Influence of γ-Radiation on Mechanical Stability to Cyclic Loads Tubular Elastic Matrix of the Aorta
Abstract
:Highlights
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospinning of Blood Vessel Prostheses
2.2. Mechanical Properties of BVP
2.3. In Vivo Study of Vascular Prostheses Samples
3. Results
3.1. Characterization of the Grafts
3.2. Mechanical tests
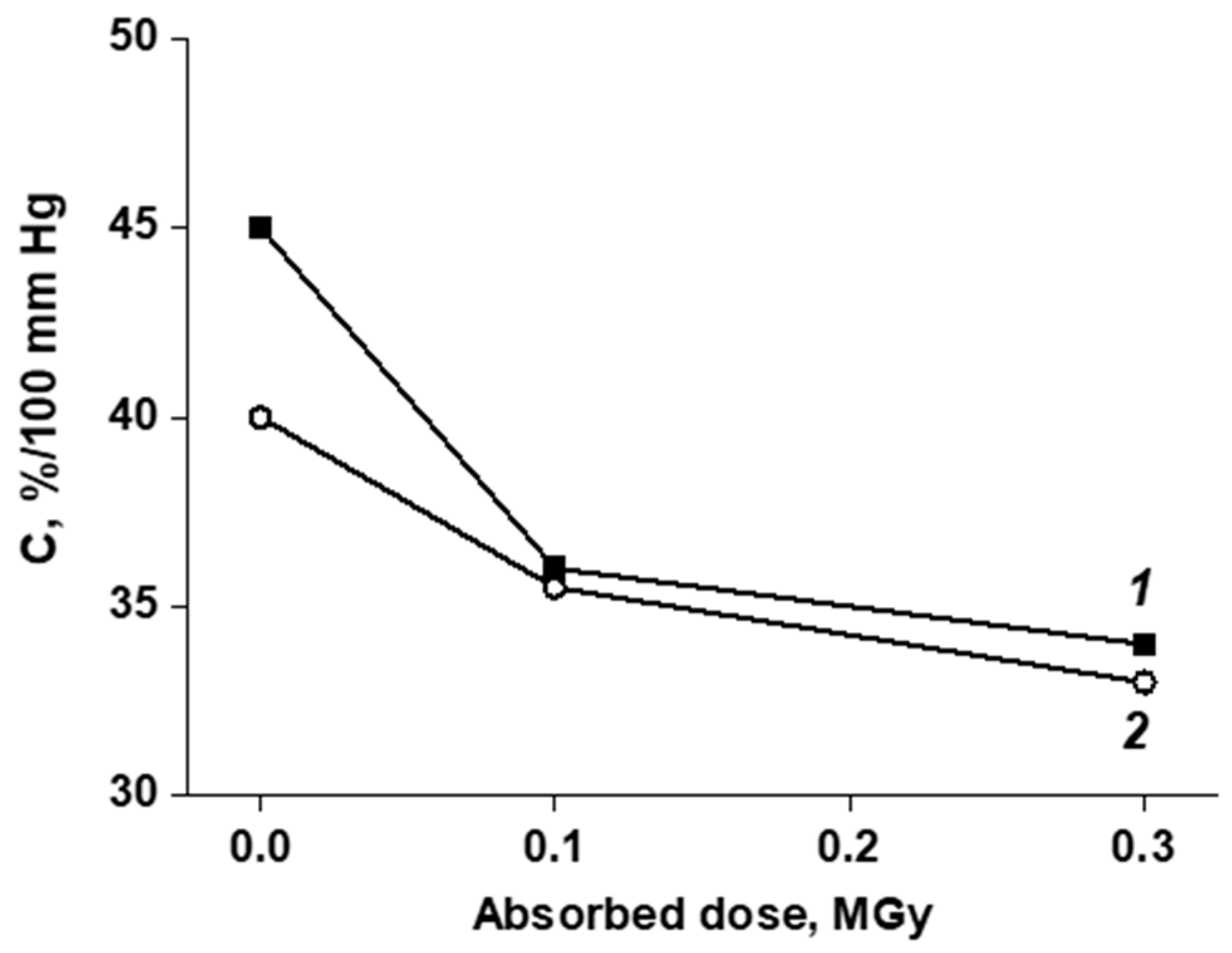
3.3. In Vivo Study of the of Vascular Prostheses Compliance
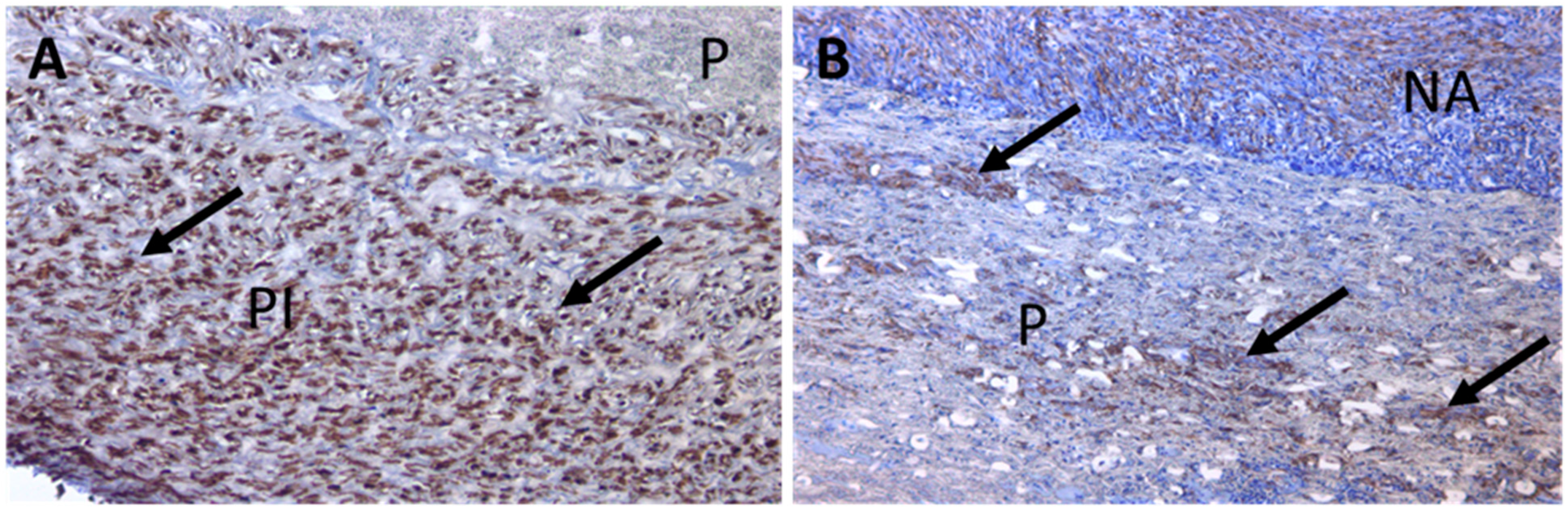
3.4. Large-Scale Prosthesis Observation after Extraction, and Histological Study of the Capsule
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VDF/HFP | vinylidene fluoride with hexafluoropropylene |
| BVP | blood vessel prostheses |
References
- Zdrahala, R.J. Small caliber vascular grafts. Part I: State of the art. J. Biomater. Appl. 1996, 10, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Zdrahala, R.J. Small caliber vascular grafts. Part II: Polyurethanes revisited. J. Biomater. Appl. 1996, 11, 37–61. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38e47. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Ong, C.h.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of Vascular Graft Studies in Large Animal Models. Tissue Eng. Part B 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, E.; Bahnini, A.; Koskas, F.; Ruotolo, C.; Blevec DLe Plissonnier, D. In situ allograft replacement of infected infrarenal aortic prosthetic grafts: Results in forty-three patients. J. Vasc. Surg. 1993, 17, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Baguneid, M.S.; Seifalian, A.M.; Salacinski, H.J.; Murray, D.; Hamilton, G.; Walker, M.G. Tissue