Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Samples Characterization
- (a)
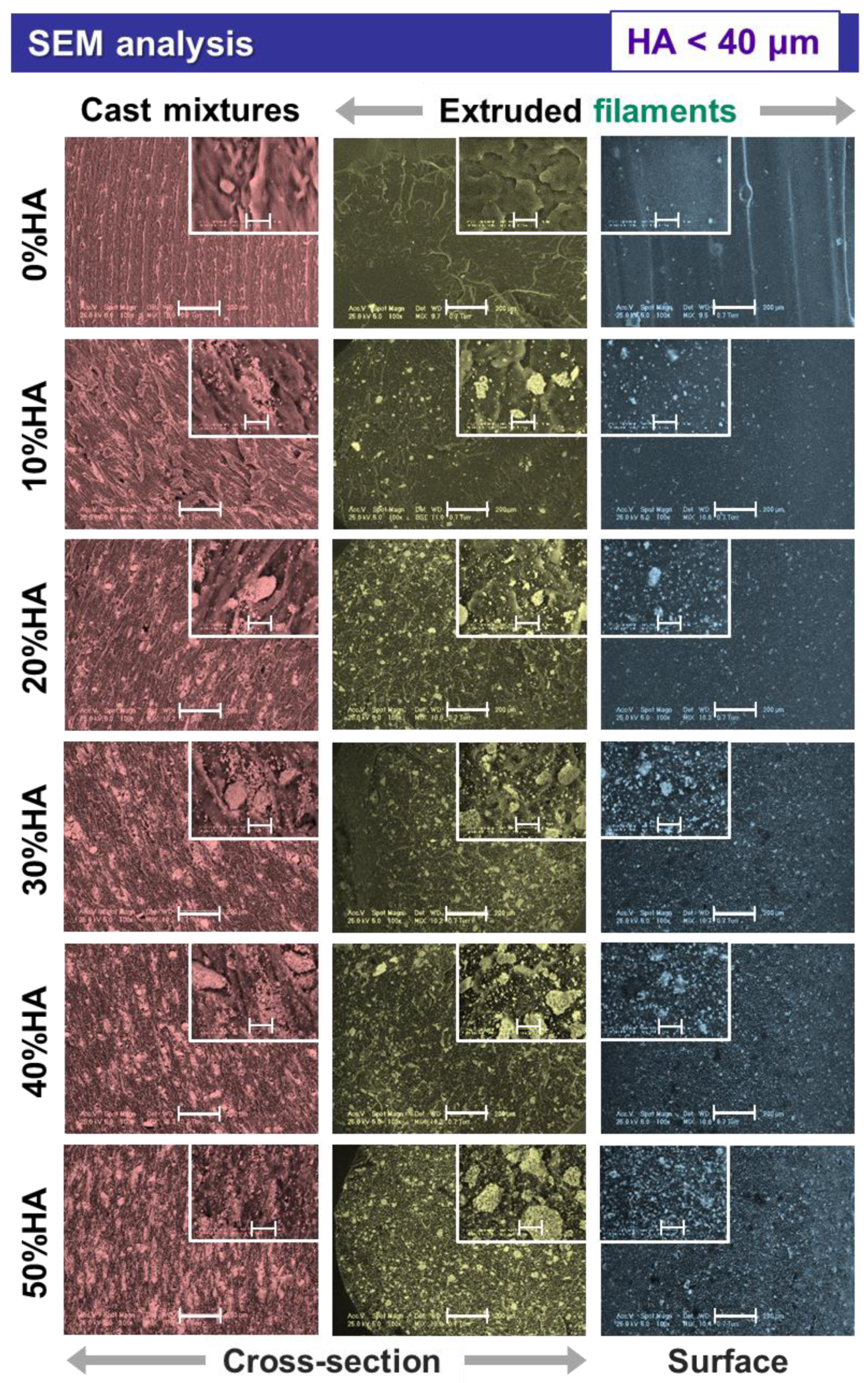
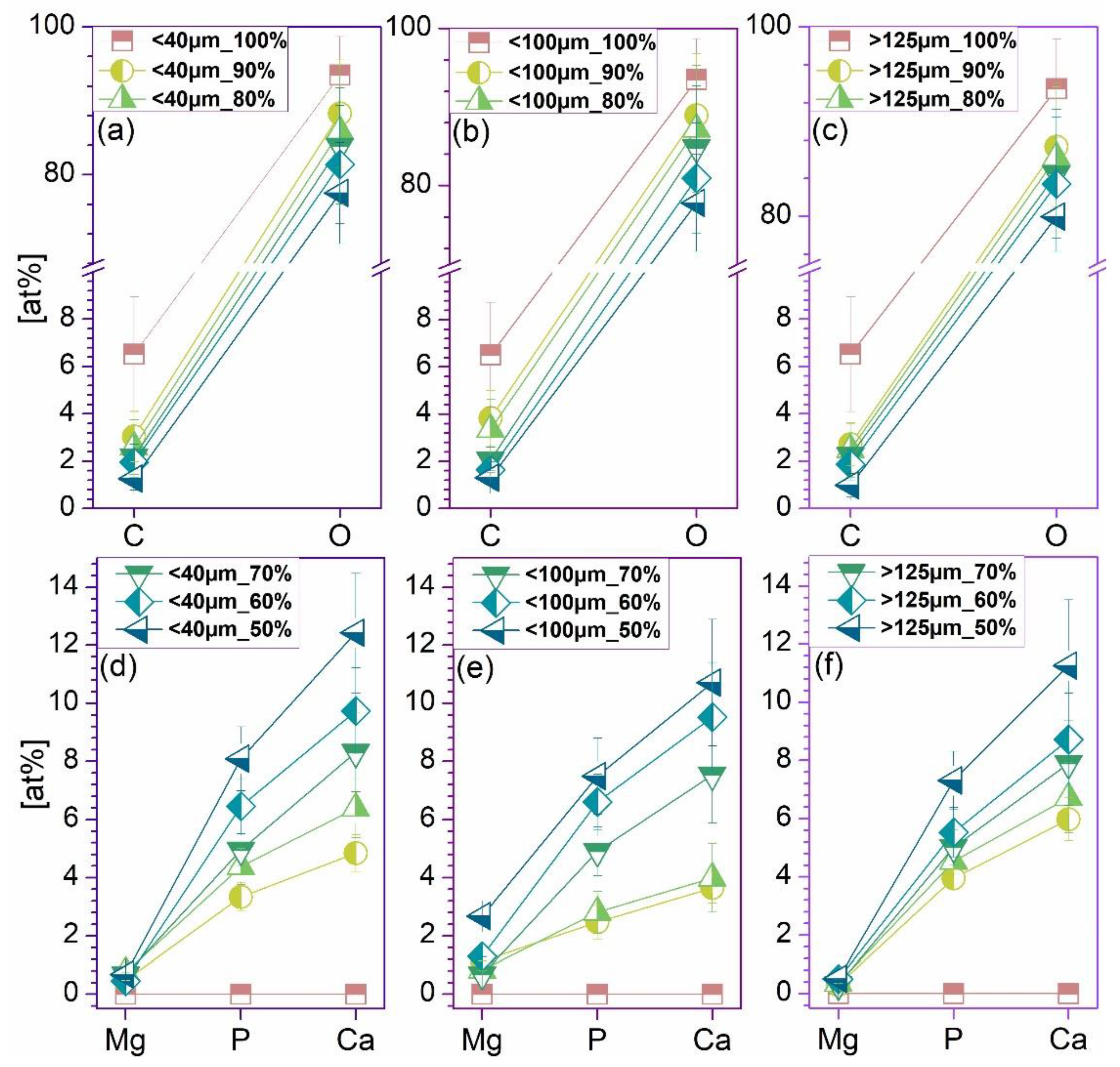
- The macro- and microstructure of the samples, in both surface and cross-sectional view, were evaluated by scanning electron microscopy (SEM), using a Phillips XL 30 ESEM TMP microscope (FEI/Phillips, Hillsboro, OR, USA) coupled with an auxiliary microanalysis EDS system (EDAX Sapphire UTW, 128 eV resolution). The acquisition of micrographs after casting and extrusion was conducted on 5 randomly selected areas. The HA particles size was inferred by processing the SEM micrographs with the help of ImageJ software (National Institutes of Health, USA).
