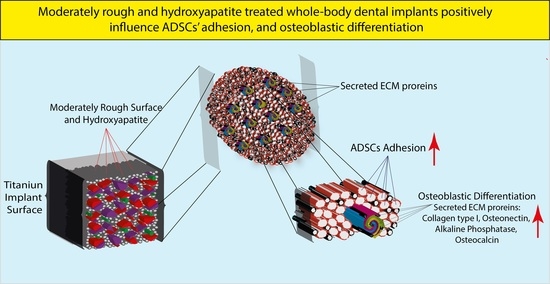
Assessing the Efficacy of Whole-Body Titanium Dental Implant Surface Modifications in Inducing Adhesion, Proliferation, and Osteogenesis in Human Adipose Tissue Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Products and Reagents
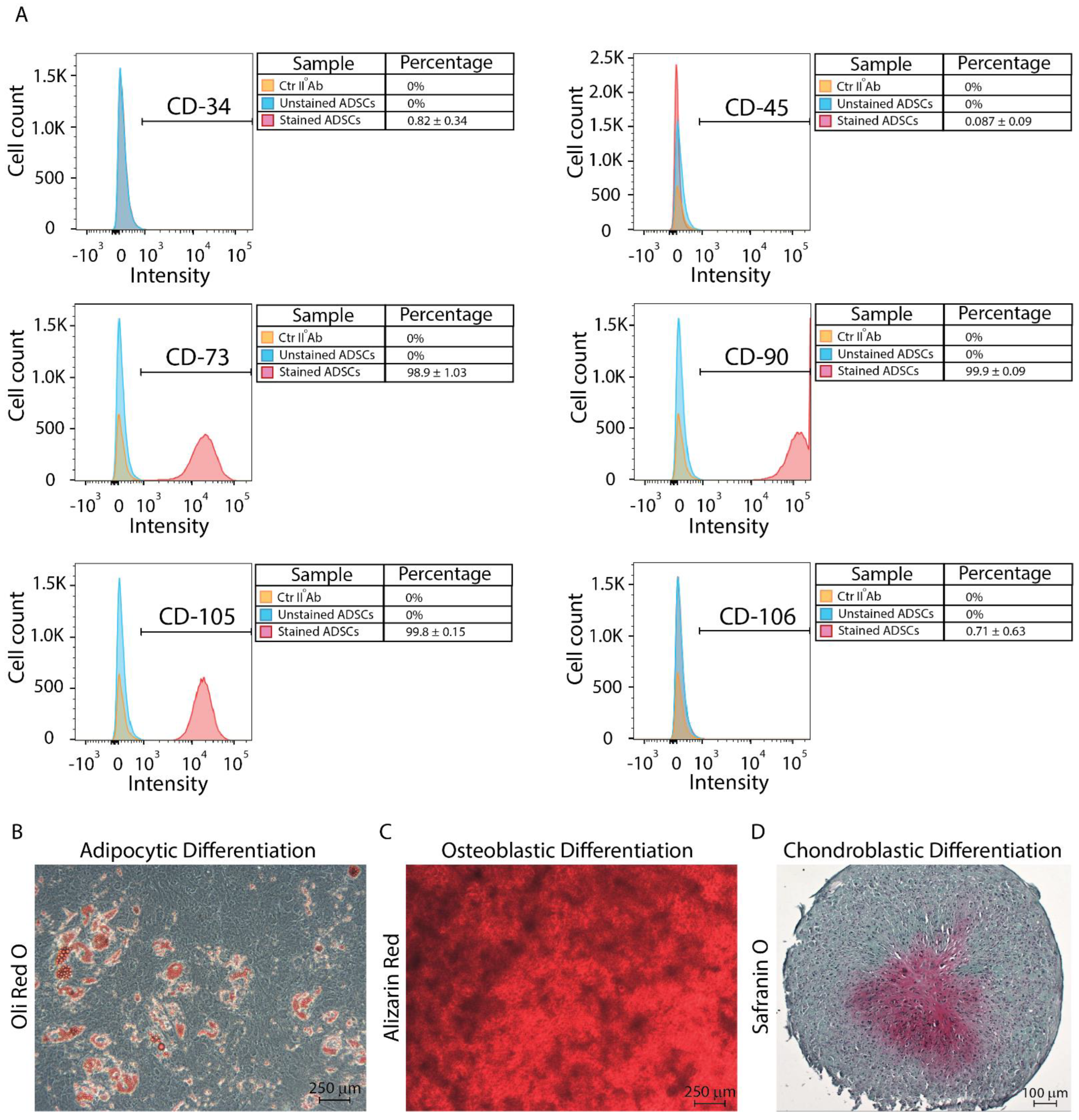
2.2. ADSC Characterization
2.3. Multi-Differentiation Assays
2.4. Characteristics of the Dental Implants
2.5. Dental Implant and Surface Characterization
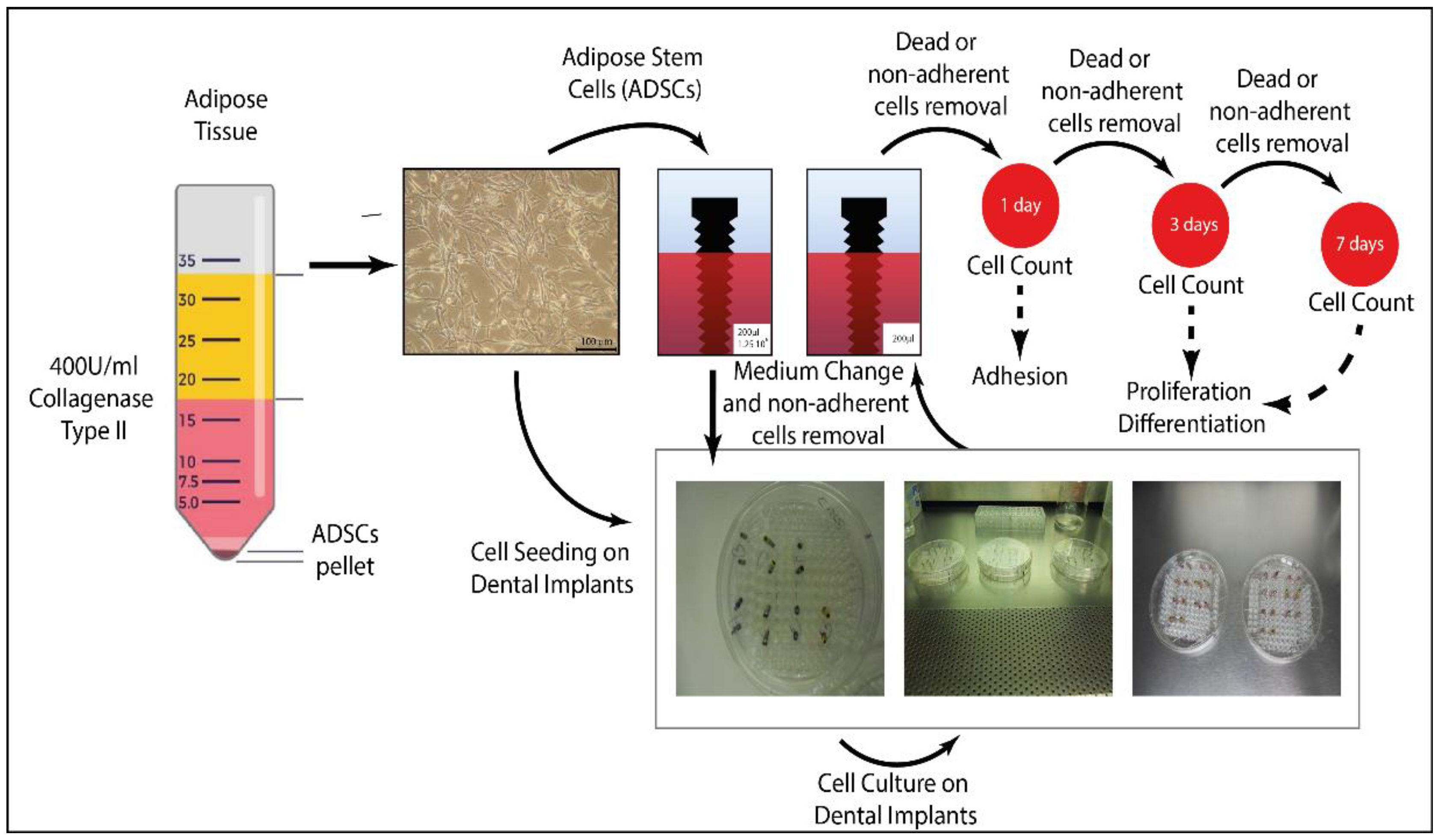
2.6. Dental Implant Seeding with ADSCs
2.7. Cell Adhesion and Proliferation
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. ADSC Isolation, Characterization, and Stemness Potential Assessment
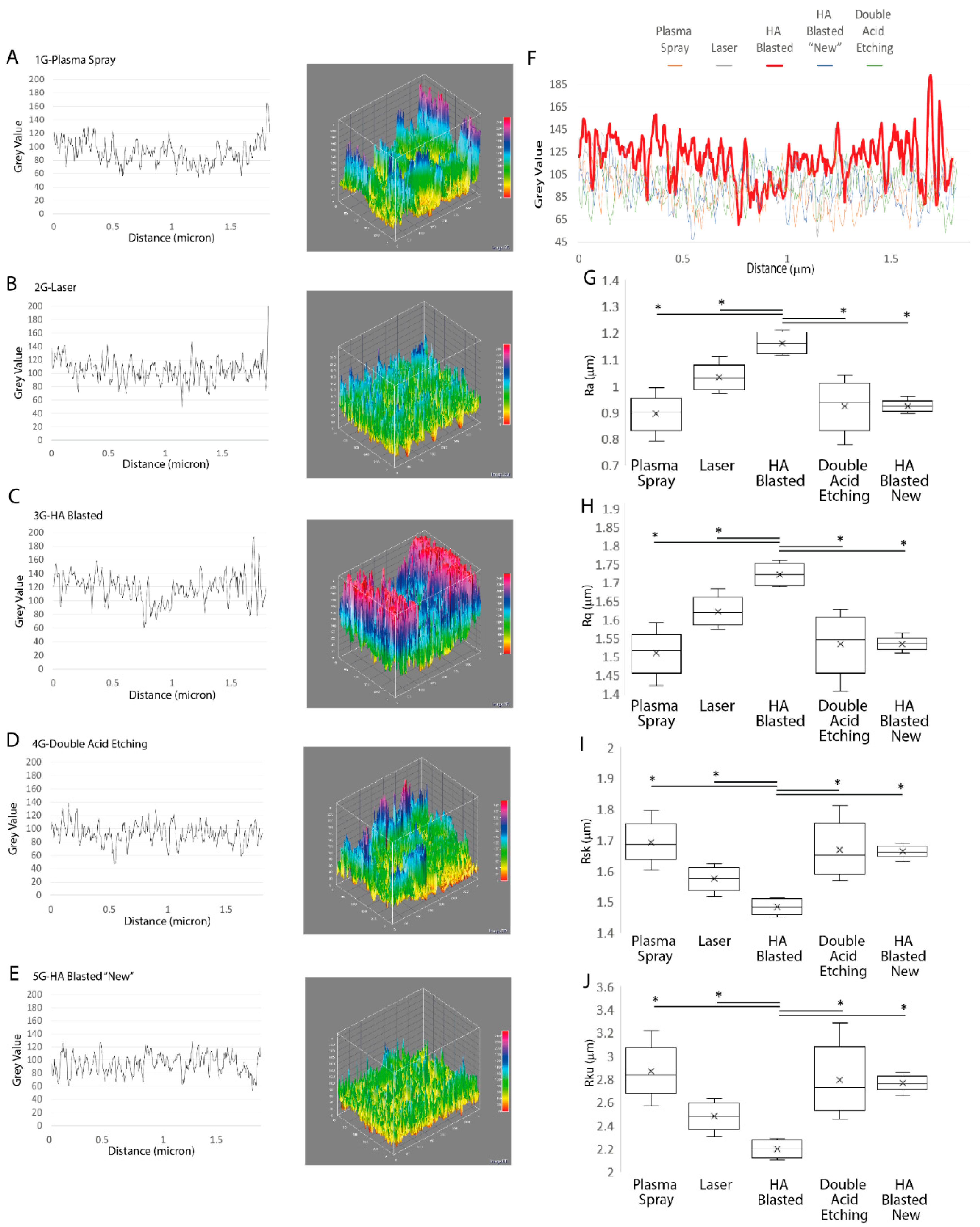
3.2. Implant Morphological Characterization
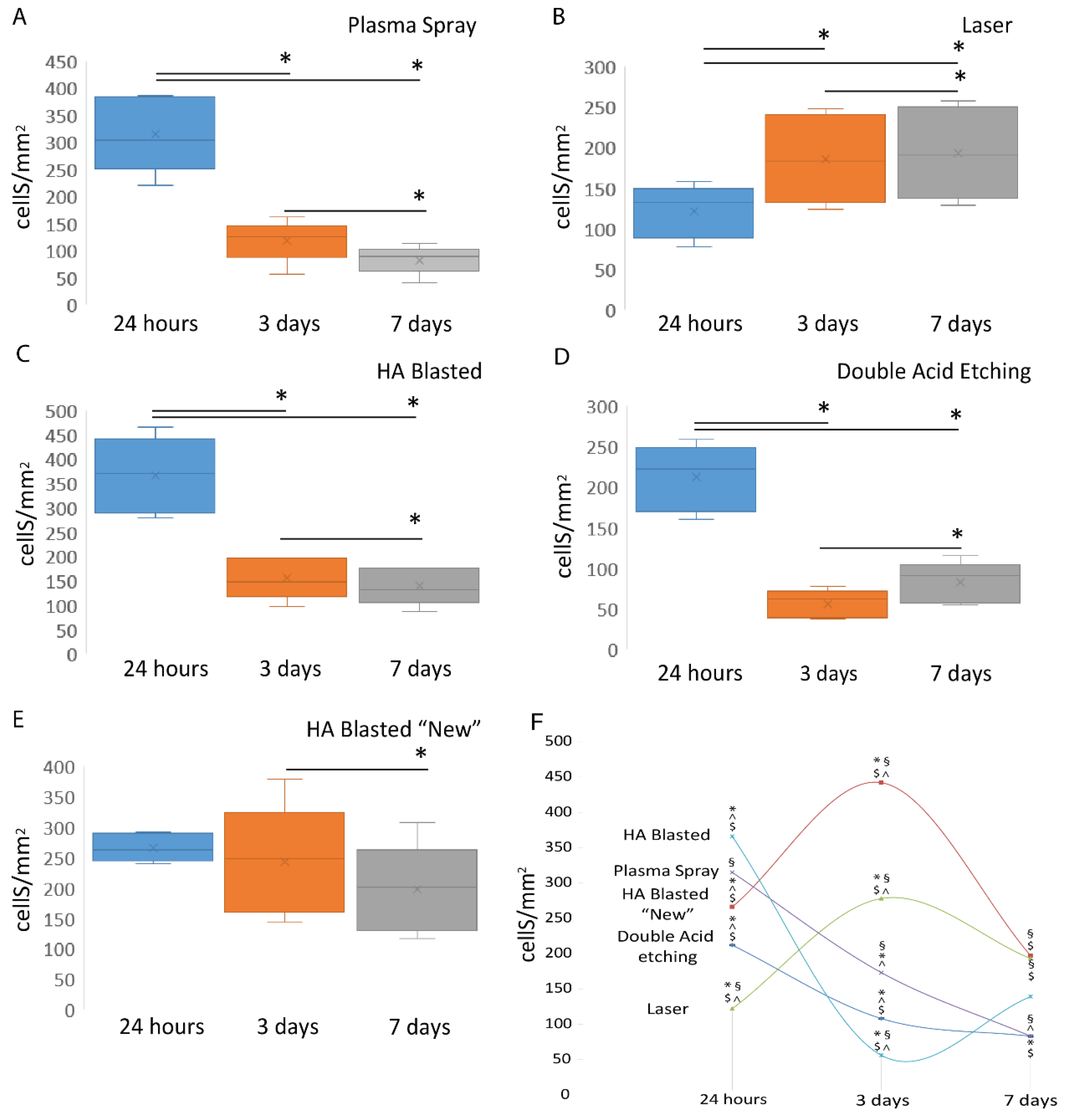
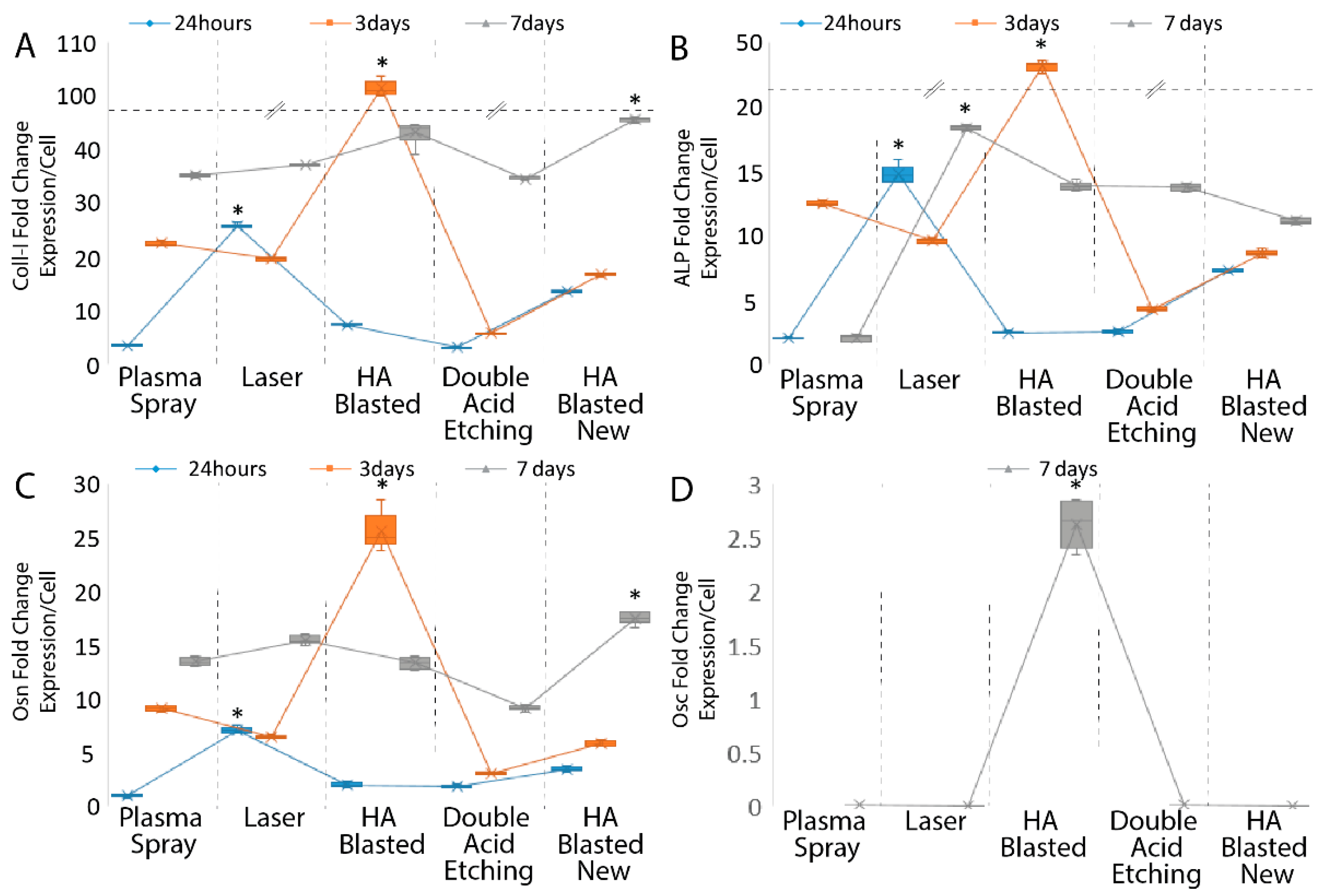
3.3. Osteoblastic Induction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dental Implants Market Size. Share & Trends Analysis Report by Type (Titanium, Zirconium), by Region (North America, Europe, Asia Pacific, Latin America, MEA), and Segment Forecasts, 2020–2027. Available online: https://www.marketresearch.com/Grand-View-Research-v4060/Dental-Implant-Size-Share-Trends-14163164/ (accessed on 25 July 2022).
- Duan, Y.; Ma, W.; Li, D.; Wang, T.; Liu, B. Enhanced Osseointegration of Titanium Implants in a Rat Model of Osteoporosis Using Multilayer Bone Mesenchymal Stem Cell Sheets. Exp. Ther. Med. 2017, 14, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated Titanium Implants. Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.F.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic Research Methods and Current Trends of Dental Implant Surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of Bone Metabolism around Dental Implants during Osseointegration and Peri-Implant Bone Loss. J. Biomed. Mater. Res. A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J. Mechanical Aspects of Dental Implants and Osseointegration: A Narrative Review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef]
- Guida, L.; Annunziata, M.; Rocci, A.; Contaldo, M.; Rullo, R.; Oliva, A. Biological Response of Human Bone Marrow Mesenchymal Stem Cells to Fluoride-Modified Titanium Surfaces. Clin. Oral Implant. Res. 2010, 21, 1234–1241. [Google Scholar] [CrossRef]
- Qahash, M.; Susin, C.; Polimeni, G.; Hall, J.; Wikesjö, U.M.E. Bone Healing Dynamics at Buccal Peri-Implant Sites. Clin. Oral Implant. Res. 2008, 19, 166–172. [Google Scholar] [CrossRef]