engineering of blood vessels. Br. J. Surg. 2006, 93, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Liao Sh He, Q.; Yang, L.; Liu Sh Zhang, Z.; Guidoin, R.; Fu, Q.; Xie, X. Toward endothelialization via vascular endothelial growth factor immobilization on cell-repelling functional polyurethanes. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Chen, P.; Zhang, Z.; Guidoin, R.; Wang, L. Preventing collapsing of vascular scaffolds: The mechanical behavior of PLA/PCL composite structure prostheses during in vitro degradation. J. Mech. Behav. Biomed. Mater. 2017, 75, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Devillard, C.D.; Marquette, C.A. Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel. Front. Bioeng. Biotechnol. 2021, 9, 721843. [Google Scholar] [CrossRef] [PubMed]
- Zhorzholiani, S.T.; Mironov, A.A.; Talygin, E.A.; Tsyganokov, Y.M.; Agafonov, A.M.; Kiknadze, G.I.; Gorodkov, A.Y.; Bokeriya, L.A. Analysis of Dynamic Geometric Configuration of the Aortic Channel from the Perspective of Tornado-Like Flow Organization of Blood Flow. Bull. Exp. Biol. Med. 2018, 164, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Zhorzholiani, S.T.; Krasheninnikov, S.V.; Shepelev, A.D.; Tenchurin, T.K.; Gorodkov, A.Y.; Bokeriya, L.A. Comparative evaluation of the dynamic biomechanical compatibility of synthetic vascular prostheses in vitro, ex vivo and in vivo. Georgian Med. News 2018, 11, 108–114. [Google Scholar]
