Hydrothermal Synthesis of Fluorapatite Coatings over Titanium Implants for Enhanced Osseointegration—An In Vivo Study in the Rabbit
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Fluorapatite-Coated Titanium Implants
2.1.1. Implants
2.1.2. Synthesis of the Hydroxyapatite and Fluorapatite Coatings by the Hydrothermal Method
2.1.3. Physical and Chemical Characterization
2.2. Biological Characterization—In Vivo Response to Bone Implantation
2.2.1. Animals
2.2.2. Surgical Procedure
2.2.3. Microtomographic Evaluation
2.3. Statistical Analysis
3. Results and Discussion
3.1. Coating Preparation and Characterization
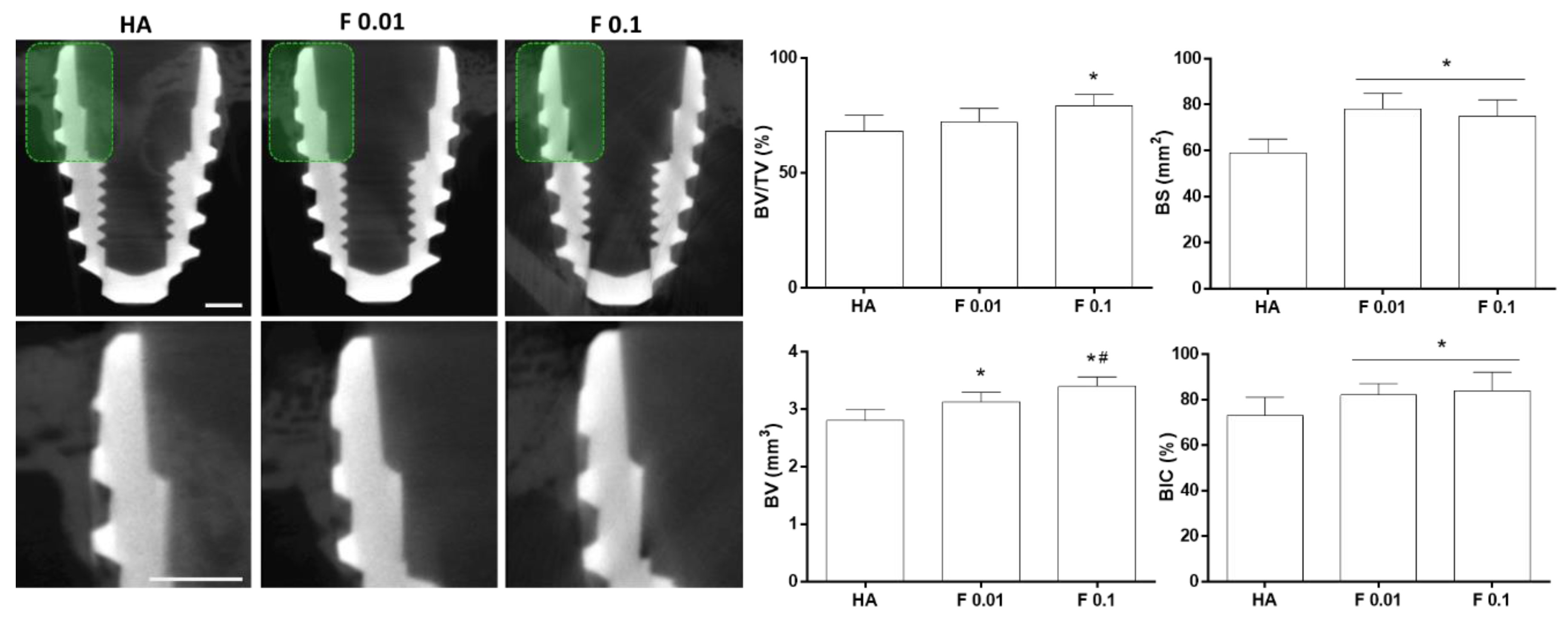
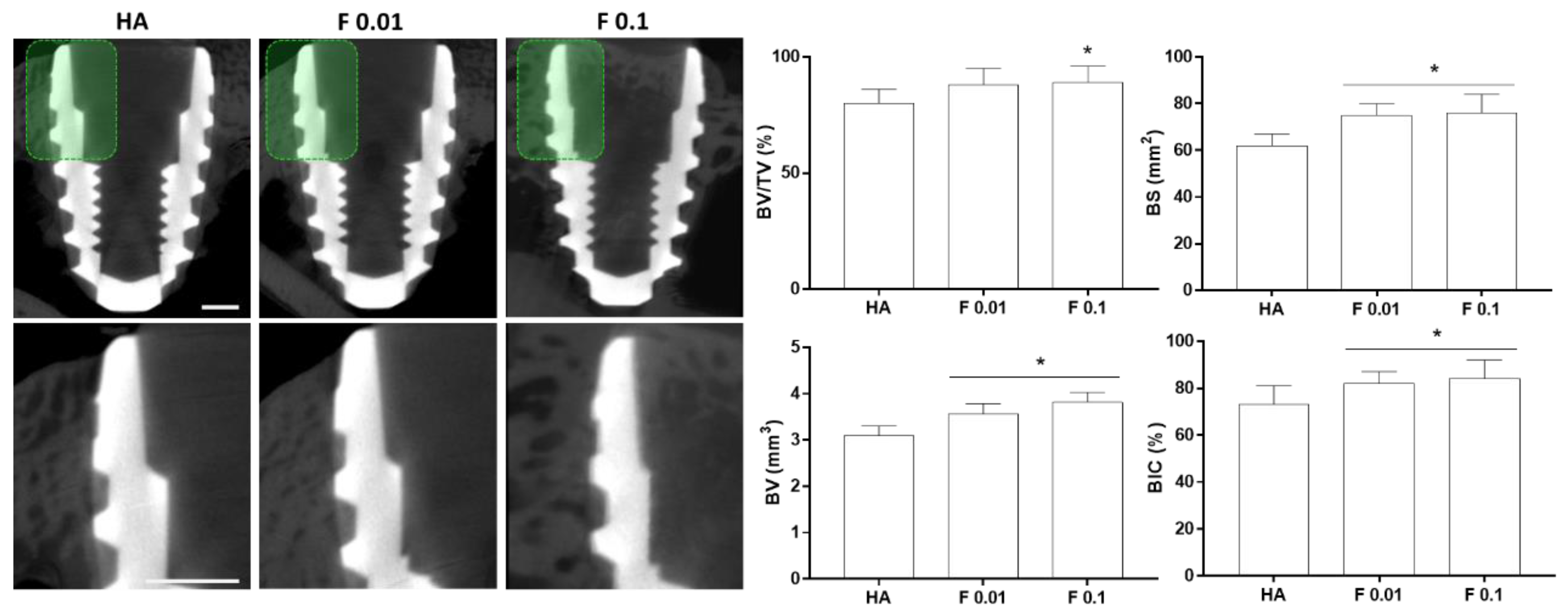
3.2. Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Chen, L. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implant. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Vahabzadeh, S.; Roy, M.; Bandyopadhyay, A.; Bose, S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015, 17, 47–55. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Moloodi, A.; Toraby, H.; Kahrobaee, S.; Razavi, M.K.; Salehi, A. Evaluation of fluorohydroxyapatite/strontium coating on titanium implants fabricated by hydrothermal treatment. Prog. Biomater. 2021, 10, 185–194. [Google Scholar] [CrossRef]
- Kien, P.; Quan, T.; Tuyet Anh, L. Coating Characteristic of Hydroxyapatite on Titanium Substrates via Hydrothermal Treatment. Coatings 2021, 11, 1226. [Google Scholar] [CrossRef]
- Arrés, M.; Salama, M.; Rechena, D.; Paradiso, P.; Reis, L.; Alves, M.M.; Botelho do Rego, A.M.; Carmezim, M.J.; Vaz, M.F.; Deus, A.M.; et al. Surface and mechanical properties of a nanostructured citrate hydroxyapatite coating on pure titanium. J. Mech. Behav. Biomed. Mater. 2020, 108, 103794. [Google Scholar] [CrossRef]
- Borkowski, L.; Przekora, A.; Belcarz, A.; Palka, K.; Jozefaciuk, G.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Ginalska, G. Fluorapatite ceramics for bone tissue regeneration: Synthesis, characterization and assessment of biomedical potential. Mater. Sci. Eng. C 2020, 116, 111211. [Google Scholar] [CrossRef]
- Li, Z.; Huang, B.; Mai, S.; Wu, X.; Zhang, H.; Qiao, W.; Luo, X.; Chen, Z. Effects of fluoridation of porcine hydroxyapatite on osteoblastic activity of human MG63 cells. Sci. Technol. Adv. Mater. 2015, 16, 035006. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Mansourianfar, M.; Fathi, M.; Bonakdar, S.; Ebrahimi, M.; Zahrani, E.M.; Hojjati-Najafabadi, A.; Li, D. Surface modification of orthopedic implants by optimized fluorine-substituted hydroxyapatite coating: Enhancing corrosion behavior and cell function. Ceram. Int. 2020, 46, 2139–2146. [Google Scholar] [CrossRef]
