Effect of Silicon Carbide Coating on Osteoblast Mineralization of Anodized Titanium Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Coating Process
2.3. Surface Characterization
2.4. Cell Viability
2.5. Scanning Electron Microscopy
2.6. Mineralization
2.7. Data Analysis
3. Results
3.1. Wettability
3.2. Surface Characterization
3.3. Cell Viability
3.4. Mineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 185–206. [Google Scholar] [CrossRef]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100-B, 9–16. [Google Scholar] [CrossRef]
- Stan, M.S.; Memet, I.; Fratila, C.; Krasicka-Cydzik, E.; Roman, I.; Dinischiotu, A. Effects of titanium-based nanotube films on osteoblast behavior in vitro. J. Biomed. Mater. Res. Part A 2015, 103, 48–56. [Google Scholar] [CrossRef]
- Subramani, K.; Pandruvada, S.N.; Puleo, D.A.; Hartsfield, J.K.; Huja, S.S. In vitro evaluation of osteoblast responses to carbon nanotube-coated titanium surfaces. Prog. Orthod. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Camargo, S.E.A.; Roy, T.; Iv, P.H.C.; Fares, C.; Ren, F.; Clark, A.E.; Esquivel-Upshaw, J.F. Novel Coatings to Minimize Bacterial Adhesion and Promote Osteoblast Activity for Titanium Implants. J. Funct. Biomater. 2020, 11, 42. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.-M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-Doped Titanium Dioxide Nanotubes Promoted Bone Formation on Titanium Implants. Int. J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef] [Green Version]
- Voltrova, B.; Hybasek, V.; Blahnova, V.; Sepitka, J.; Lukasova, V.; Vocetkova, K.; Sovkova, V.; Matejka, R.; Fojt, J.; Joska, L.; et al. Different diameters of titanium dioxide nanotubes modulate Saos-2 osteoblast-like cell adhesion and osteogenic differentiation and nanomechanical properties of the surface. RSC Adv. 2019, 9, 11341–11355. [Google Scholar] [CrossRef]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; Van Der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Soler, M.D.; Hsu, S.-M.; Fares, C.; Ren, F.; Jenkins, R.J.; Gonzaga, L.; Clark, A.E.; O’Neill, E.; Neal, D.; Esquivel-Upshaw, J.F. Titanium Corrosion in Peri-Implantitis. Materials 2020, 13, 5488. [Google Scholar] [CrossRef]
- Safioti, L.M.; Kotsakis, G.; Pozhitkov, A.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium Are Associated with Peri-Implantitis—A Cross-Sectional Study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Ren, F.; Chen, Z.; Kim, M.; Fares, C.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Novel Coating to Minimize Corrosion of Glass-Ceramics for Dental Applications. Materials 2020, 13, 1215. [Google Scholar] [CrossRef] [Green Version]
- Fares, C.; Hsu, S.-M.; Xian, M.; Xia, X.; Ren, F.; Mecholsky, J.J.J.; Gonzaga, L.; Esquivel-Upshaw, J. Demonstration of a SiC Protective Coating for Titanium Implants. Materials 2020, 13, 3321. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Roy, T.; Xia, X.; Fares, C.; Hsu, S.-M.; Ren, F.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Novel Coatings to Minimize Corrosion of Titanium in Oral Biofilm. Materials 2021, 14, 342. [Google Scholar] [CrossRef]
- Afonso Camargo, S.E.; Mohiuddeen, A.S.; Fares, C.; Partain, J.L.; Carey, P.H.; Ren, F.; Hsu, S.-M.; Clark, A.E.; Esquivel-Upshaw, J.F. Anti-Bacterial Properties and Biocompatibility of Novel SiC Coating for Dental Ceramic. J. Funct. Biomater. 2020, 11, 33. [Google Scholar] [CrossRef]