- Allayarov, S.R.; Confer, M.P.; Demidov, S.V.; Allayarova, U.Y.; Mishenko, D.V.; Klimanova, E.N.; Dixon, D.A. Investigation of γ-irradiated polyvinylidene fluoride and its acute toxicity. J. Fluor. Chem. 2021, 251, 109885. [Google Scholar] [CrossRef]
- Ivanov, V.S. Radiation Chemistry of Polymers; VSP: Leiden, The Netherlands, 1992. [Google Scholar]
- Gorodkov, A.Y.; Zhorzholiani, S.T.; Agafonov, A.V.; Krasheninnikov, S.V.; Mamagulashvili, V.G.; Tenchurin, T.K.; Shepelev, A.D.; Chvalun, S.N. Fabrication of matrices of nonwoven porcine carotid arteries and investigation of their functional properties. Fibre Chem. 2019, 50, 556–561. [Google Scholar] [CrossRef]
- Bokeria, L.A.; Gorodkov, A.Y.; Grigoriev, T.E.; Agafonov, A.V.; Zhorzholiani, S.T.; Mamagulashvili, V.G.; Savinov, D.V.; Tenchurin, T.H.; Chvalun, S.N.; Shepelev, A.D. Non-Woven Prosthesis of Aorta and Large Arterial Vessels and a Method for Preparing it. RU Patent 184 164 U1, 5 June 2018. [Google Scholar]
- Sokolov, A.V. Modeling of mechanical behavior of blood vessels. Bull. Russ. Acad. Sci. Phys. 2010, 6, 15. [Google Scholar]
- Singh, C.; Wong, C.S.; Wang, X. Medical Textiles as Vascular Implants and Their Success to Mimic Natural Arteries. J. Funct. Biomater. 2015, 6, 500–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kancevicha, V.; Lukyanchikovs, A.; Auzans, A. Structure of Elastic Woven Vascular Implants. Scientific Journal of Riga Technical University Material Science. Text. Cloth. Technol. 2011, 6, 9–12. [Google Scholar]