- Ripamonti, U.; Roden, L.C.; Ferretti, C.; Klar, R.M. Biomimetic Matrices Self-Initiating the Induction of Bone Formation. J. Craniofacial Surg. 2011, 22, 1859–1870. [Google Scholar] [CrossRef] [Green Version]
- Löberg, J.; Gretzer, C.; Mattisson, I.; Ahlberg, E. Electronic Properties of Anodized TiO2 Electrodes and the Effect on in Vitro Response. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 826–839. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Tolde, Z.; Vanek, P.; Nehasil, V.; Doubková, M.; Trávníčková, M.; Drahokoupil, J.; Buixaderas, E.; Borodavka, F.; Novakova, J.; et al. Beta-Titanium Alloy Covered by Ferroelectric Coating–Physicochemical Properties and Human Osteoblast-Like Cell Response. Coatings 2021, 11, 210. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; de Fátima Balderrama, Í.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface Roughness of Titanium Disks Influences the Adhesion, Proliferation and Differentiation of Osteogenic Properties Derived from Human. Int. J. Implant Dent. 2020, 6, 46. [Google Scholar] [CrossRef]
- Kassem, R.; Samara, A.; Biadsee, A.; Masarwa, S.; Mtanis, T.; Ormianer, Z. A Comparative Evaluation of the Strain Transmitted through Prostheses on Implants with Two Different Macro-Structures and Connection during Insertion and Loading Phase: An In Vitro Study. Materials 2022, 15, 4954. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral Implant Surfaces: Part 1—Review Focusing on Topographic and Chemical Properties of Different Surfaces and in Vivo Responses to Them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral Implant Surfaces: Part 2—Review Focusing on Clinical Knowledge of Different Surfaces. Int. J. Prosthodont. 2004, 17, 544–564. [Google Scholar] [PubMed]
- Cipriano, A.F.; De Howitt, N.; Gott, S.C.; Miller, C.; Rao, M.P.; Liu, H. Bone Marrow Stromal Cell Adhesion and Morphology on Micro- and Sub-Micropatterned Titanium. J. Biomed. Nanotechnol. 2014, 10, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Zanicotti, D.G.; Duncan, W.J.; Seymour, G.J.; Coates, D.E. Effect of Titanium Surfaces on the Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Int. J. Oral Maxillofac. Implant. 2018, 33, e77–e87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrektsson, T.; Wennerberg, A. On Osseointegration in Relation to Implant Surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 4–7. