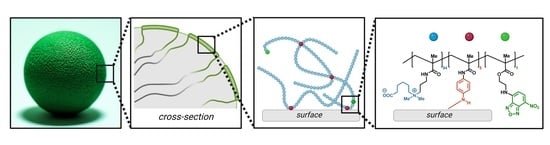
Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abbreviations
2.2. Adsorbents
2.3. Pre-conditioning
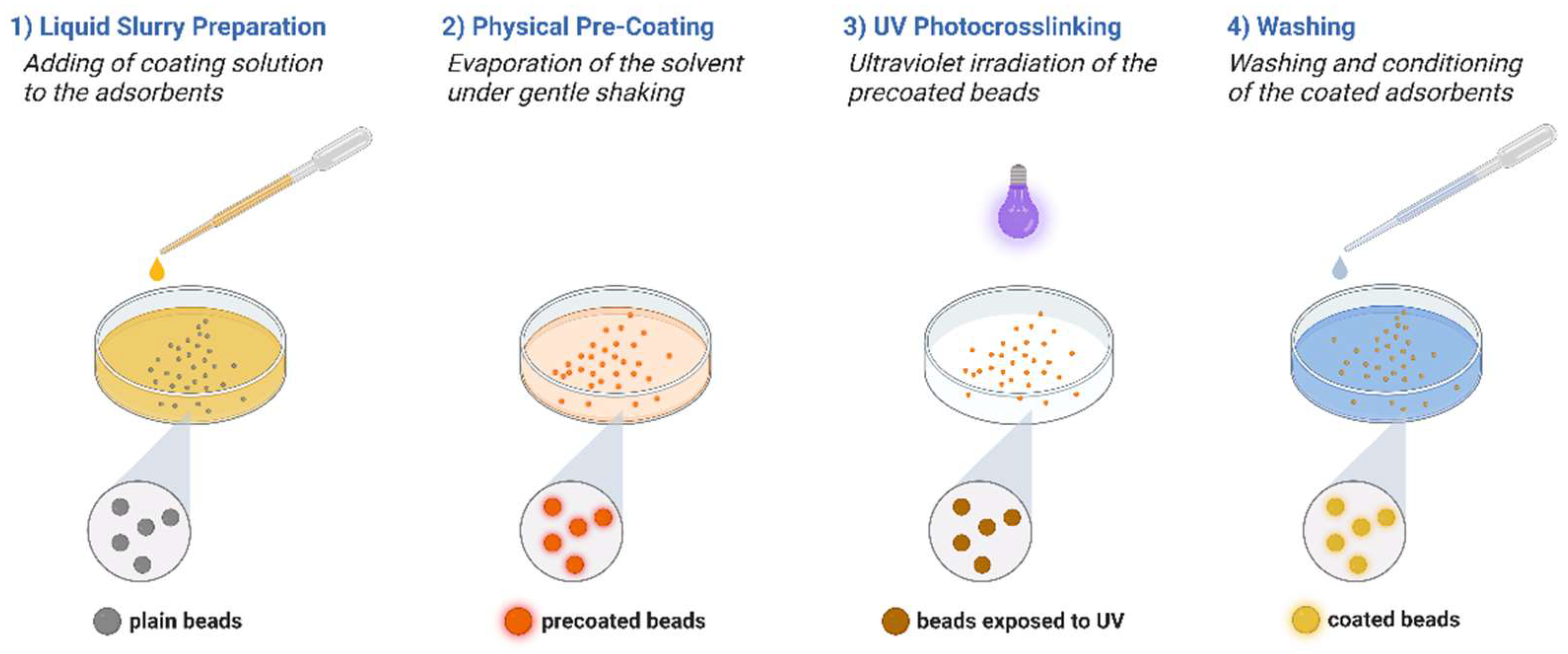
2.4. Physical Coating and UV Crosslinking
2.5. Characterization of Blood Compatibility
2.6. Blood Compatibility of Coated CG161c Beads
2.7. Recirculation of Whole Blood over DALI Columns
2.8. Stimulation of Whole Blood with Lipopolysaccharide (LPS)
2.9. Cytokine Adsorption and Quantification
2.10. Adsorption of Lipoproteins
3. Results
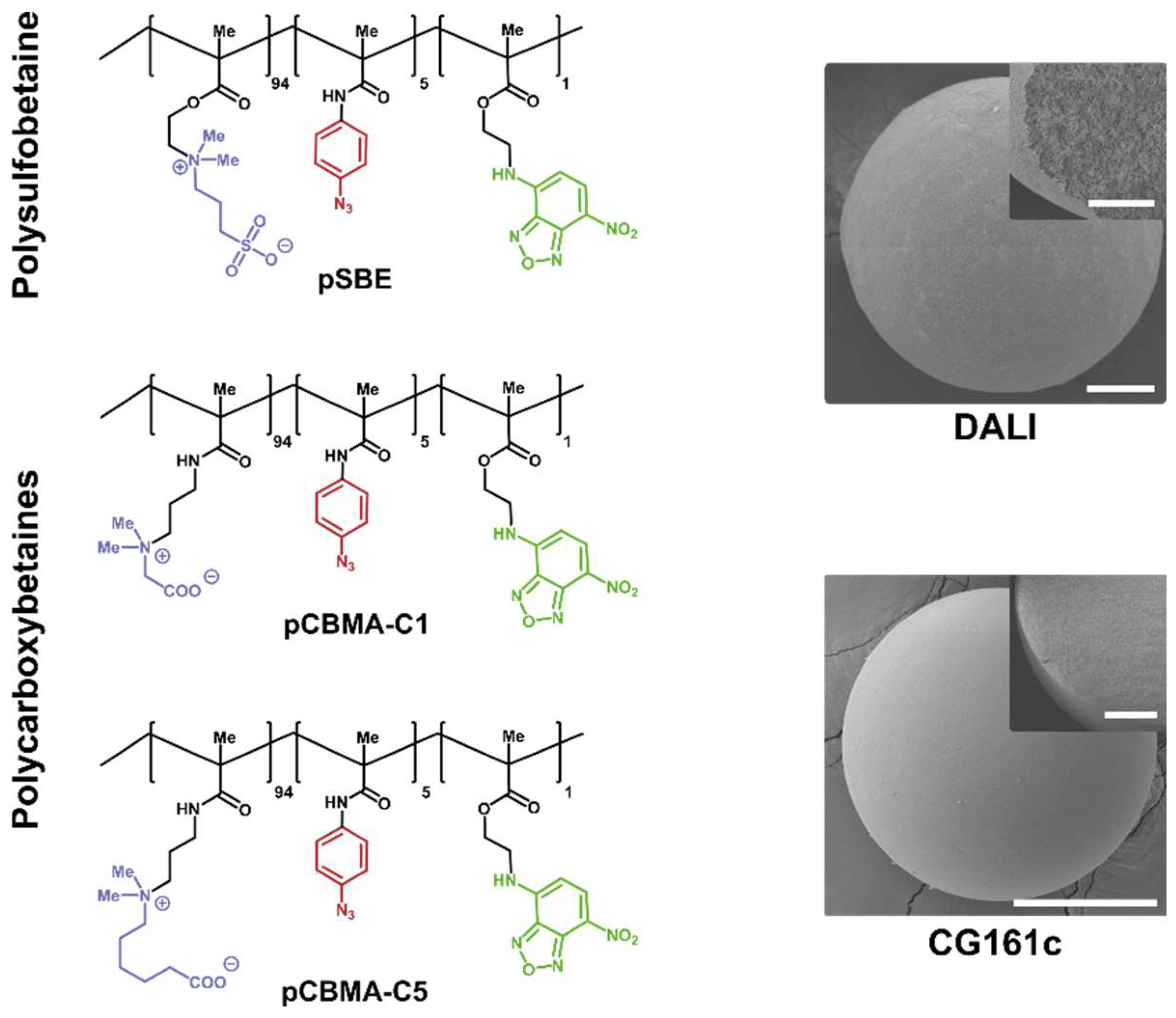
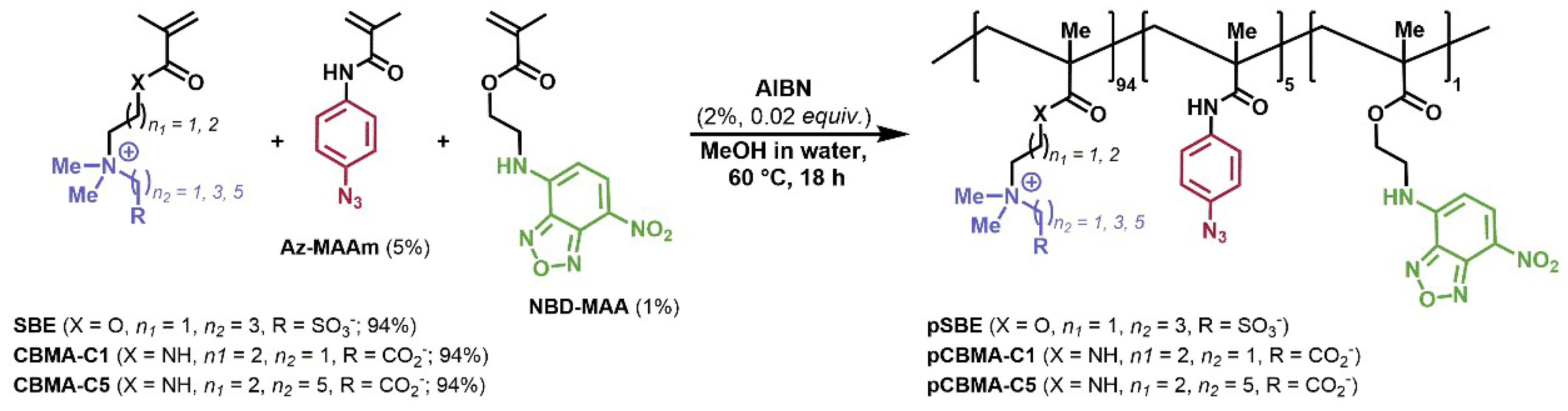
3.1. Synthesis of Monomers and Copolymers
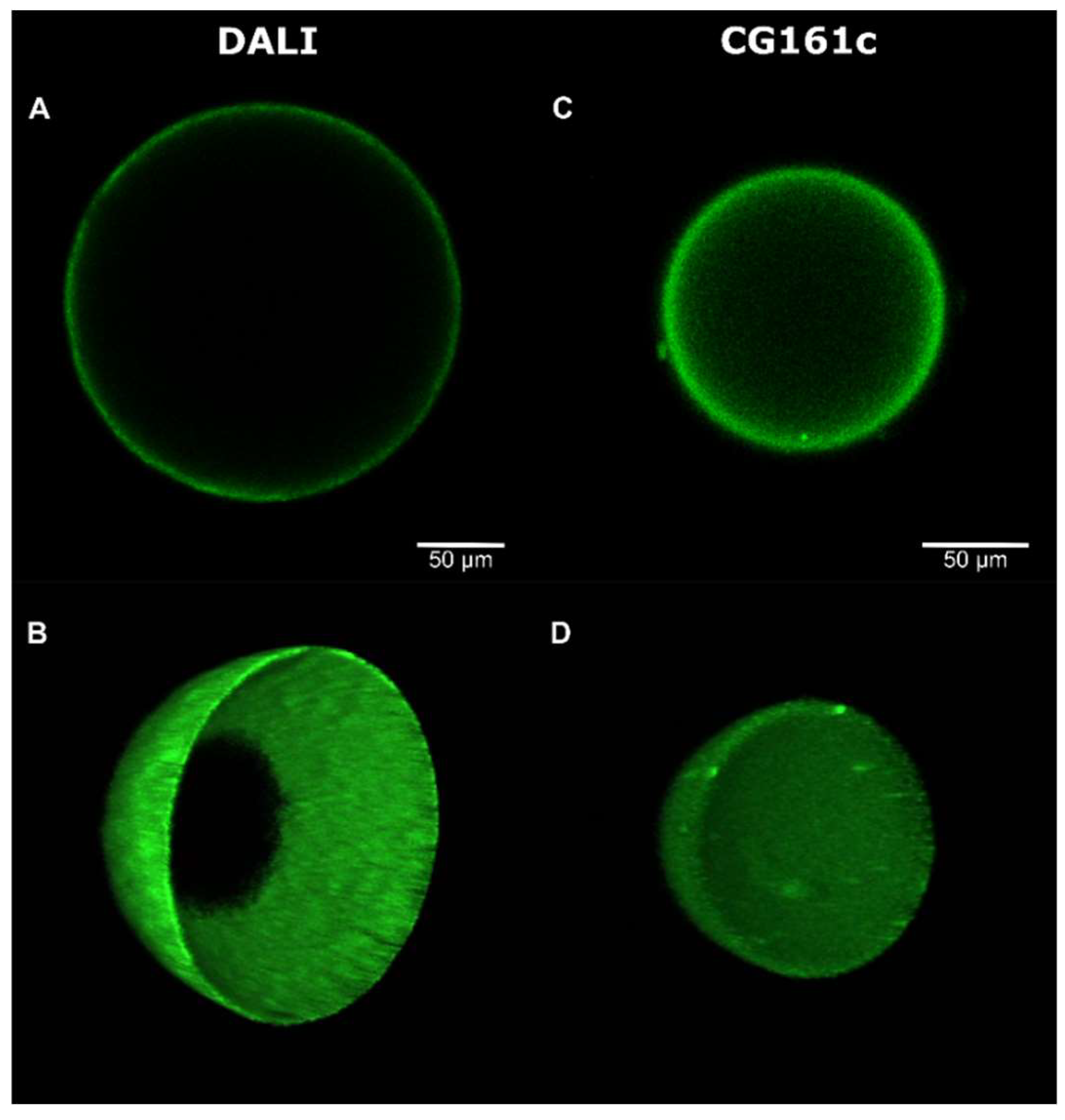
3.2. Deposition, Stability, and Microscopic Analysis of the Coating
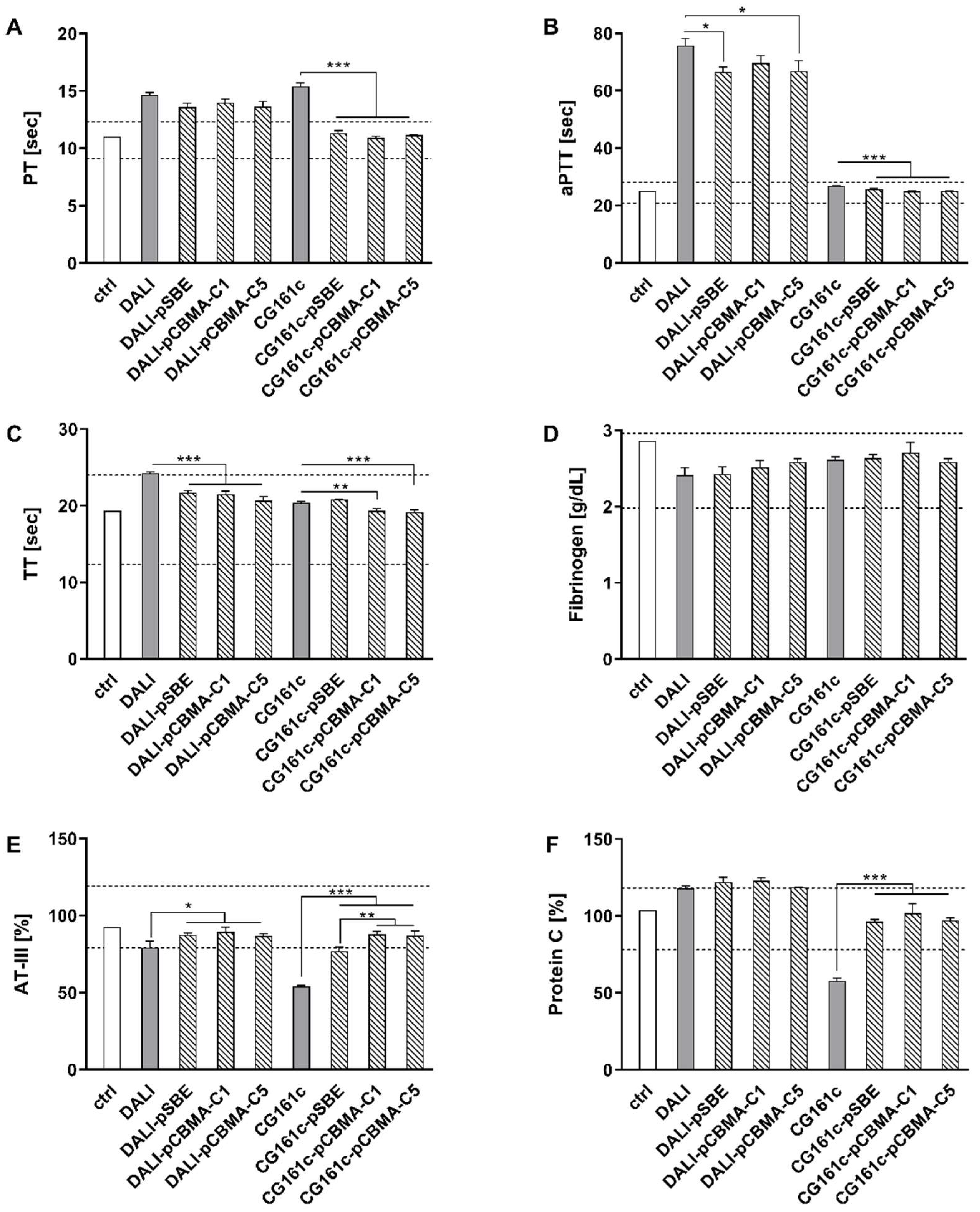
3.3. Effect of Coating on Blood Compatibility
3.4. Adsorption of Cytokines and LDL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mickiewicz, A.; Borowiec-Wolna, J.; Bachorski, W.; Gilis-Malinowska, N.; Gałąska, R.; Raczak, G.; Chmara, M.; Wasąg, B.; Jaguszewski, M.J.; Fijałkowski, M.; et al. Long-Term Lipoprotein Apheresis in the Treatment of Severe Familial Hypercholesterolemia Refractory to High Intensity Statin Therapy: Three Year Experience at a Lipoprotein Apheresis Center. Cardiol. J. 2019, 26, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldmann, E.; Parhofer, K.G. Apheresis for Severe Hypercholesterolaemia and Elevated Lipoprotein(A). Pathology 2019, 51, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, S.N.; Afanasieva, O.I.; Ezhov, M.V. Therapeutic Apheresis for Management of Lp(a) Hyperlipoproteinemia. Curr. Atheroscler. Rep. 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Ankawi, G.; Neri, M.; Zhang, J.; Breglia, A.; Ricci, Z.; Ronco, C. Extracorporeal Techniques for the Treatment of Critically Ill Patients with Sepsis beyond Conventional Blood Purification Therapy: The Promises and the Pitfalls 11 Medical and Health Sciences 1107 Immunology. Crit. Care 2018, 22, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonavia, A.; Groff, A.; Karamchandani, K.; Singbartl, K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018, 46, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood Compatible Materials: State of the Art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef]