- Agafonov, A.V.; Talygin, E.A.; Bockeria, L.A.; Gorodkov, A.Y. The Hydrodynamics of a Swirling Blood Flow in the Left Heart and Aorta. Acta Nat. 2021, 13, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Hiob, M.A.; Crouch, G.W.; Weiss, A.S. School Elastomers in vascular tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Akentjew, T.L.; Terraza, C.; Suazo, C.; Maksimcuka, J.; Wilkens, C.A.; Vargas, F.; Zavala, G.; Ocaña, M.; Enrione, J.; García-Herrera, C.M.; et al. Rapid fabrication of reinforced and cell-laden vascular grafts structurally inspired by human coronary arteries. Nat. Commun. 2019, 10, 3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, I.; Figliuzzi, M.; Azzollini, N.; Catto, V.; Farè, S.; Tanzi, M.C.; Alessandrino, A.; Freddi, G.; Remuzzi, A. In vivo regeneration of elastic lamina on fibroin biodegradable vascular scaffold. Int. J. Artif. Organs 2013, 36, 166–174. [Google Scholar] [CrossRef]
- Greenwald, S.E.; Berry, C.L. Improving vascular grafts: The importance of mechanical and haemodynamic properties. J. Pathol. 2000, 190, 292–299. [Google Scholar] [CrossRef]
- Qin, K.; Wu, Y.; Pan, Y.; Wang, K.; Kong, D.; Zhao, Q. Implantation of Electrospun Vascular Grafts with Optimized Structure in a Rat Model. J. Vis. Exp. 2018, 136, 57340. [Google Scholar] [CrossRef] [Green Version]
- Gras, C.; Stoiber, M.; Röhrich, M.; Moscato, F.; Bergmeister, H.; Schima, H. Electrospinning of small diameter vascular grafts with preferential fiber directions and comparison of their mechanical behavior with native rat aortas. Mater. Sci. Eng. C 2021, 124, 112085. [Google Scholar] [CrossRef]