- (b)
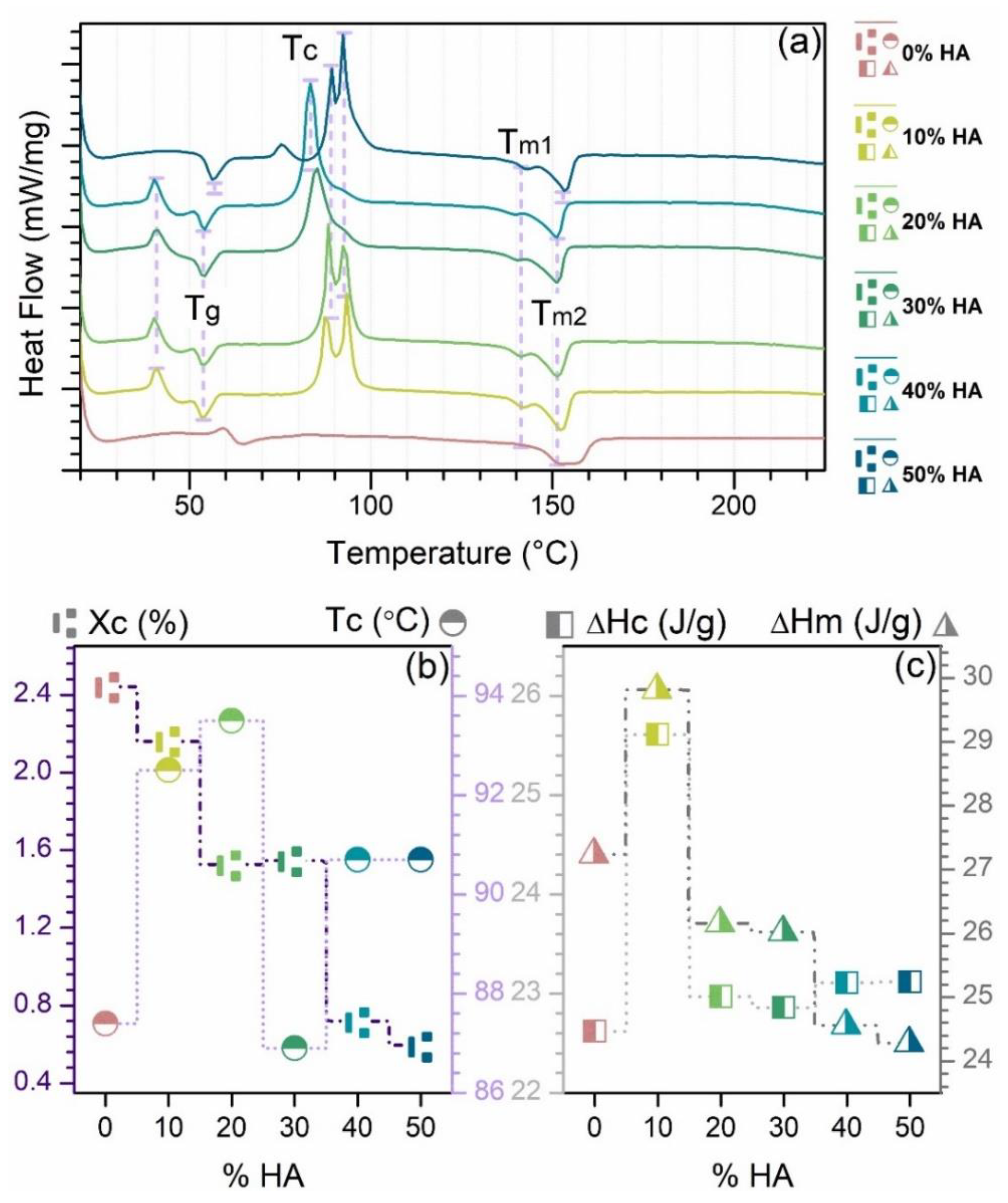
- The differential scanning calorimetry (DSC) curves were acquired using Netzsch DSC 204 F1 Phoenix equipment (Netzsch, Selb, Germany). The samples were heated from room temperature (RT) up to 300 °C, at a heating rate of 5 °C/min in air ambient.
- (c)
- The wettability was evaluated by water contact angle measurements using a Krüss Drop Shape Analyzer—DSA100 (A. Krüss Optronic GmbH, Hamburg, Germany). The experiments were performed with three wetting agents (water, diiodomethane (DIM), and ethylene glycol (EG)) at 20 ± 1 °C and room humidity of 45 ± 5%. The images were captured 1 s after the wetting agent droplet deposition. The results (average of 5 determinations/sample) were then processed with the ImageJ 1.50 software (National Institutes of Health, USA). The surface free energy was calculated by the Owens, Wendt, Rabel, and Kaelble (OWRK) method [40].