- Rezaee, T.; Bouxsein, M.L.; Karim, L. Increasing fluoride content deteriorates rat bone mechanical properties. Bone 2020, 136, 115369. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Bruker-MicroCT MN074 Osteointegration: Analysis of Bone around a Metal Implant. In Bruker-MicroCT Method Note; Bruker Micro-Ct Academy: Kontich, Belgium, 2005; pp. 1–26.
- Vidal, C.; Alves, P.; Alves, M.M.; Carmezim, M.J.; Fernandes, M.H.; Grenho, L.; Inácio, P.L.; Ferreira, F.B.; Santos, T.G.; Santos, C. Fabrication of a biodegradable and cytocompatible magnesium/nanohydroxyapatite/fluorapatite composite by upward friction stir processing for biomedical applications. J. Mech. Behav. Biomed. Mater. 2022, 129, 105137. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Tseng, Y.-H.; Chan, J.C.C. Morphology Control of Fluorapatite Crystallites by Citrate Ions. Cryst. Growth Des. 2010, 10, 4240–4242. [Google Scholar] [CrossRef]
- Bhadang, K.A.; Gross, K.A. Influence of fluorapatite on the properties of thermally sprayed hydroxyapatite coatings. Biomaterials 2004, 25, 4935–4945. [Google Scholar] [CrossRef]
- Qu, H.; Wei, M. The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater. 2006, 2, 113–119. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramirez-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivanchenko, P.; Martra, G.; Lin, F.-H.; Delgado-López, J.M.; et al. Combined Effect of Citrate and Fluoride Ions on Hydroxyapatite Nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Jha, L.J.; Best, S.M.; Knowles, J.C.; Rehman, I.; Santos, J.D.; Bonfield, W. Preparation and characterization of fluoride-substituted apatites. J. Mater. Sci. Mater. Med. 1997, 8, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Caligari Conti, M.; Xerri, G.; Peyrouzet, F.; Wismayer, P.S.; Sinagra, E.; Mantovani, D.; Vella, D.; Buhagiar, J. Optimisation of fluorapatite coating synthesis applied to a biodegradable substrate. Surf. Eng. 2019, 35, 255–265. [Google Scholar] [CrossRef]
- Bulina, N.V.; Makarova, S.V.; Prosanov, I.Y.; Vinokurova, O.B.; Lyakhov, N.Z. Structure and thermal stability of fluorhydroxyapatite and fluorapatite obtained by mechanochemical method. J. Solid State Chem. 2020, 282, 121076. [Google Scholar] [CrossRef]
