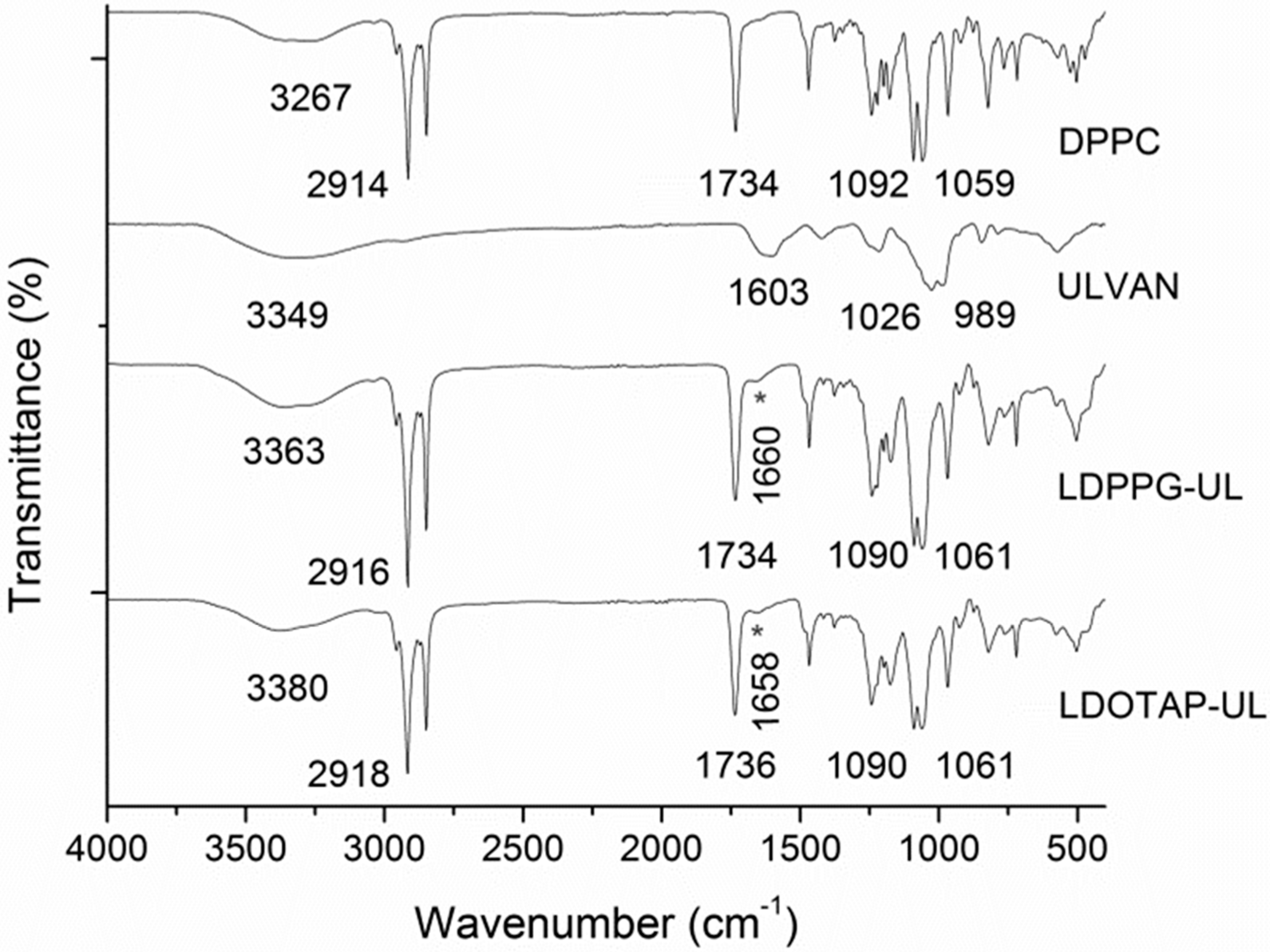
| System | Culvan (mg/mL) | Tonset.m (°C) a | Tm (°C) b | ΔT1/2.m (°C) c | ΔHm (J/mol) d | Tonset.s (°C) a | Ts (°C) b | ΔT1/2.s (°C) c | ΔHs (J/mol) d |
|---|---|---|---|---|---|---|---|---|---|
| DPPC: ulvan | 0 | 40.9 | 41.9 | 1.33 | −398.0 | 34.2 | 36.5 | 2.08 | −36.6 |
| DPPC: ulvan | 0.5 | 40.8 | 41.7 | 1.29 | −378.0 | 33.9 | 36.2 | 1.98 | −38.9 |
| DPPC: ulvan | 1 | 41.2 | 42.5 | 1.84 | −226.1 | 36.0 | 37.5 | 1.83 | −37.4 |
| DPPC: ulvan | 2.5 | 41.2 | 42.5 | 1.84 | −371.0 | 36.0 | 37.5 | 1.93 | −16.2 |
| DPPC: ulvan | 5 | 41.4 | 43.0 | 1.89 | −371.0 | 33.8 | 37.8 | 1.55 | −8.9 |
| DPPC: ulvan | 7.5 | 41.3 | 42.9 | 2.02 | −362.0 | 32.2 | 34.5 | 5.20 | −2.7 |
| DPPC: ulvan | 10 | 41.2 | 43.3 | 1.91 | −398.3 | 36.8 | 37.4 | 1.79 | −2.6 |
| DPPC:DPPG: ulvan | 0 | 42.0 | 43.1 | 0.74 | −287.4 | 34.9 | 36.9 | 1.19 | −41.2 |
| DPPC:DPPG: ulvan | 0.5 | 41.9 | 42.9 | 0.76 | −276.8 | - | - | - | - |
| DPPC:DPPG: ulvan | 1 | 40.1 | 41.9 | 1.37 | −98.5 | - | - | - | - |