- (d)
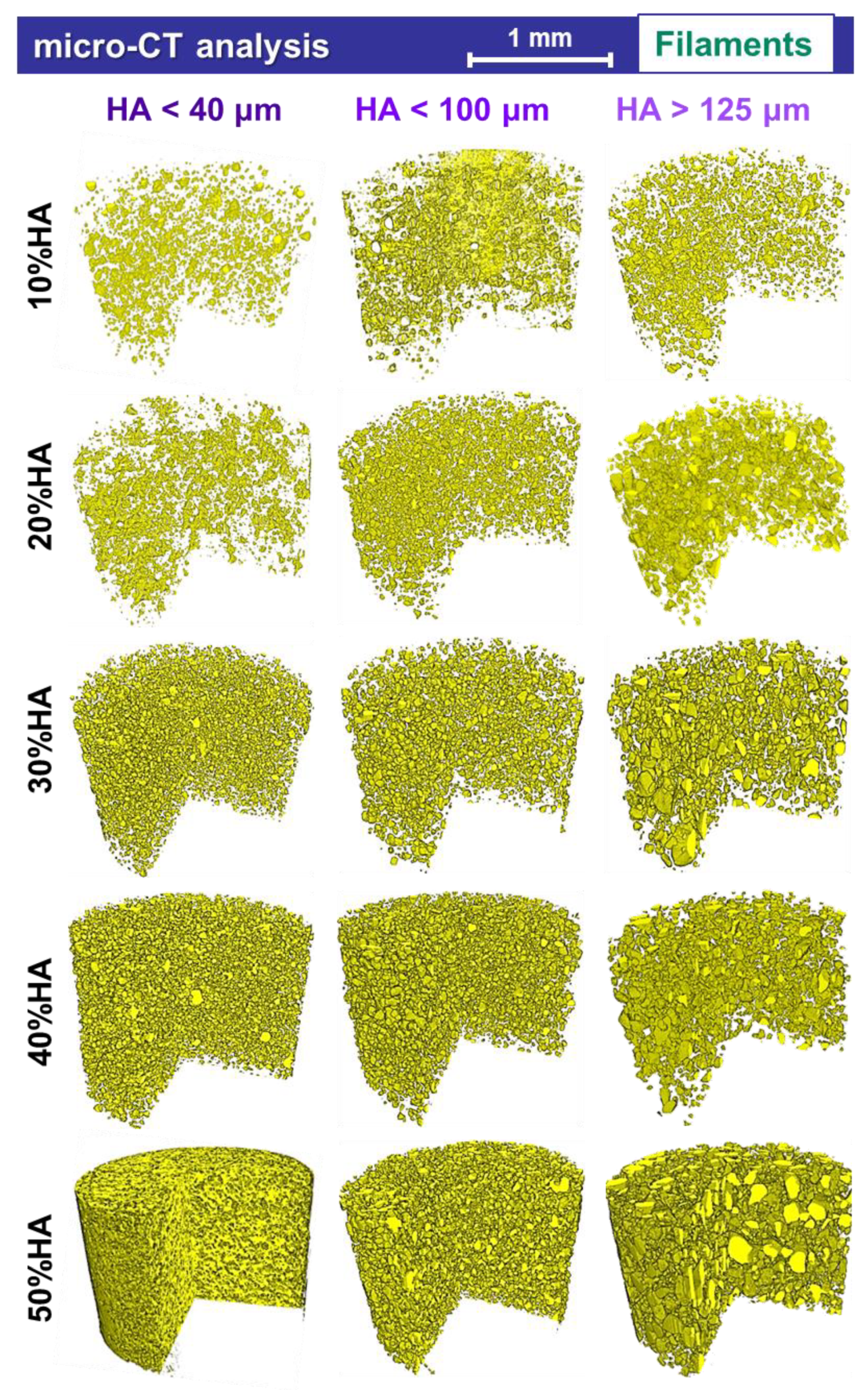
- The micro-CT analysis of the extruded filaments was conducted on a Brucker micro-CT SkyScaner 1272 model. The products were cut into specimens with ~4 mm length from the middle of each sample/filament wire. The scanning was performed without any filter, at a voltage of 50 kV, a current of 175 µA, a rotation set of 0.2°, and 4 average frames per capture. Each sample was rotated to 360°. Images were processed using a NRecon 1.7.1.6 reconstruction software from Bruker micro-CT.