- Slimen, J.B.; Hidouri, M.; Ghouma, M.; Salem, E.B.; Dorozhkin, S.V. Sintering of Potassium Doped Hydroxy-Fluorapatite Bioceramics. Coatings 2021, 11, 858. [Google Scholar] [CrossRef]
- Wei, M.; Evans, J.H.; Bostrom, T.; Grøndahl, L. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J. Mater. Sci. Mater. Med. 2003, 14, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Alhilou, A.; Do, T.; Mizban, L.; Clarkson, B.H.; Wood, D.J.; Katsikogianni, M.G. Physicochemical and Antibacterial Characterization of a Novel Fluorapatite Coating. ACS Omega 2016, 1, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.H.; Alves, M.M.; Cebotarenco, M.; Ribeiro, I.A.C.; Grenho, L.; Gomes, P.S.; Carmezim, M.J.; Santos, C.F. Citrate zinc hydroxyapatite nanorods with enhanced cytocompatibility and osteogenesis for bone regeneration. Mater. Sci. Eng. C 2020, 115, 111147. [Google Scholar] [CrossRef]
- Deng, L.; Ou, J.; Yang, H.; Wen, G.; Huang, T. The surface regulation of calcite for defluoridation by fluorapatite-induced crystallization. J. Water Process Eng. 2021, 41, 102082. [Google Scholar] [CrossRef]
- Joseph Nathanael, A.; Mangalaraj, D.; Hong, S.I.; Masuda, Y.; Rhee, Y.H.; Kim, H.W. Influence of fluorine substitution on the morphology and structure of hydroxyapatite nanocrystals prepared by hydrothermal method. Mater. Chem. Phys. 2013, 137, 967–976. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Fan, Q.; Hu, Q.; Shi, B. Comparative study of hydroxyapatite, fluor-hydroxyapatite and Si-substituted hydroxyapatite nanoparticles on osteogenic, osteoclastic and antibacterial ability. RSC Adv. 2019, 9, 16106–16118. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef]
- Stübinger, S.; Dard, M. The Rabbit as Experimental Model for Research in Implant Dentistry and Related Tissue Regeneration. J. Investig. Surg. 2013, 26, 266–282. [Google Scholar] [CrossRef]
- Shin, D.; Blanchard, S.B.; Ito, M.; Chu, T.-M.G. Peripheral quantitative computer tomographic, histomorphometric, and removal torque analyses of two different non-coated implants in a rabbit model. Clin. Oral Implant. Res. 2011, 22, 242–250. [Google Scholar] [CrossRef]
- Seong, W.-J.; Grami, S.; Jeong, S.C.; Conrad, H.J.; Hodges, J.S. Comparison of Push-In versus Pull-Out Tests on Bone-Implant Interfaces of Rabbit Tibia Dental Implant Healing Model. Clin. Implant Dent. Relat. Res. 2013, 15, 460–469. [Google Scholar] [CrossRef]
- Garbieri, T.F.; Martin, V.; dos Santos, C.F.; Gomes, P.D.S.; Fernandes, M.H. The Embryonic Chick Femur Organotypic Model as a Tool to Analyze the Angiotensin II Axis on Bone Tissue. Pharmaceuticals 2021, 14, 469. [Google Scholar] [CrossRef]