- Salacinski, H.J.; Goldner, S.; Giudiceandrea, A.; Hamilton, G.; Seifalian, A.M.; Edwards, A.; Carson, R.J. The mechanical behavior of vascular grafts: A review. J. Biomater. 2001, 15, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.J.; Santos, M.; Fernandez-Colino, A.; Pinedo, G.; Girotti, A. Recent contributions of elastin-like recombinamers to biomedicine and nanotechnology. Curr. Top. Med. Chem. 2014, 14, 819–836. [Google Scholar] [CrossRef] [Green Version]
- MacEwan, S.R.; Chilkoti, A. Elastin-like polypeptides: Biomedical applications of tunable biopolymers. Biopolymers 2010, 94, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.; Fernández-Colino, A.; López, I.M.; Rodríguez-Cabello, J.C.; Arias, F.J. Elastin-like recombinamers: Biosynthetic strategies and biotechnological applications. Biotechnol. J. 2011, 6, 1174–1186. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Wolf, F.; Rütten, S.; Schmitz-Rode, T.; Rodríguez-Cabello, J.C.; Jockenhoevel, S.; Mela, P. Small caliber compliant vascular grafts based on elastin-like recombinamers for in situ tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 340. [Google Scholar] [CrossRef] [Green Version]
- Maleckis, K.; Kamenskiy, A.; Lichter, E.Z.; Oberley-Deegan, R.; Dzenis, Y.; MacTaggart, J. Mechanically tuned vascular graft demonstrates rapid endothelialization and integration into the porcine iliac artery wall. Acta Biomater. 2021, 125, 126–137. [Google Scholar] [CrossRef]
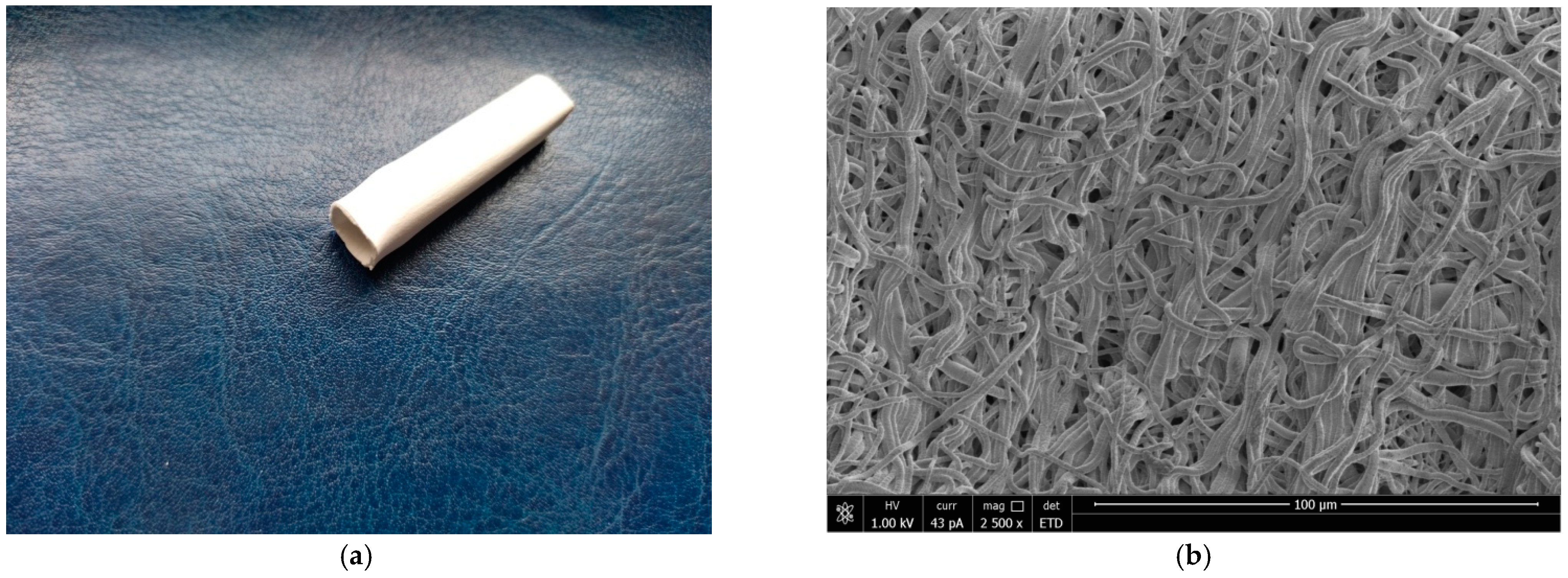

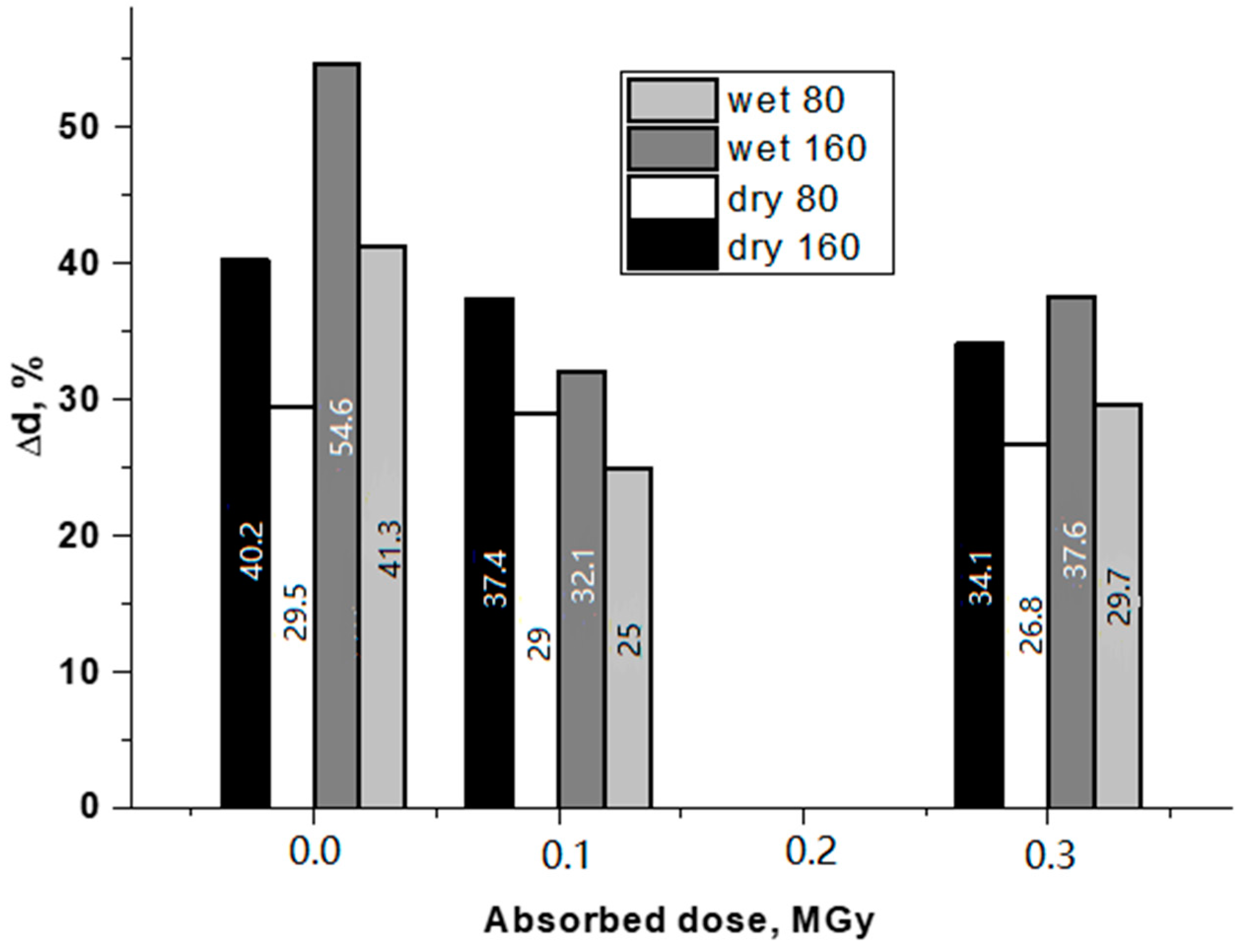




| Sample of BVP | Strength, MPa | Breaking Strain, % | Modulus of Elasticity (10–25%), MPa | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| Control | 1.59 ± 0.15 | 1.32 ± 0.16 | 722 ± 5 | 749 ± 15 | 0.55 ± 0.02 | 0.45 ± 0.03 |