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, M.; Guida, L.; Perillo, L.; Aversa, R.; Passaro, I.; Oliva, A. Biological Response of Human Bone Marrow Stromal Cells to Sandblasted Titanium Nitride-Coated Implant Surfaces. J. Mater. Sci. Mater. Med. 2008, 19, 3585–3591. [Google Scholar] [CrossRef]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double Acid Etching Treatment of Dental Implants for Enhanced Biological Properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz Filho, H.O.; Wennerberg, A. The Influence of Surface Treatment on the Implant Roughness Pattern. J. Appl. Oral Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulangara, K.; Yang, J.; Chellappan, M.; Yang, Y.; Leong, K.W. Nanotopography Alters Nuclear Protein Expression, Proliferation and Differentiation of Human Mesenchymal Stem/Stromal Cells. PLoS ONE 2014, 9, e114698. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Guan, B.; Hu, P.; Qi, X.; Wang, P.; Kong, Y.; Liu, Z.; Gao, P.; Li, R.; Zhang, X.; et al. Topographical Cues of Direct Metal Laser Sintering Titanium Surfaces Facilitate Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells through Epigenetic Regulation. Cell Prolif. 2018, 51, e12460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albouy, J.-P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Implant Surface Characteristics Influence the Outcome of Treatment of Peri-Implantitis: An Experimental Study in Dogs. J. Clin. Periodontol. 2011, 38, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface Topography Enhances Differentiation of Mesenchymal Stem Cells towards Osteogenic and Adipogenic Lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef]
- Steger, M.; Abagnale, G.; Bremus-Köbberling, E.; Wagner, W.; Gillner, A. Nanoscale Biofunctionalization of Polymer Surfaces by Laser Treatment for Controlled Cellular Differentiation. In Optically Induced Nanostructures: Biomedical and Technical Applications; Konig, K., Ostendorf, A., Eds.; Walter de Gruyter Inc.: Berlin, Germany; Boston, MA, USA, 2015. [Google Scholar]
- Svanborg, L.M.; Andersson, M.; Wennerberg, A. Surface Characterization of Commercial Oral Implants on the Nanometer Level. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 462–469. [Google Scholar] [CrossRef]
- Wennerberg, A.; Ide-Ektessabi, A.; Hatkamata, S.; Sawase, T.; Johansson, C.; Albrektsson, T.; Martinelli, A.; Södervall, U.; Odelius, H. Titanium Release from Implants Prepared with Different Surface Roughness. Clin. Oral Implant. Res. 2004, 15, 505–512. [Google Scholar] [CrossRef]
- Wang, L.; Aghvami, M.; Brunski, J.; Helms, J. Biophysical Regulation of Osteotomy Healing: An Animal Study. Clin. Implant Dent. Relat. Res. 2017, 19, 590–599. [Google Scholar] [CrossRef]