- Francisco, I.; Vale, F.; Martin, V.; Fernandes, M.H.; Gomes, P.S. From Blood to Bone—The Osteogenic Activity of L-PRF Membranes on the Ex Vivo Embryonic Chick Femur Development Model. Materials 2021, 14, 7830. [Google Scholar] [CrossRef]
- Araújo, R.; Martin, V.; Ferreira, R.; Fernandes, M.H.; Gomes, P.S. A new ex vivo model of the bone tissue response to the hyperglycemic environment—The embryonic chicken femur organotypic culture in high glucose conditions. Bone 2022, 158, 116355. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, D.; Zhou, R.; Zhang, Y. 3D X-ray micro-computed tomography imaging for the microarchitecture evaluation of porous metallic implants and scaffolds. Micron 2021, 142, 102994. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology—A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef]
- Alsabeeha, N.H.M.; Ma, S.; Atieh, M.A. Hydroxyapatite-coated oral implants: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 2012, 27, 1123–1130. [Google Scholar] [PubMed]
- Yang, S.; Lee, S.; Bajpai, I.; Kim, S. Hydrothermal treatment of Ti surface to enhance the formation of low crystalline hydroxyl carbonate apatite. Biomater. Res. 2015, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-S.; Chang, C.-C.; Lin, P.-C.; Lin, S.-P.; Wang, C.-L. Direct growth of structurally controllable hydroxyapatite coating on Ti-6Al-4V through a rapid hydrothermal synthesis. Appl. Surf. Sci. 2021, 556, 149672. [Google Scholar] [CrossRef]
- Bhadang, K.A.; Holding, C.A.; Thissen, H.; McLean, K.M.; Forsythe, J.S.; Haynes, D.R. Biological responses of human osteoblasts and osteoclasts to flame-sprayed coatings of hydroxyapatite and fluorapatite blends. Acta Biomater. 2010, 6, 1575–1583. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol–gel method: Dissolution behaviour and biological properties after crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Knowles, J.C. Fluor-hydroxyapatite sol–gel coating on titanium substrate for hard tissue implants. Biomaterials 2004, 25, 3351–3358. [Google Scholar] [CrossRef]
- Gross, K.A.; Rodríguez-Lorenzo, L.M. Sintered hydroxyfluorapatites. Part I: Sintering ability of precipitated solid solution powders. Biomaterials 2004, 25, 1375–1384. [Google Scholar] [CrossRef]
- Gao, Y.; Karpukhina, N.; Law, R.V. Phase segregation in hydroxyfluorapatite solid solution at high temperatures studied by combined XRD/solid state NMR. RSC Adv. 2016, 6, 103782–103790. [Google Scholar] [CrossRef]