| DPPC:DPPG: ulvan | 2.5 | 41.0 | 42.3 | 1.37 | −92.0 | - | - | - | - |
| DPPC:DPPG: ulvan | 5 | 41.0 | 42.5 | 1.67 | −164.2 | - | - | - | - |
| DPPC:DPPG: ulvan | 7.5 | 41.8 | 42.8 | 1.11 | −43.4 | - | - | - | - |
| DPPC:DPPG: ulvan | 10 | 41.2 | 42.8 | 3.47 | −122.8 | - | - | - | - |
| DPPC:DOTAP: ulvan | 0 | 33.2 | 36.6 | 3.25 | −398.3 | - | - | - | - |
| DPPC:DOTAP: ulvan | 0.5 | 33.4 | 36.9 | 3.48 | −402.3 | - | - | - | - |
| DPPC:DOTAP: ulvan | 1 | 33.8 | 37.1 | 3.50 | −414.2 | - | - | - | - |
| DPPC:DOTAP: ulvan | 2.5 | 34.1 | 37.1 | 3.62 | −449.8 | - | - | - | - |
| DPPC:DOTAP: ulvan | 5 | 34.8 | 37.0 | 3.15 | −464.4 | - | - | - | - |
| DPPC:DOTAP: ulvan | 7.5 | 34.9 | 37.5 | 3.40 | −477.5 | - | - | - | - |
| DPPC:DOTAP: ulvan | 10 | 34.4 | 37.3 | 3.40 | −494.5 | - | - | - | - |
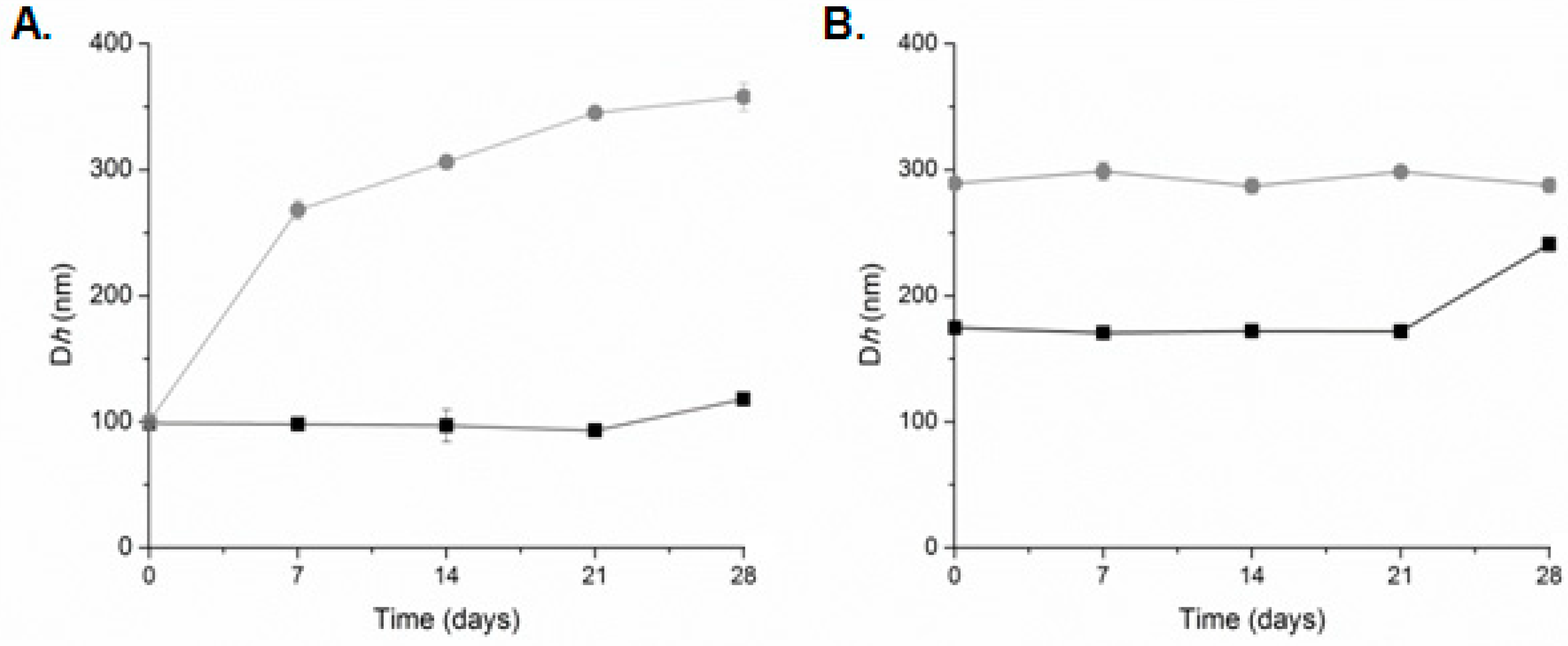
| System | Dispersion Medium | Culvan (mg/mL) | Dh (nm) a | PDI b | ζ-Potential (mV) |
|---|---|---|---|---|---|
| UL | HPLC grade water | 1 | 578.8 ± 76.0 | 1.000 ± 0.000 | −15.7 ± 1.6 |
| LDPPC | HPLC grade water | - | 123.3 ± 15.8 | 0.652 ± 0.053 | 6.5 ± 1.9 |