- Leucht, P.; Kim, J.-B.; Wazen, R.; Currey, J.A.; Nanci, A.; Brunski, J.B.; Helms, J.A. Effect of Mechanical Stimuli on Skeletal Regeneration around Implants. Bone 2007, 40, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, Y.; Perez, K.C.; Hyman, S.; Brunski, J.B.; Tulu, U.; Bao, C.; Salmon, B.; Helms, J.A. Effects of Condensation on Peri-Implant Bone Density and Remodeling. J. Dent. Res. 2017, 96, 413–420. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Karoussis, I.K.; Fourmousis, I. A Comprehensive and Critical Review of Dental Implant Placement in Diabetic Animals and Patients. Clin. Oral Implant. Res. 2006, 17, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Spelat, R.; Falini, G.; Gallelli, A.; D’Aurizio, F.; Puppato, E.; Pandolfi, M.; Beltrami, A.P.; Cesselli, D.; Beltrami, C.A.; et al. Adipose Tissue-Derived Stem Cell in Vitro Differentiation in a Three-Dimensional Dental Bud Structure. Am. J. Pathol. 2011, 178, 2299–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.A.; Parrett, B.M.; Conejero, J.A.; Laser, J.; Chen, J.; Kogon, A.J.; Nanda, D.; Grant, R.T.; Breitbart, A.S. Biological Alchemy: Engineering Bone and Fat from Fat-Derived Stem Cells. Ann. Plast. Surg. 2003, 50, 610–617. [Google Scholar] [CrossRef]
- Hattori, H.; Masuoka, K.; Sato, M.; Ishihara, M.; Asazuma, T.; Takase, B.; Kikuchi, M.; Nemoto, K.; Ishihara, M. Bone Formation Using Human Adipose Tissue-Derived Stromal Cells and a Biodegradable Scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Hicok, K.C.; Du Laney, T.V.; Zhou, Y.S.; Halvorsen, Y.-D.C.; Hitt, D.C.; Cooper, L.F.; Gimble, J.M. Human Adipose-Derived Adult Stem Cells Produce Osteoid in Vivo. Tissue Eng. 2004, 10, 371–380. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef] [Green Version]
- Haeri, M.; Haeri, M. ImageJ Plugin for Analysis of Porous Scaffolds Used in Tissue Engineering. J. Open Res. Softw. 2015, 3, e1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, J.; He, L.; Huang, H.; Weng, J. Bio-Surface Coated Titanium Scaffolds with Cancellous Bone-like Biomimetic Structure for Enhanced Bone Tissue Regeneration. Acta Biomater. 2020, 114, 431–448. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Piattelli, A.; Sammartino, G.; Wang, H.-L. New Biomaterials and Regenerative Medicine Strategies in Periodontology, Oral Surgery, Esthetic and Implant Dentistry 2018. Biomed. Res. Int. 2019, 2019, 1363581. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin. Implant Dent. Relat. Res. 2013, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On Implant Surfaces: A Review of Current Knowledge and Opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Albouy, J.