- Everett, E.T. Fluoride’s Effects on the Formation of Teeth and Bones, and the Influence of Genetics. J. Dent. Res. 2011, 90, 552–560. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zeng, X.; Ma, L.L.; Weng, W.; Yan, W.; Qian, M. Osteoblastic cell response on fluoridated hydroxyapatite coatings. Acta Biomater. 2007, 3, 191–197. [Google Scholar] [CrossRef]
- Pan, L.; Shi, X.; Liu, S.; Guo, X.; Zhao, M.; Cai, R.; Sun, G. Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway. Toxicol. Lett. 2014, 225, 34–42. [Google Scholar] [CrossRef]
- Huo, L.; Liu, K.; Pei, J.; Yang, Y.; Ye, Y.; Liu, Y.; Sun, J.; Han, H.; Xu, W.; Gao, Y. Flouride Promotes Viability and Differentiation of Osteoblast-Like Saos-2 Cells Via BMP/Smads Signaling Pathway. Biol. Trace Elem. Res. 2013, 155, 142–149. [Google Scholar] [CrossRef]
- Pei, J.; Li, B.; Gao, Y.; Wei, Y.; Zhou, L.; Yao, H.; Wang, J.; Sun, D. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATc1 gene expression. Environ. Toxicol. 2014, 29, 588–595. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Liu, K.; Wang, W.; Xu, C.; Zhang, Y.; Liu, Y. Role of inhibition of osteogenesis function by Sema4D/Plexin-B1 signaling pathway in skeletal fluorosis in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 712–715. [Google Scholar] [CrossRef]
- Zipkin, I.; Bernick, S.; Menczel, J. A Morphological Study of the Effect of Fluoride on the Periodontium of the Hydrocortisone-Treated Rat. Periodontics 1965, 3, 111–114. [Google Scholar]
- Cook, F.J.; Seagrove-Guffey, M.; Mumm, S.; Veis, D.J.; McAlister, W.H.; Bijanki, V.N.; Wenkert, D.; Whyte, M.P. Non-endemic skeletal fluorosis: Causes and associated secondary hyperparathyroidism (case report and literature review). Bone 2021, 145, 115839. [Google Scholar] [CrossRef]
- Wu, S.; Xia, B.; Mai, S.; Feng, Z.; Wang, X.; Liu, Y.; Liu, R.; Li, Z.; Xiao, Y.; Chen, Z.; et al. Sodium Fluoride under Dose Range of 2.4–24 μM, a Promising Osteoimmunomodulatory Agent for Vascularized Bone Formation. ACS Biomater. Sci. Eng. 2019, 5, 817–830. [Google Scholar] [CrossRef]
- Nagendra, A.H.; Bose, B.; Shenoy, P.S. Recent advances in cellular effects of fluoride: An update on its signalling pathway and targeted therapeutic approaches. Mol. Biol. Rep. 2021, 48, 5661–5673. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.; Zou, J.; Franklin, R.B.; Reynolds, M.A. The status of citrate in the hydroxyapatite/collagen complex of bone; and Its role in bone formation. J. Regen. Med. Tissue Eng. 2014, 3, 4. [Google Scholar] [CrossRef]