- Reviakine, I.; Jung, F.; Braune, S.; Brash, J.L.; Latour, R.; Gorbet, M.; van Oeveren, W. Stirred, Shaken, or Stagnant: What Goes on at the Blood–Biomaterial Interface. Blood Rev. 2017, 31, 11–21. [Google Scholar] [CrossRef]
- Weiss, R.; Spittler, A.; Schmitz, G.; Fischer, M.B.; Weber, V. Thrombocyte Adhesion and Release of Extracellular Microvesicles Correlate with Surface Morphology of Adsorbent Polymers for Lipid Apheresis. Biomacromolecules 2014, 15, 2648–2655. [Google Scholar] [CrossRef]
- Weiss, R.; Eichhorn, T.; Spittler, A.; Micusik, M.; Fischer, M.B.; Weber, V. Release and Cellular Origin of Extracellular Vesicles during Circulation of Whole Blood over Adsorbent Polymers for Lipid Apheresis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 636–646. [Google Scholar] [CrossRef]
- Weiss, R.; Fischer, M.B.; Weber, V. The Impact of Citrate Concentration on Adhesion of Platelets and Leukocytes to Adsorbents in Whole Blood Lipoprotein Apheresis. J. Clin. Apher. 2017, 32, 375–383. [Google Scholar] [CrossRef]
- Gubensek, J.; Strobl, K.; Harm, S.; Weiss, R.; Eichhorn, T.; Buturovic-Ponikvar, J.; Weber, V.; Hartmann, J. Influence of Citrate Concentration on the Activation of Blood Cells in an in Vitro Dialysis Setup. PLoS One 2018, 13, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Lauková, L.; Weiss, R.; Semak, V.; Weber, V. Desialylation of Platelet Surface Glycans Enhances Platelet Adhesion to Adsorbent Polymers for Lipoprotein Apheresis. Int. J. Artif. Organs 2021, 44, 378–384. [Google Scholar] [CrossRef]
- Semak, V.; Fischer, M.B.; Weber, V. Biomimetic Principles to Develop Blood Compatible Surfaces. Int. J. Artif. Organs 2017, 40, 22–30. [Google Scholar] [CrossRef]
- Lim, C.-M.; Li, M.-X.; Joung, Y.K. Surface-Modifying Polymers for Blood-Contacting Polymeric Biomaterials. In Biomimicked Biomaterials: Advances in Tissue Engineering and Regenerative Medicine; Chun, H.J., Reis, R.L., Motta, A., Khang, G., Eds.; Springer: Singapore, 2020; pp. 189–198. ISBN 978-981-15-3262-7. [Google Scholar]
- Erfani, A.; Seaberg, J.; Aichele, C.P.; Ramsey, J.D. Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules 2020, 21, 2557–2573. [Google Scholar] [CrossRef]