- (e)
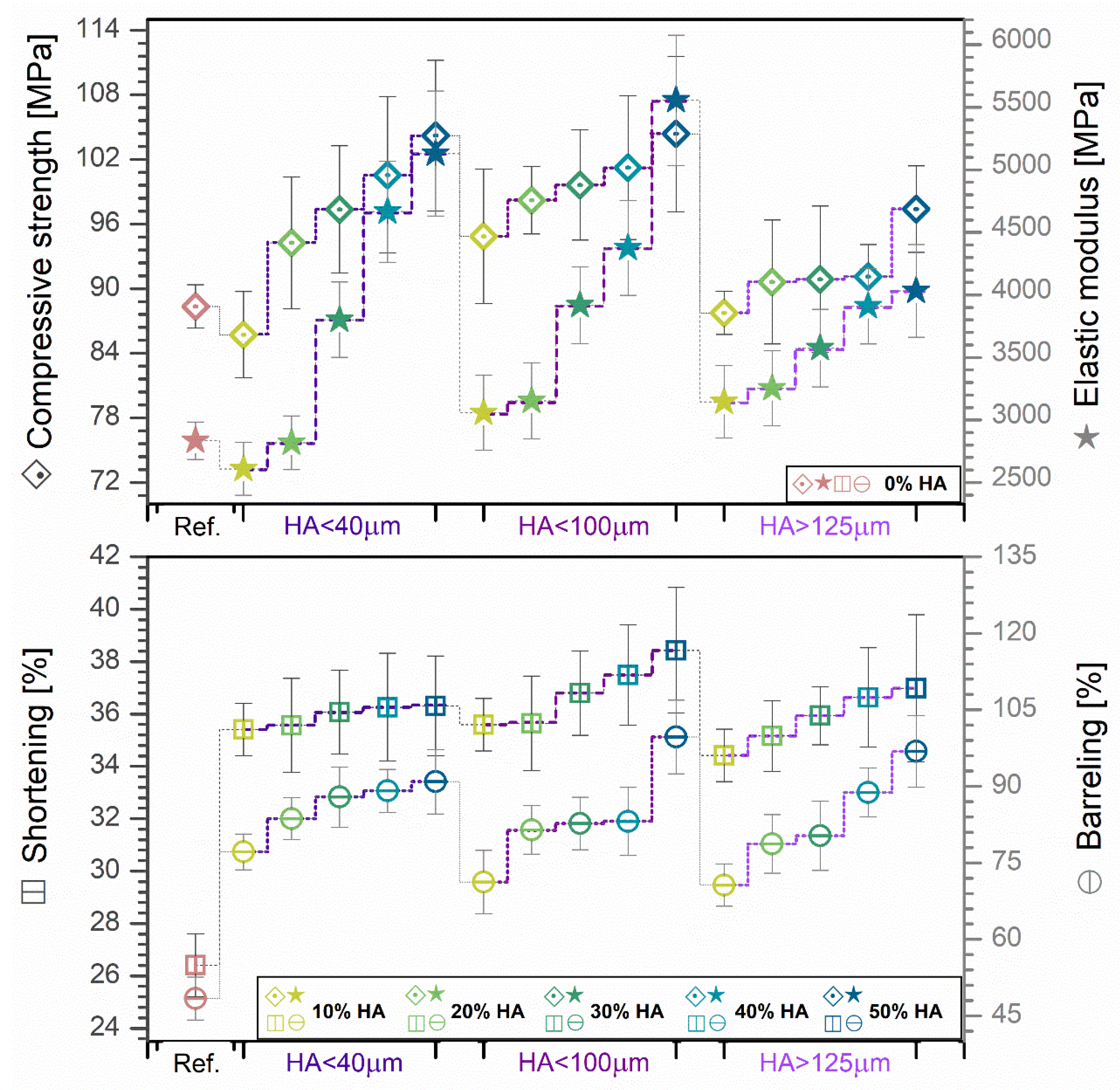
- The compression test was performed on the cast mixtures pellets using a universal test machine Walter + Bai AG, Loehningen (Schaffhausen, Switzerland), type LFV300. The used test speed was 1 mm/min with an acquisition rate of 0.01 s. The shortening and barreling deformation degrees were calculated by measuring the cast mixtures pellets (diameter and height) before and after performing the compression tests. The results represent the average of three sets of measurements/sample type.