-P.; Abrahamsson, I.; Berglundh, T. Spontaneous Progression of Experimental Peri-Implantitis at Implants with Different Surface Characteristics: An Experimental Study in Dogs. J. Clin. Periodontol. 2012, 39, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Kato, H.; Neo, M.; Oka, M.; Kim, H.M.; Kokubo, T.; Nakamura, T. Alkali- and Heat-Treated Porous Titanium for Orthopedic Implants. J. Biomed. Mater. Res. 2001, 54, 198–208. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; David, S.R.N.; Zulhilmi, N.R.; Sodhi Dhaliwal, S.K.; Knights, J.; de Albuquerque Junior, R.F. Contamination of Titanium Dental Implants: A Narrative Review. SN Appl. Sci. 2020, 2, 1011. [Google Scholar] [CrossRef]
- Faccioni, F.; Bevilacqua, L.; Porrelli, D.; Khoury, A.; Faccioni, P.; Turco, G.; Frassetto, A.; Maglione, M. Ultrasonic Instrument Effects on Different Implant Surfaces: Profilometry, Energy-Dispersive X-Ray Spectroscopy, and Microbiology In Vitro Study. Int. J. Oral Maxillofac. Implant. 2021, 36, 520–528. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, B.; Tan, P.; Wang, M.; Abotaleb, B.; Zhu, S.; Jiang, N. Promoting Osseointegration of Titanium Implants through Magnesium- and Strontium-Doped Hierarchically Structured Coating. J. Mater. Res. Technol. 2022, 16, 1547–1559. [Google Scholar] [CrossRef]
- Stricker, A.; Bergfeldt, T.; Fretwurst, T.; Addison, O.; Schmelzeisen, R.; Rothweiler, R.; Nelson, K.; Gross, C. Impurities in Commercial Titanium Dental Implants—A Mass and Optical Emission Spectrometry Elemental Analysis. Dent. Mater. 2022, 38, 1395–1403. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and Alendronic Acid on Titanium Surfaces: Adhesion, Proliferation and Differentiation of Bone Marrow-Derived Stem Cells. J. Biomed. Mater. Res. A 2020, 108, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular Responses Evoked by Different Surface Characteristics of Intraosseous Titanium Implants. Biomed. Res. Int. 2015, 2015, 171945. [Google Scholar] [CrossRef] [Green Version]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix--Cytoskeleton Crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Cukierman, E.; Pankov, R.; Yamada, K.M. Cell Interactions with Three-Dimensional Matrices. Curr. Opin. Cell Biol 2002, 14, 633–639. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal Adhesion Regulation of Cell Behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, J.; Brewer, E.; Tu, Q.; Yu, L.; Tang, J.; Krebsbach, P.; Wieland, M.; Chen, J. Osterix Enhances BMSC-Associated Osseointegration of Implants. J. Dent. Res. 2009, 88, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Spelat, R.; Shaw, G.; Coleman, C.M.; Chen, X.Z.; Connolly, D.; Palamá, E.M.F.; Gentili, C.; Contessotto, P.; Murphy, M.J. Regenerative and Anti-Inflammatory Potential of Regularly Fed, Starved Cells and Extracellular Vesicles In Vivo. Cells 2022, 11, 2696. [Google Scholar] [CrossRef]