- Morganti, C.; Bonora, M.; Marchi, S.; Ferroni, L.; Gardin, C.; Wieckowski, M.R.; Giorgi, C.; Pinton, P.; Zavan, B. Citrate Mediates Crosstalk between Mitochondria and the Nucleus to Promote Human Mesenchymal Stem Cell In Vitro Osteogenesis. Cells 2020, 9, 1034. [Google Scholar] [CrossRef]
- Ma, C.; Tian, X.; Kim, J.P.; Xie, D.; Ao, X.; Shan, D.; Lin, Q.; Hudock, M.R.; Bai, X.; Yang, J. Citrate-based materials fuel human stem cells by metabonegenic regulation. Proc. Natl. Acad. Sci. USA 2018, 115, E11741–E11750. [Google Scholar] [CrossRef] [PubMed]
- Shares, B.H.; Busch, M.; White, N.; Shum, L.; Eliseev, R.A. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J. Biol. Chem. 2018, 293, 16019–16027. [Google Scholar] [CrossRef] [PubMed]
- Schafrum Macedo, A.; Cezaretti Feitosa, C.; Yoiti Kitamura Kawamoto, F.; Vinicius Tertuliano Marinho, P.; dos Santos Dal-Bó, Í.; Fiuza Monteiro, B.; Prado, L.; Bregadioli, T.; Antonio Covino Diamante, G.; Ricardo Auada Ferrigno, C. Animal modeling in bone research—Should we follow the White Rabbit? Anim. Model. Exp. Med. 2019, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, E.; Martin, V.; Colaço, B.; Fernandes, M.H.; Santos, C.; Gomes, P.S. Hydrothermal Synthesis of Fluorapatite Coatings over Titanium Implants for Enhanced Osseointegration—An In Vivo Study in the Rabbit. J. Funct. Biomater. 2022, 13, 241. https://doi.org/10.3390/jfb13040241
Santiago E, Martin V, Colaço B, Fernandes MH, Santos C, Gomes PS. Hydrothermal Synthesis of Fluorapatite Coatings over Titanium Implants for Enhanced Osseointegration—An In Vivo Study in the Rabbit. Journal of Functional Biomaterials. 2022; 13(4):241. https://doi.org/10.3390/jfb13040241
Chicago/Turabian StyleSantiago, Eduardo, Victor Martin, Bruno Colaço, Maria Helena Fernandes, Catarina Santos, and Pedro S. Gomes. 2022. "Hydrothermal Synthesis of Fluorapatite Coatings over Titanium Implants for Enhanced Osseointegration—An In Vivo Study in the Rabbit" Journal of Functional Biomaterials 13, no. 4: 241. https://doi.org/10.3390/jfb13040241
APA StyleSantiago, E., Martin, V., Colaço, B., Fernandes, M. H., Santos, C., & Gomes, P. S. (2022). Hydrothermal Synthesis of Fluorapatite Coatings over Titanium Implants for Enhanced Osseointegration—An In Vivo Study in the Rabbit. Journal of Functional Biomaterials, 13(4), 241. https://doi.org/10.3390/jfb13040241