3. Results and Discussion
3.1. Morpho-Compositional Evaluation
3.2. Differential Scanning Calorimetry (DSC)
3.3. Contact Angle and Surface Energy Investigations
3.4. Micro-CT Reconstruction
3.5. Mechanical Features Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manoj, A.; Bhuyan, M.; Banik, S.R.; Sankar, M.R. Review on particle emissions during fused deposition modeling of acrylonitrile butadiene styrene and polylactic acid polymers. Mater. Today Proc. 2021, 44, 1375–1383. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Jiang, J.; Wu, Y.; Zhu, P.; Chen, G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivity in vivo for bone regeneration. Biomed. Mater. 2019, 14, 065003. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocanu, A.-C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.-C.; Voicu, I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vladescu, A.; Badea, M.; Padmanabhan, S.C.; Paraschiv, G.; Floroian, L.; Gaman, L.; Morris, M.A.; Marty, J.-L.; Cotrut, C.M. Nanomaterials for Medical Applications and Their Antimicrobial Advantages. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–431. [Google Scholar] [CrossRef]
- Miculescu, F.; Miculescu, M.; Ciocan, L.; Ernuteanu, A.; Antoniac, I.; Pencea, I.; Matei, E. Comparative Studies Regarding Heavy Elements Concentration In Human Cortical Bone. Dig. J. Nanomater. Biostruct. 2011, 6, 1117–1127. [Google Scholar]
- Nevado, P.; Lopera, A.; Bezzon, V.; Fulla, M.; Palacio, J.; Zaghete, M.; Biasotto, G.; Montoya, A.; Rivera, J.; Robledo, S.; et al. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater. Sci. Eng. C 2020, 114, 111013. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Zahedi, M.; Khan, A.; Usman, H. 3D printing and morphological characterisation of polymeric composite scaffolds. Eng. Struct. 2020, 216, 110752. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, M.-D. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Pandele, A.M.; Andronescu, C.; Ghebaur, A.; Garea, S.A.; Iovu, H. New Biocompatible Mesoporous Silica/Polysaccharide Hybrid Materials as Possible Drug Delivery Systems. Materials 2019, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Dascalu, C.-A.; Miculescu, F.; Mocanu, A.-C.; Constantinescu, A.E.; Butte, T.M.; Pandele, A.M.; Ciocoiu, R.-C.; Voicu, S.I.; Ciocan, L.T. Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications. Coatings 2020, 10, 283. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Miculescu, M.; Batalu, N.D.; Ciocoiu, R.C.; Voicu, I.; Stan, G.E.; Thakur, V.K. Synthesis and Characterization of Jellified Composites from Bovine Bone-Derived Hydroxyapatite and Starch as Precursors for Robocasting. ACS Omega 2018, 3, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Çetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Stan, G.; Popa, A.; Bojin, D. Bioreactivity evaluation in simulated body fluid of magnetron sputtered glass and glass-ceramic coatings: A ftir spectroscopy study. Dig. J. Nanomater. Biostruct. 2010, 5, 557–566. [Google Scholar]
- Popa, A.-C.; Stan, G.; Husanu, M.-A.; Mercioniu, I.; Santos, L.; Fernandes, H.; Ferreira, J. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T.; et al. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.; Ciocan, L.; Niculescu, M.; Corobea, M.; Rada, M.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, M.; Machedon-Pisu, T.; Maidaniuc, A.; Ciocoiu, R.C.; Ioniță, M.; Pasuk, I.; Stan, G.E.; Miculescu, F. Internal and external surface features of newly developed porous ceramics with random interconnected 3D channels by a fibrous sacrificial porogen method. Appl. Surf. Sci. 2019, 489, 226–238. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Miculescu, F.; Miculescu, M.; Ciocoiu, R.C.; Pandele, A.M.; Stan, G.E.; Cîmpean, A.; Voicu, I.; Ciocan, L.-T. Comprehensive analysis of compatible natural fibre as sacrificial porogen template for tailored ceramic 3D bioproducts destined for hard tissue reconstruction. Ceram. Int. 2021, 47, 5318–5334. [Google Scholar] [CrossRef]
- Backes, E.H.; de Nóbile Pires, L.; Selistre-De-Araujo, H.S.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Development and characterization of printable PLA/β-TCP bioactive composites for bone tissue applications. J. Appl. Polym. Sci. 2021, 138, 49759. [Google Scholar] [CrossRef]