- Spelat, R.; Ferro, F.; Contessotto, P.; Warren, N.J.; Marsico, G.; Armes, S.P.; Pandit, A. A Worm Gel-Based 3D Model to Elucidate the Paracrine Interaction between Multiple Myeloma and Mesenchymal Stem Cells. Mater. Today Bio 2020, 5, 100040. [Google Scholar] [CrossRef]
- Contessotto, P.; Pandit, A. Therapies to Prevent Post-Infarction Remodelling: From Repair to Regeneration. Biomaterials 2021, 275, 120906. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; Ueland, T.; Broch, K.; Vincent, R.P.; Cross, G.F.; Dahl, C.P.; Aukrust, P.; Gullestad, L.; Hov, J.R.; Trøseid, M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017, 23, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Marsico, G.; Jin, C.; Abbah, S.A.; Brauchle, E.M.; Thomas, D.; Rebelo, A.L.; Orbanić, D.; Chantepie, S.; Contessotto, P.; Papy-Garcia, D.; et al. Elastin-like Hydrogel Stimulates Angiogenesis in a Severe Model of Critical Limb Ischemia (CLI): An Insight into the Glyco-Host Response. Biomaterials 2021, 269, 120641. [Google Scholar] [CrossRef]
- Limongi, T.; Brigo, L.; Tirinato, L.; Pagliari, F.; Gandin, A.; Contessotto, P.; Giugni, A.; Brusatin, G. Three-Dimensionally Two-Photon Lithography Realized Vascular Grafts. Biomed. Mater. 2021, 16, 035013. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Shaw, G.; Duffy, N.; Islam, M.N.; O’Shea, P.M.; O’Toole, D.; Howard, L.; Murphy, J.M. Survival/Adaptation of Bone Marrow-Derived Mesenchymal Stem Cells After Long-Term Starvation Through Selective Processes. Stem Cells 2019, 37, 813–827. [Google Scholar] [CrossRef] [PubMed]
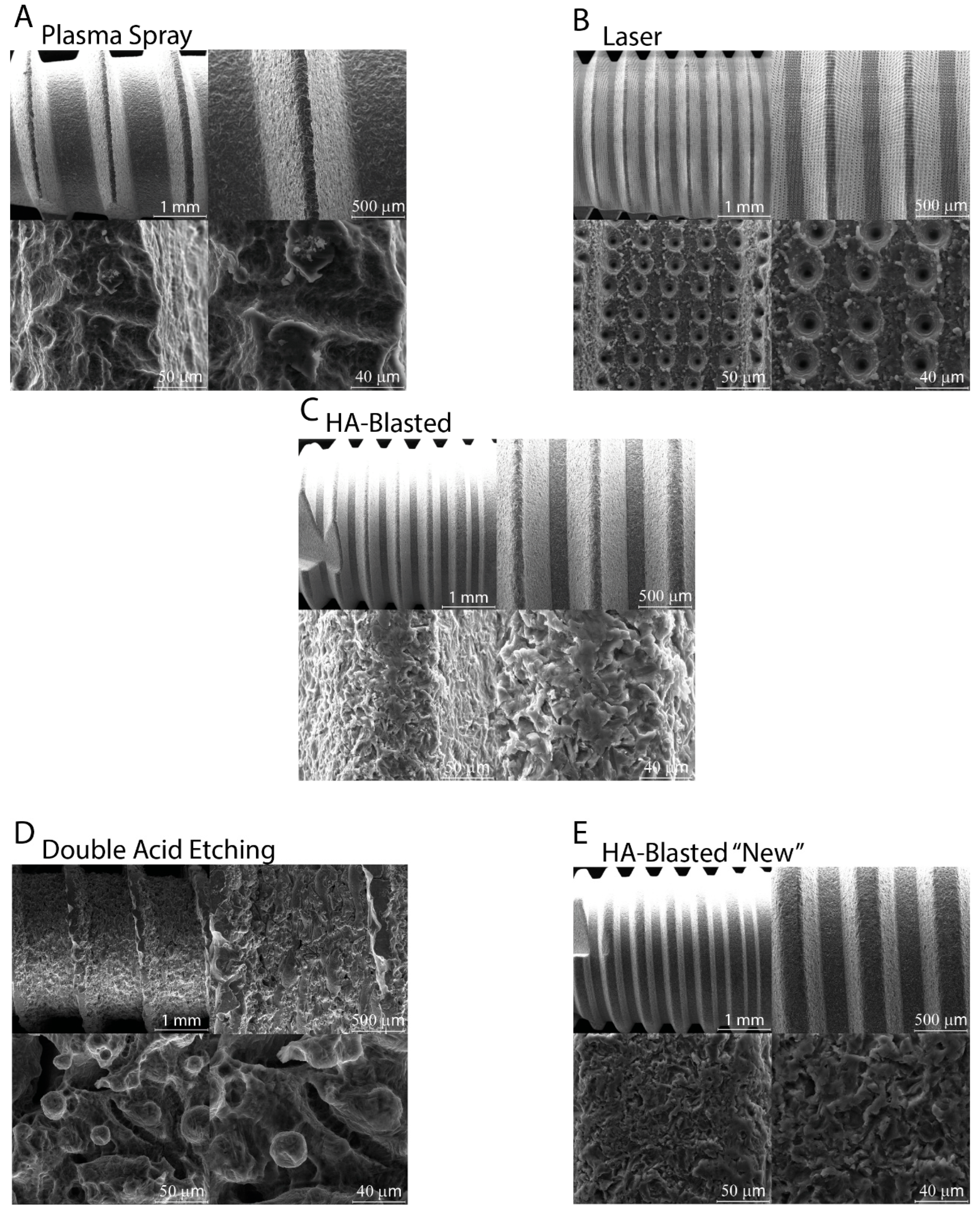

| Surface Treatment | Plasma Spray | Laser | HA-Blasted and Bland Acid Etching | Double Acid Etching | HA-Blasted “New” under Patent |
|---|---|---|---|---|---|
| Composition | Grade 4 titanium | Grade 4 titanium | Ti6Al4V | Grade 4 titanium | Ti6Al4V |
| Nominal chemical composition [38] | Ti 99% | Ti 99% | Ti 90% | Ti 99% | Ti 90% |
| Fe 0.3% | Fe 0.3% | Fe 0.25% | Fe 0.3% | Fe 0.25% | |
| O 0.4% | O 0.4% | O 0.2% max | O 0.4% | O 0.2% max | |
| C 0.1% | C 0.1% | C 0.0% | C 0.1% | C 0.0% | |
| N 0.05% | N 0.05% | N 0.0% | N 0.05% | N 0.0% | |
| H 0.015% | H 0.015% | H 0.0% | H 0.015% | H 0.0% | |
| Al 0.0% | Al 0.0% | Al 6.4% | Al 0.0% | Al 6.4% | |
| V 0.0% | V 0.0% | V 4.12% | V 0.0% | V 4.12% |
| Plasma Spray | Laser | HA-Blasted | Double Acid Etching | HA-Blasted “New” | |