| LDPPG | HPLC grade water | - | 73.8 ± 7.9 | 0.362 ± 0.007 | −34.9 ± 4.3 |
| LDOTAP | HPLC grade water | - | 69.8 ± 5.9 | 0.318 ± 0.005 | 60.2 ± 2.8 |
| LDPPG-UL | HPLC grade water | 1 | 98.9 ± 1.5 | 0.443 ± 0.002 | −23.8 ± 4.5 |
| LDOTAP-UL | HPLC grade water | 1 | 174.7 ± 1.8 | 0.393 ± 0.030 | 66.2 ± 4.0 |
| UL | PBS | 1 | 432.9 ± 17.0 | 1.000 ± 0.000 | −10.1 ± 0.1 |
| DPPC-UL | PBS | 1 | 165.9 ± 2.3 | 0.752 ± 0.022 | −5.9 ± 1.6 |
| LDPPG-UL | PBS | 1 | 100.4 ± 1.9 | 0.428 ± 0.009 | −25.6 ± 0.8 |
| LDOTAP-UL | PBS | 1 | 289.2 ± 0.9 | 0.482 ± 0.081 | 35.6 ± 1.9 |
| FA-LDPPG | HPLC grade water | - | 71.0 ± 3.5 | 0.572 ± 0.014 | −32.1 ± 0.6 |
| FA-DOTAP | HPLC grade water | - | 72.1 ± 1.4 | 0.563 ± 0.009 | 62.9 ± 5.4 |
| FA-LDPPG-UL | HPLC grade water | 1 | 65.2 ± 4.1 | 0.512 ± 0.011 | −20.0 ± 1.8 |
| FA-LDOTAP-UL | HPLC grade water | 1 | 188.6 ± 2.7 | 0.507 ± 0.015 | 54.3 ± 1.7 |
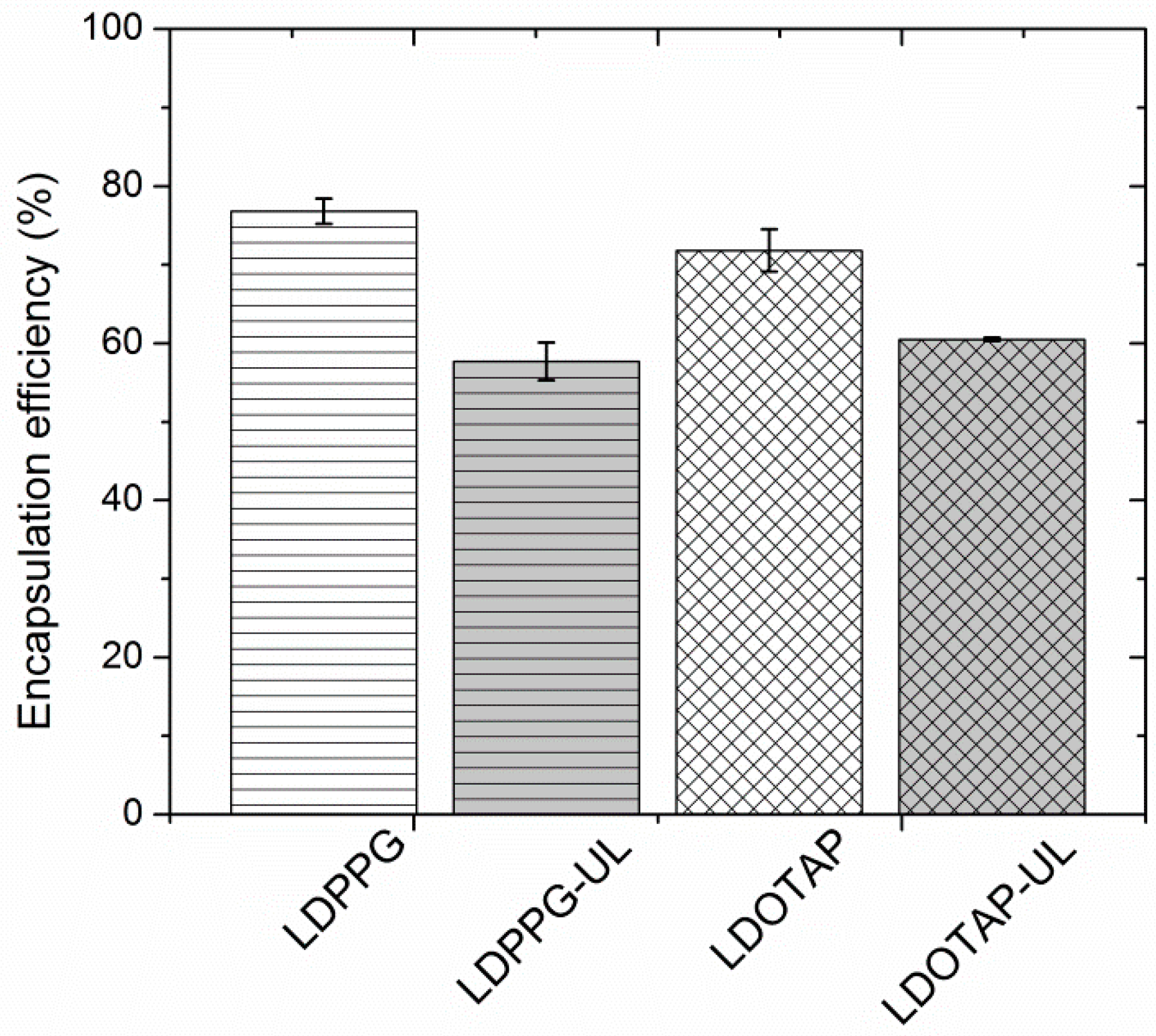
| CLIPOSOMES (μg/mL) a | MICFA (μg/mL) b | |
|---|---|---|
| FA-LDPPG | 6.3 | 0.2 |
| FA-LDPPG-UL | 5.2 | 0.2 |
| FA-LDOTAP | 5.0 | 0.1 |
| FA-LDOTAP-UL | 6.2 | 0.1 |
| FA | - | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tziveleka, L.-A.; Pippa, N.; Ioannou, E.; Demetzos, C.; Roussis, V. Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms. J. Funct. Biomater. 2022, 13, 186. https://doi.org/10.3390/jfb13040186
Tziveleka L-A, Pippa N, Ioannou E, Demetzos C, Roussis V. Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms. Journal of Functional Biomaterials. 2022; 13(4):186. https://doi.org/10.3390/jfb13040186
Chicago/Turabian StyleTziveleka, Leto-Aikaterini, Natassa Pippa, Efstathia Ioannou, Costas Demetzos, and Vassilios Roussis. 2022. "Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms" Journal of Functional Biomaterials 13, no. 4: 186. https://doi.org/10.3390/jfb13040186
APA StyleTziveleka, L.-A., Pippa, N., Ioannou, E., Demetzos, C., & Roussis, V. (2022). Development of Ulvan-Containing Liposomes as Antibacterial Drug Delivery Platforms. Journal of Functional Biomaterials, 13(4), 186. https://doi.org/10.3390/jfb13040186