- Babilotte, J.; Guduric, V.; Le Nihouannen, D.; Naveau, A.; Fricain, J.-C.; Catros, S. 3D printed polymer–mineral composite biomaterials for bone tissue engineering: Fabrication and characterization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2579–2595. [Google Scholar] [CrossRef]
- Sotorrío, G.; Alonso, J.; Olsson, N.; Tenorio, J. Printability of materials for extrusion 3D printing technologies: A review of material requirements and testin. Mater. De Constr. 2021, 71, e267. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Orozco-Díaz, C.A.; Moorehead, R.D.; Reilly, G.C.; Gilchrist, F.; Miller, C.A. Characterization of a composite polylactic acid-hydroxyapatite 3D-printing filament for bone-regeneration. Biomed. Phys. Eng. Express 2020, 6, 025007. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.; Ghazali, S.; Heim, H.P.; Feldmann, M. Impact modified PLA-hydroxyapatite composites—Thermo-mechanical properties. Compos. Part A Appl. Sci. Manuf. 2018, 107, 326–333. [Google Scholar] [CrossRef]
- Yeon, Y.K.; Park, H.S.; Lee, J.M.; Lee, J.S.; Lee, Y.J.; Sultan, T.; Bin Seo, Y.; Lee, O.J.; Kim, S.H.; Park, C.H. New concept of 3D printed bone clip (polylactic acid/hydroxyapatite/silk composite) for internal fixation of bone fractures. J. Biomater. Sci. Polym. Ed. 2018, 29, 894–906. [Google Scholar] [CrossRef]
- Nadarajan, V.; Phang, S.W.; Choo, H.L. Fabrication of 3D-printed bone scaffold of natural hydroxyapatite from fish bones in polylactic acid composite. AIP Conf. Proc. 2020, 2233, 040004. [Google Scholar] [CrossRef]
- Sahmani, S.; Khandan, A.; Esmaeili, S.; Saber-Samandari, S.; Nejad, M.G.; Aghdam, M. Calcium phosphate-PLA scaffolds fabricated by fused deposition modeling technique for bone tissue applications: Fabrication, characterization and simulation. Ceram. Int. 2020, 46, 2447–2456. [Google Scholar] [CrossRef]
- Ferri, J.; Jordá, J.; Montanes, N.; Fenollar, O.; Balart, R. Manufacturing and characterization of poly(lactic acid) composites with hydroxyapatite. J. Thermoplast. Compos. Mater. 2018, 31, 865–881. [Google Scholar] [CrossRef] [Green Version]
- Custodio, C.L.; Broñola, P.J.M.; Cayabyab, S.R.; Lagura, V.U.; Celorico, J.R.; Basilia, B.A. Powder Loading Effects on the Physicochemical and Mechanical Properties of 3D Printed Poly Lactic Acid/Hydroxyapatite Biocomposites. Int. J. Bioprinting 2021, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Bojin, D.; Ciocan, L.; Antoniac, I.; Miculescu, M.; Miculescu, N. Experimental researches on biomaterial-tissue interface interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- ASTM E11-20; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM International: West Conshohockin, PA, USA, 2017.
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-Structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farid, T.; Herrera, V.N.; Kristiina, O. Investigation of crystalline structure of plasticized poly (lactic acid)/Banana nanofibers composites. IOP Conf. Series Mater. Sci. Eng. 2018, 369, 012031. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Yu, C.-X.; Li, Y.; Zhu, Q.-Q.; Zhou, L.; Cao, C.; Yu, T.-T.; Du, F.-P. Research on the changes in wettability of rice (Oryza sativa.) leaf surfaces at different development stages using the OWRK method. Pest Manag. Sci. 2014, 70, 462–469. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Li, Y.; Yang, M.; Yuan, Q. Experimental Investigations for Optimizing the Extrusion Parameters on FDM PLA Printed Parts. J. Mater. Eng. Perform. 2019, 28, 169–182. [Google Scholar] [CrossRef]
- Miculescu, F.; Luță, C.; Constantinescu, A.E.; Maidaniuc, A.; Mocanu, A.-C.; Miculescu, M.; Voicu, Ș.I.; Ciocan, L.T. Considerations and Influencing Parameters in EDS Microanalysis of Biogenic Hydroxyapatite. J. Funct. Biomater. 2020, 11, 82. [Google Scholar] [CrossRef]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 225–233. [Google Scholar]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.; Miculescu, M.; Burhala, B. A study on the influence of the primary electron beam on nanodimensional layers analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 335–345. [Google Scholar]