- Sanchez-Cano, C.; Carril, M. Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces. Int. J. Mol. Sci. 2020, 21, 1007. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic Materials for Antifouling Membrane Surface Construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Jiang, S.; Ishihara, K.; Ji, J. Special Issue on Zwitterionic Materials. Acta Biomater. 2016, 40, iv. [Google Scholar] [CrossRef]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why Do Phospholipid Polymers Reduce Protein Adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- de los Santos Pereira, A.; Sheikh, S.; Blaszykowski, C.; Pop-Georgievski, O.; Fedorov, K.; Thompson, M.; Rodriguez-Emmenegger, C. Antifouling Polymer Brushes Displaying Antithrombogenic Surface Properties. Biomacromolecules 2016, 17, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From Surface Properties and Bioactivity Profiles to Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Racovita, S.; Trofin, M.A.; Loghin, D.F.; Zaharia, M.M.; Bucatariu, F.; Mihai, M.; Vasiliu, S. Polybetaines in Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 9321. [Google Scholar] [CrossRef] [PubMed]
- Azha, S.F.; Shamsudin, M.S.; Bonilla-Petriciolet, A.; Ismail, S. Kinetics, Process Design and Implementation of Zwitterionic Adsorbent Coating for Dipolar Dyes Removal in Wastewater Treatment Industry. Environ. Technol. Innov. 2021, 23, 101763. [Google Scholar] [CrossRef]
- Azha, S.F.; Shamsudin, M.S.; Shahadat, M.; Ismail, S. Low Cost Zwitterionic Adsorbent Coating for Treatment of Anionic and Cationic Dyes. J. Ind. Eng. Chem. 2018, 67, 187–198. [Google Scholar] [CrossRef]
- Weers, J.G.; Rathman, J.F.; Axe, F.U.; Crichlow, C.A.; Foland, L.D.; Scheuing, D.R.; Wiersema, R.J.; Zielske, A.G. Effect of the Intramolecular Charge Separation Distance on the Solution Properties of Betaines and Sulfobetaines. Langmuir 1991, 7, 854–867. [Google Scholar] [CrossRef]
- Gritsan, N.; Platz, M. Photochemistry of Azides: The Azide/Nitrene Interface. In Organic Azides; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 311–372. ISBN 9780470682517. [Google Scholar]
- Ito, Y.; Hasuda, H.; Sakuragi, M.; Tsuzuki, S. Surface Modification of Plastic, Glass and Titanium by Photoimmobilization of Polyethylene Glycol for Antibiofouling. Acta Biomater. 2007, 3, 1024–1032. [Google Scholar] [CrossRef]