| 0.1 MGy | 1.56 ± 0.14 | 1.54 ± 0.15 | 653 ± 6 | 592 ± 7 | 0.59 ± 0.02 | 0.60 ± 0.02 |
| 0.2 MGy | 1.48 ± 0.14 | - | 551 ± 12 | - | 0.57 ± 0.02 | - |
| 0.3 MGy | 1.01 ± 0.11 | 0.96 ± 0.11 | 340 ± 5 | 335 ± 7 | 0.54 ± 0.02 | 0.53 ± 0.02 |
| 0.4 MGy | 0.78 ± 0.09 | - | 187 ± 7 | - | 1.42 ± 0.02 | - |
| Sample | Compliance, %/100 mmHg | |
|---|---|---|
| 3 Days | 30 Days | |
| Native aorta | 29 ± 1 | 24 ± 1 |
| BVP | 28 ± 1 | 22 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorodkov, A.Y.; Tsygankov, Y.M.; Shepelev, A.D.; Krasheninnikov, S.V.; Zhorzholiani, S.T.; Agafonov, A.V.; Mamagulashvili, V.G.; Savinov, D.V.; Tenchurin, T.K.; Chvalun, S.N. Influence of γ-Radiation on Mechanical Stability to Cyclic Loads Tubular Elastic Matrix of the Aorta. J. Funct. Biomater. 2022, 13, 192. https://doi.org/10.3390/jfb13040192
Gorodkov AY, Tsygankov YM, Shepelev AD, Krasheninnikov SV, Zhorzholiani ST, Agafonov AV, Mamagulashvili VG, Savinov DV, Tenchurin TK, Chvalun SN. Influence of γ-Radiation on Mechanical Stability to Cyclic Loads Tubular Elastic Matrix of the Aorta. Journal of Functional Biomaterials. 2022; 13(4):192. https://doi.org/10.3390/jfb13040192
Chicago/Turabian StyleGorodkov, Alexander Yu., Yuriy M. Tsygankov, Alexey D. Shepelev, Sergey V. Krasheninnikov, Shota T. Zhorzholiani, Andrey V. Agafonov, Vissarion G. Mamagulashvili, Dmitriy V. Savinov, Timur Kh. Tenchurin, and Sergey N. Chvalun. 2022. "Influence of γ-Radiation on Mechanical Stability to Cyclic Loads Tubular Elastic Matrix of the Aorta" Journal of Functional Biomaterials 13, no. 4: 192. https://doi.org/10.3390/jfb13040192