- Persson, M.; Lorite, G.S.; Cho, S.-W.; Tuukkanen, J.; Skrifvars, M. Melt Spinning of Poly(lactic acid) and Hydroxyapatite Composite Fibers: Influence of the Filler Content on the Fiber Properties. ACS Appl. Mater. Interfaces 2013, 5, 6864–6872. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, S.I.; Finlayson, C.E.; Best, S.M.; Cameron, R.E. The influence of hydroxyapatite (HA) microparticles (m) and nanoparticles (n) on the thermal and dynamic mechanical properties of poly-l-lactide. Polymer 2011, 52, 2883–2890. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Chow, L.C.; Yang, M.; Mitchell, J.W. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly(lactic acid). Int. J. Polym. Sci. 2014, 2014, 827028. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Schmid, M.; Hauser, C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods 2017, 6, 31. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Hołysz, L.; Chodkowski, M.; Guinarte, D.C. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids Interfaces 2021, 5, 4. [Google Scholar] [CrossRef]
- Fernández, V.; Khayet, M. Evaluation of the surface free energy of plant surfaces: Toward standardizing the procedure. Front. Plant Sci. 2015, 6, 510. [Google Scholar] [CrossRef] [Green Version]
- Vafaei, S.; Tuck, C.; Ashcroft, I.; Wildman, R. Surface microstructuring to modify wettability for 3D printing of nano-filled inks. Chem. Eng. Res. Des. 2016, 109, 414–420. [Google Scholar] [CrossRef]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on LDPE with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.E.; Tite, T.; Popa, A.-C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D.; et al. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]
- Mitra, J.; Tripathi, G.; Sharma, A.; Basu, B. Scaffolds for bone tissue engineering: Role of surface patterning on osteoblast response. RSC Adv. 2013, 3, 11073–11094. [Google Scholar] [CrossRef]
- Patterson, B.M.; Cordes, N.L.; Henderson, K.; Mertens, J.C.E.; Clarke, A.J.; Hornberger, B.; Merkle, A.; Etchin, S.; Tkachuk, A.; Leibowitz, M.; et al. In Situ Laboratory-Based Transmission X-Ray Microscopy and Tomography of Material Deformation at the Nanoscale. Exp. Mech. 2016, 56, 1585–1597. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the Use of X-ray Computed Tomography in Assessment of 3D-Printed Components. J. Nondestruct. Eval. 2020, 39, 1–17. [Google Scholar] [CrossRef]
- Fernández, M.P.; Witte, F.; Tozzi, G. Applications of X-ray computed tomography for the evaluation of biomaterial-mediated bone regeneration in critical-sized defects. J. Microsc. 2020, 277, 179–196. [Google Scholar] [CrossRef]
- Bossa, N.; Chaurand, P.; Vicente, J.; Borschneck, D.; Levard, C.; Aguerre-Chariol, O.; Rose, J. Micro- and nano-X-ray computed-tomography: A step forward in the characterization of the pore network of a leached cement paste. Cem. Concr. Res. 2015, 67, 138–147. [Google Scholar] [CrossRef]
- Popovtzer, R.; Agrawal, A.; Kotov, N.; Popovtzer, A.; Balter, J.; Carey, T.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [Green Version]
- Marzuki, A.P.; Mohd Salleh, F.; Rosli, M.N.S.; Tharazi, I.; Abdullah, A.H.; Abdul Halim, N.H. Rheological, mechanical and physical properties of poly-lactic acid (PLA)/hydroxyapatites (HA) composites prepared by an injection moulding process. J. Mech. Eng. 2022, 19, 17–40. [Google Scholar] [CrossRef]
- Galea, L.; Alexeev, D.; Bohner, M.; Doebelin, N.; Studart, A.R.; Aneziris, C.G.; Graule, T. Textured and hierarchically structured calcium phosphate ceramic blocks through hydrothermal treatment. Biomaterials 2015, 67, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mocanu, A.-C.; Miculescu, F.; Dascălu, C.-A.; Voicu, Ș.I.; Pandele, M.-A.; Ciocoiu, R.-C.; Batalu, D.; Dondea, S.; Mitran, V.; Ciocan, L.-T. Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications. J. Funct. Biomater. 2022, 13, 199. https://doi.org/10.3390/jfb13040199
Mocanu A-C, Miculescu F, Dascălu C-A, Voicu ȘI, Pandele M-A, Ciocoiu R-C, Batalu D, Dondea S, Mitran V, Ciocan L-T. Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications. Journal of Functional Biomaterials. 2022; 13(4):199. https://doi.org/10.3390/jfb13040199
Chicago/Turabian StyleMocanu, Aura-Cătălina, Florin Miculescu, Cătălina-Andreea Dascălu, Ștefan Ioan Voicu, Mădălina-Andreea Pandele, Robert-Cătălin Ciocoiu, Dan Batalu, Sorina Dondea, Valentina Mitran, and Lucian-Toma Ciocan. 2022. "Influence of Ceramic Particles Size and Ratio on Surface—Volume Features of the Naturally Derived HA-Reinforced Filaments for Biomedical Applications" Journal of Functional Biomaterials 13, no. 4: 199. https://doi.org/10.3390/jfb13040199