- Mosnáček, J.; Osička, J.; Popelka, A.; Zavahir, S.; Ben-Hamadou, R.; Kasák, P. Photochemical Grafting of Polysulfobetaine onto Polyethylene and Polystyrene Surfaces and Investigation of Long-Term Stability of the Polysulfobetaine Layer in Seawater. Polym. Adv. Technol. 2018, 29, 1930–1938. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Popelka, A.; Mičušík, M.; Sláviková, M.; Krupa, I.; Mosnáček, J.; Tkáč, J.; Lacík, I.; Kasák, P. Photoimmobilization of Zwitterionic Polymers on Surfaces to Reduce Cell Adhesion. J. Colloid Interface Sci. 2017, 500, 294–303. [Google Scholar] [CrossRef]
- Makiyama, T.; Nakamura, H.; Nagasaka, N.; Yamashita, H.; Honda, T.; Yamaguchi, N.; Nishida, A.; Murayama, T. Trafficking of Acetyl-C16-Ceramide-NBD with Long-Term Stability and No Cytotoxicity into the Golgi Complex. Traffic 2015, 16, 476–492. [Google Scholar] [CrossRef]
- Dräger, L.J.; Julius, U.; Kraenzle, K.; Schaper, J.; Toepfer, M.; Zygan, K.; Otto, V.; Steinhagen-Thiessen, E. DALI-the First Human Whole-Blood Low-Density Lipoprotein and Lipoprotein (a) Apheresis System in Clinical Use: Procedure and Clinical Results. Eur. J. Clin. Invest. 1998, 28, 994–1002. [Google Scholar] [CrossRef]
- Najam, O.; Lambert, G.; Ray, K.K. The Past, Present and Future of Lipid-Lowering Therapy. Clin. Lipidol. 2015, 10, 481–498. [Google Scholar] [CrossRef]
- Bulut, M.; Nisli, K.; Dindar, A. The Effect of DALI Lipid Apheresis in the Prognosis of Homozygous Familial Hypercholesterolemia: Seven Patients´ Experience at a DALI Apheresis Center. Ann. Pediatr. Cardiol. 2020, 13, 111–116. [Google Scholar] [CrossRef]
- Rimmelé, T.; Kellum, J.A. Clinical Review: Blood Purification for Sepsis. Crit. Care 2011, 15, 205. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine Release Syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Zhou, Y.; Ma, J.; Xia, P.; Qin, Y.; Li, X. Is There a Role for Blood Purification Therapies Targeting Cytokine Storm Syndrome in Critically Severe COVID-19 Patients? Ren. Fail. 2020, 42, 483–488. [Google Scholar] [CrossRef]
- Ronco, C.; Reis, T. Kidney Involvement in COVID-19 and Rationale for Extracorporeal Therapies. Nat. Rev. Nephrol. 2020, 16, 308–310. [Google Scholar] [CrossRef] [Green Version]
- NIH; CytoSorbents; Inc CytoSorb® Reduction of Free Hemoglobin/Acute Kidney Injury During Cardiac Surgery (REFRESH II-AKI). Available online: https://clinicaltrials.gov/ct2/show/NCT03384875 (accessed on 27 September 2022).