|---|---|---|---|---|---|
| Particle area (μm2) | 8.59 ± 23.18 | 11.91 ± 41.22 | 20.69 ± 57.53 | 10 ± 22.2 | 13.19 ± 29.09 |
| Mean distance between particles (μm) | 4.55 ± 1.54 | 5.65 ± 2.16 | 6.95 ± 2.5 | 5 ± 1.45 | 5.8 ± 1.63 |
| Nearest Particle Distance (μm) | 3.14 ± 1.27 | 3.57 ± 1.75 | 4.49 ± 2.18 | 3.35 ± 1.27 | 3.92 ± 1.61 |
| Average Wall Thickness between particles (μm) | 1.82 ± 1.37 | 2.52 ± 2.5 | 3.13 ± 2.54 | 1.98 ± 1.51 | 2.37 ± 1.82 |
| Particles per surface | 518 ± 67 | 316 ± 39 | 222 ± 29 | 464 ± 78 | 337 ± 94 |
| Plasma Spray | Laser | HA-Blasted | Double Acid Etching | HA-Blasted New | |
|---|---|---|---|---|---|
| Ra (μm) | 0.89 ± 0.07 | 1.03 ± 0.05 | 1.16 ± 0.04 | 0.92 ± 0.09 | 0.92 ± 0.02 |
| Rq (μm) | 1.5 ± 0.06 | 1.62 ± 0.04 | 1.72 ± 0.03 | 1.53 ± 0.08 | 1.53 ± 0.02 |
| Rsk (μm) | 1.69 ± 0.07 | 1.57 ± 0.04 | 1.48 ± 0.02 | 1.66 ± 0.09 | 1.66 ± 0.02 |
| Rku (μm) | 2.86 ± 0.24 | 2.47 ± 0.12 | 2.19 ± 0.07 | 2.78 ± 0.31 | 2.76 ± 0.07 |
| Reference Implant | Implant Type | 24 h | 3 Days | 7 Days |
|---|---|---|---|---|
| Plasma Spray | Laser | <0.001 | 0.01 | <0.001 |
| HA-Blasted | 0.152 | 0.003 | 0.125 | |
| Double Acid Etching | 0.234 | 0.054 | 0.999 | |
| HA-Blasted “New” | 0.197 | <0.001 | <0.001 | |
| Laser | Plasma Spray | <0.001 | 0.01 | <0.001 |
| HA-Blasted | <0.001 | <0.001 | 0.153 | |
| Double Acid Etching | <0.001 | 0.034 | 0.049 | |
| HA-Blasted “New” | <0.001 | <0.001 | 999 | |
| HA-Blasted | Plasma Spray | 0.152 | 0.003 | 0.125 |
| Laser | <0.001 | <0.001 | 0.153 | |
| Double Acid Etching | <0.001 | <0.001 | 0.075 | |
| HA-Blasted “New” | <0.001 | <0.001 | 0.113 | |
| Double Acid Etching | Plasma Spray | 0.234 | 0.054 | 0.999 |
| Laser | <0.001 | 0.034 | 0.049 | |
| HA-Blasted | <0.001 | <0.001 | 0.075 | |
| HA Blasted “New” | <0.001 | <0.001 | 0.001 | |
| HA-Blasted “New” | Plasma Spray | 0.197 | <0.001 | <0.001 |
| Laser | <0.001 | <0.001 | 0.999 | |
| HA-Blasted | <0.001 | <0.001 | 0.113 | |
| Double Acid Etching | <0.001 | <0.001 | 0.001 |
| Coll-I | ALP | Osn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference Implant | Implant Type | 24 h | 3 days | 7 days | 24 h | 3 days | 7 days | 24 h | 3 days | 7 days |
| Plasma Spray | Laser | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HA-Blasted | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | |
| Double Acid Etching | <0.001 | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HA-Blasted “New” | <0.001 | 0.314 | <0.001 | 0.004 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | |
| Laser | Plasma Spray | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| HA-Blasted | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Double Acid Etching | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HA-Blasted “New” | <0.001 | 0.025 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 1 | 0.022 | |
| HA-Blasted | Plasma Spray | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 |
| Laser | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Double Acid Etching | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.218 | 1 | |
| HA-Blasted “New” | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | |
| Double Acid Etching | Plasma Spray | <0.001 | <0.001 | <0.001 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Laser | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HA-Blasted | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.218 | 1 | |
| HA-Blasted “New” | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| HA-Blasted “New” | Plasma Spray | <0.001 | 0.314 | <0.001 | 0.004 | <0.001 | <0.001 | 1 | <0.001 | <0.001 |
| Laser | <0.001 | 0.025 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | 1 | 0.022 | |
| HA-Blasted | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | |
| Double Acid Etching | <0.001 | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferro, F.; Azzolin, F.; Spelat, R.; Bevilacqua, L.; Maglione, M. Assessing the Efficacy of Whole-Body Titanium Dental Implant Surface Modifications in Inducing Adhesion, Proliferation, and Osteogenesis in Human Adipose Tissue Stem Cells. J. Funct. Biomater. 2022, 13, 206. https://doi.org/10.3390/jfb13040206
Ferro F, Azzolin F, Spelat R, Bevilacqua L, Maglione M. Assessing the Efficacy of Whole-Body Titanium Dental Implant Surface Modifications in Inducing Adhesion, Proliferation, and Osteogenesis in Human Adipose Tissue Stem Cells. Journal of Functional Biomaterials. 2022; 13(4):206. https://doi.org/10.3390/jfb13040206
Chicago/Turabian StyleFerro, Federico, Federico Azzolin, Renza Spelat, Lorenzo Bevilacqua, and Michele Maglione. 2022. "Assessing the Efficacy of Whole-Body Titanium Dental Implant Surface Modifications in Inducing Adhesion, Proliferation, and Osteogenesis in Human Adipose Tissue Stem Cells" Journal of Functional Biomaterials 13, no. 4: 206. https://doi.org/10.3390/jfb13040206
APA StyleFerro, F., Azzolin, F., Spelat, R., Bevilacqua, L., & Maglione, M. (2022). Assessing the Efficacy of Whole-Body Titanium Dental Implant Surface Modifications in Inducing Adhesion, Proliferation, and Osteogenesis in Human Adipose Tissue Stem Cells. Journal of Functional Biomaterials, 13(4), 206. https://doi.org/10.3390/jfb13040206