- Träger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption Treatment of Patients with Acute Infective Endocarditis during Surgery with Cardiopulmonary Bypass - A Case Series. Int. J. Artif. Organs 2017, 40, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.H.; Rinoesl, H.; Dragosits, K.; Ristl, R.; Hoffelner, F.; Opfermann, P.; Lamm, C.; Preißing, F.; Wiedemann, D.; Hiesmayr, M.J.; et al. Effect of Hemoadsorption During Cardiopulmonary Bypass Surgery - a Blinded, Randomized, Controlled Pilot Study Using a Novel Adsorbent. Crit. Care 2016, 20, 96. [Google Scholar] [CrossRef]
- Eichhorn, T.; Rauscher, S.; Hammer, C.; Gröger, M.; Fischer, M.B.; Weber, V. Polystyrene-Divinylbenzene-Based Adsorbents Reduce Endothelial Activation and Monocyte Adhesion Under Septic Conditions in a Pore Size-Dependent Manner. Inflammation 2016, 39, 1737–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harm, S.; Schildböck, C.; Hartmann, J. Cytokine Removal in Extracorporeal Blood Purification: An in Vitro Study. Blood Purif. 2020, 49, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Watkins, B.F.; Bermes, E.W.J. An Evaluation of a Spectrophotometric Scanning Technique for Measurement of Plasma Hemoglobin. Ann. Clin. Lab. Sci. 1981, 11, 126–131. [Google Scholar] [PubMed]
- Eichhorn, T.; Fische, M.B.; Weber, V. Mechanisms of Endothelial Activation in Sepsis and Cell Culture Models to Study the Heterogeneous Host Response. Int. J. Artif. Organs 2017, 40, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Unsworth, L.D. Multi-Functional Initiator and Poly(Carboxybetaine Methacrylamides) for Building Biocompatible Surfaces Using “Nitroxide Mediated Free Radical Polymerization” Strategies. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1051–1060. [Google Scholar] [CrossRef]
- Jia, E.; Liang, B.; Lin, Y.; Su, Z. Hemocompatibility of Polyzwitterion-Modified Titanium Dioxide Nanotubes. Nanotechnology 2021, 32, 305704. [Google Scholar] [CrossRef]
- Lieber, E.; Rao, C.N.R.; Chao, T.S.; Hoffman, C.W.W. Infrared Spectra of Organic Azides. Anal. Chem. 1957, 29, 916–918. [Google Scholar] [CrossRef]
- Cegielska, B.; Kacprzak, K.M. Simple and Convenient Protocol for Staining of Organic Azides on TLC Plates by Ninhydrin. A New Application of an Old Reagent. Chem. Analityczna 2009, 54, 807–812. [Google Scholar]
- Cheng, Q.; Asha, A.B.; Liu, Y.; Peng, Y.-Y.; Diaz-Dussan, D.; Shi, Z.; Cui, Z.; Narain, R. Antifouling and Antibacterial Polymer-Coated Surfaces Based on the Combined Effect of Zwitterions and the Natural Borneol. ACS Appl. Mater. Interfaces 2021, 13, 9006–9014. [Google Scholar] [CrossRef]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.-C.; Lorca, T.; Castro, A. Loss of Human Greatwall Results in G2 Arrest and Multiple Mitotic Defects Due to Deregulation of the Cyclin B-Cdc2/PP2A Balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, J.D.; Gibson, G.A.; Watkins, S.C.; Kellum, J.A.; Federspiel, W.J. IL-6 Adsorption Dynamics in Hemoadsorption Beads Studied Using Confocal Laser Scanning Microscopy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 390–396. [Google Scholar] [CrossRef]
- Liu, Q.; Chiu, A.; Wang, L.H.; An, D.; Zhong, M.; Smink, A.M.; de Haan, B.J.; de Vos, P.; Keane, K.; Vegge, A.; et al. Zwitterionically Modified Alginates Mitigate Cellular Overgrowth for Cell Encapsulation. Nat. Commun. 2019, 10, 5262. [Google Scholar] [CrossRef] [Green Version]
- Schönemann, E.; Koc, J.; Karthaüser, J.F.; Ozcan, O.; Schanzenbach, D.; Schardt, L.; Rosenhahn, A.; Laschewsky, A. Sulfobetaine Methacrylate Polymers of Unconventional Polyzwitterion Architecture and Their Antifouling Properties. Biomacromolecules 2021, 22, 1494–1508. [Google Scholar] [CrossRef]
- ISO:10993-4; Biological Evaluation of Medical Devices — Part 4: Selection of Tests for Interactions with Blood. ISO: Geneva, Switzerland, 2017.
- Lippi, G. Systematic Assessment of the Hemolysis Index: Pros and Cons (Chapter 6). In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 71, pp. 157–170. [Google Scholar]
- Harm, S.; Gabor, F.; Hartmann, J. Characterization of Adsorbents for Cytokine Removal from Blood in an In Vitro Model. J. Immunol. Res. 2015, 2015, 484736. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Jiang, S. Effect of Carbon Spacer Length on Zwitterionic Carboxybetaines. J. Phys. Chem. B 2013, 117, 1357–1366. [Google Scholar] [CrossRef]
- Kathmann, E.E.; White, L.A.; McCormick, L. Water Soluble Polymers: 70. Effects of Methylene versus Propylene Spacers in the PH and Electrolyte Responsiveness of Zwitterionic Copolymers Incorporating Carboxybetaine Monomers. Polymer (Guildf). 1997, 38, 879–886. [Google Scholar] [CrossRef]
- Lacík, I.; Sobolčiak, P.; Stach, M.; Chorvát, D.; Kasák, P. Propagation Rate Coefficient for Sulfobetaine Monomers by PLP−SEC. Polymer (Guildf). 2016, 87, 38–49. [Google Scholar] [CrossRef]
- Ukita, R.; Wu, K.; Lin, X.; Carleton, N.M.; Naito, N.; Lai, A.; Do-Nguyen, C.C.; Demarest, C.T.; Jiang, S.; Cook, K.E. Zwitterionic Poly-Carboxybetaine Coating Reduces Artificial Lung Thrombosis in Sheep and Rabbits. Acta Biomater. 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Julius, U.; Siegert, G.; Gromeier, S. Intraindividual Comparison of the Impact of Two Selective Apheresis Methods (DALI and HELP) on the Coagulation System. Int. J. Artif. Organs 2000, 23, 199–206. [Google Scholar] [CrossRef]
- Horbett, T.A. Fibrinogen Adsorption to Biomaterials. J. Biomed. Mater. Res. Part A 2018, 106, 2777–2788. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Yuan, J. Design of Hemocompatible and Antifouling PET Sheets with Synergistic Zwitterionic Surfaces. J. Colloid Interface Sci. 2016, 480, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Leukocyte Activation and Leukocyte Procoagulant Activities after Blood Contact with Polystyrene and Polyethylene Glycol–Immobilized Polystyrene Beads. J. Lab. Clin. Med. 2001, 137, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Peng, L.; Song, E.; Song, Y. Polystyrene Nanoplastics Induce Neutrophil Extracellular Traps in Mice Neutrophils. Chem. Res. Toxicol. 2022, 35, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Eichhorn, T.; Harm, S.; Weber, V. Adsorption of the Inflammatory Mediator High-Mobility Group Box 1 by Polymers with Different Charge and Porosity. Biomed Res. Int. 2014, 2014, 238160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harm, S.; Falkenhagen, D.; Hartmann, J. Pore Size - A Key Property for Selective Toxin Removal in Blood Purification. Int. J. Artif. Organs 2014, 37, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, A.R.; Mohammadsaleh, F. Synthesis of Aryl Azides from Aryl Halides Promoted by Cu2O/Tetraethylammonium Prolinate. Tetrahedron Lett. 2014, 55, 6799–6802. [Google Scholar] [CrossRef]
- Warneke, J.; Wang, Z.; Zeller, M.; Leibfritz, D.; Plaumann, M.; Azov, V. Methacryloyl Chloride Dimers: From Structure Elucidation to a Manifold of Chemical Transformations. Tetrahedron 2014, 70, 6515–6521. [Google Scholar] [CrossRef]
- Kurt, M.; Babu, P.C.; Sundaraganesan, N.; Cinar, M.; Karabacak, M. Molecular Structure, Vibrational, UV and NBO Analysis of 4-Chloro-7-Nitrobenzofurazan by DFT Calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1162–1170. [Google Scholar] [CrossRef]
- Anastassopoulou, J.D. Mass and FT-IR Spectra of Quaternary Ammonium Surfactants. In Chemistry and Properties of Biomolecular Systems; Rizzarelli, E., Theophanides, T., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 1–9. ISBN 978-94-011-3620-4. [Google Scholar]
- Abraham, S.; So, A.; Unsworth, L.D. Poly(Carboxybetaine Methacrylamide) Modified Nanoparticles: A Model System for Studying the Effect of Chain Chemistry on Film Properties, Adsorbed Protein Conformation and Clot Formation Kinetics. Biomacromolecules 2011, 12, 3567–3580. [Google Scholar] [CrossRef]
- Weiss, R.; Gröger, M.; Rauscher, S.; Fendl, B.; Eichhorn, T.; Fischer, M.B.; Spittler, A.; Weber, V. Differential Interaction of Platelet-Derived Extracellular Vesicles with Leukocyte Subsets in Human Whole Blood. Sci. Rep. 2018, 8, 6598. [Google Scholar] [CrossRef] [Green Version]
- Fendl, B.; Weiss, R.; Eichhorn, T.; Linsberger, I.; Afonyushkin, T.; Puhm, F.; Binder, C.J.; Fischer, M.B.; Weber, V. Extracellular Vesicles Are Associated with C-Reactive Protein in Sepsis. Sci. Rep. 2021, 11, 6996. [Google Scholar] [CrossRef]
- George, S.K.; Lauková, L.; Weiss, R.; Semak, V.; Fendl, B.; Weiss, V.U.; Steinberger, S.; Allmaier, G.; Tripisciano, C.; Weber, V. Comparative Analysis of Platelet-derived Extracellular Vesicles Using Flow Cytometry and Nanoparticle Tracking Analysis. Int. J. Mol. Sci. 2021, 22, 3839. [Google Scholar] [CrossRef]
- Takayama, T.; Mitsumori, T.; Kawano, M.; Sekine, A.; Uekusa, H.; Ohashi, Y.; Sugawara, T. Direct Observation of Arylnitrene Formation in the Photoreaction of Arylazide Crystals. Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 639–646. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]



| CG161c-pSBE | CG161c-pCBMA-C1 | CG161c-pCBMA-C5 | |
|---|---|---|---|
| IL-6 | 67 ± 6% | 39 ± 4% | 63 ± 1% |
| TNF-α | 29 ± 2% | 25 ± 6% | 35 ± 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semak, V.; Eichhorn, T.; Weiss, R.; Weber, V. Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis. J. Funct. Biomater. 2022, 13, 216. https://doi.org/10.3390/jfb13040216
Semak V, Eichhorn T, Weiss R, Weber V. Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis. Journal of Functional Biomaterials. 2022; 13(4):216. https://doi.org/10.3390/jfb13040216
Chicago/Turabian StyleSemak, Vladislav, Tanja Eichhorn, René Weiss, and Viktoria Weber. 2022. "Polyzwitterionic Coating of Porous Adsorbents for Therapeutic Apheresis" Journal of Functional Biomaterials 13, no. 4: 216. https://doi.org/10.3390/jfb13040216