- Esquivel-Upshaw, J.F.; Ren, F.; Camargo, S.C.A.E. Methods And Compositions For Medical Implants Having Anti-Bacterial Coatings; U.S.P.T. Office: Gainesville, FL, USA, 2020. [Google Scholar]
- Esquivel-Upshaw, J.F.; Ren, F.; Clark, A.E.; Batich, C.C.P. Quaternized TiN Anti-Bacterial Coating for Dental Implants; U.S.P.T. Office: Gainesville, FL, USA, 2018. [Google Scholar]
- Hsu, S.-M.; Ren, F.; Batich, C.D.; Clark, A.E.; Neal, D.; Esquivel-Upshaw, J.F. Effect of pH Cycling Frequency on Glass–Ceramic Corrosion. Materials 2020, 13, 3655. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279–280. [Google Scholar] [CrossRef]
- Honda, M.; Kikushima, K.; Kawanobe, Y.; Konishi, T.; Mizumoto, M.; Aizawa, M. Enhanced early osteogenic differentiation by silicon-substituted hydroxyapatite ceramics fabricated via ultrasonic spray pyrolysis route. J. Mater. Sci. Mater. Electron. 2012, 23, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Slamovich, E.B.; Webster, T.J. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J. Biomed. Mater. Res. Part A 2007, 85A, 157–166. [Google Scholar] [CrossRef]
- Hsu, S.M.; Fares, C.; Xia, X.; Rasel, M.A.J.; Ketter, J.; Camargo, S.E.A.; Haque, M.A.; Ren, F.; Esquivel-Upshaw, J. In vitro corrosion of SiC-coated anodized Ti nano-tubular surfaces. Funct. Biomater 2021, 12, 52. [Google Scholar] [CrossRef]
- Nuss, K.M.R.; von Rechenberg, B. Biocompatibility Issues with Modern Implants in Bone-A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Iglič, A.; Kulkarni, M.; Flasker, A.; Lokar, M.; Mrak-Poljšak, K.; Mazare, A.; Artenjak, A.; Cucnik, S.; Kralj, S.; Velikonja, A.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef] [Green Version]
- Alves-Rezende, M.C.R.; Capalbo, L.C.; Limírio, J.P.J.D.O.; Capalbo, B.C.; Limírio, P.H.J.O.; Rosa, J.L. The role of TiO 2 nanotube surface on osseointegration of titanium implants: Biomechanical and histological study in rats. Microsc. Res. Tech. 2020, 83, 817–823. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, H.; Qiao, Y.; Zhang, Z.; Sun, J. Enhanced Performance of Osteoblasts by Silicon Incorporated Porous TiO2 Coating. J. Mater. Sci. Technol. 2012, 28, 109–117. [Google Scholar] [CrossRef]
- Toffoli, A.; Parisi, L.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Thermal treatment to increase titanium wettability induces selective proteins adsorption from blood serum thus affecting osteoblasts adhesion. Mater. Sci. Eng. C 2019, 107, 110250. [Google Scholar] [CrossRef]
- Ghezzi, B.; Lagonegro, P.; Pece, R.; Parisi, L.; Bianchi, M.; Tatti, R.; Verucchi, R.; Attolini, G.; Quaretti, M.; Macaluso, G.M. Osteoblast adhesion and response mediated by terminal –SH group charge surface of SiOxCy nanowires. J. Mater. Sci. Mater. Med. 2019, 30, 43. [Google Scholar] [CrossRef]
- Khan, M.; Donos, N.; Salih, V.; Brett, P. The enhanced modulation of key bone matrix components by modified Titanium implant surfaces. Bone 2012, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Elhassani, R.; Partain, J.; Hsu, S.M.; Cracium, V.; Ren, F.; Esquivel-Upshaw, J. Anneling and N2 plasma treatment to minimize corrosion of SiC-coated glass-ceramics. Materials 2020, 13, 2375. [Google Scholar] [CrossRef] [PubMed]
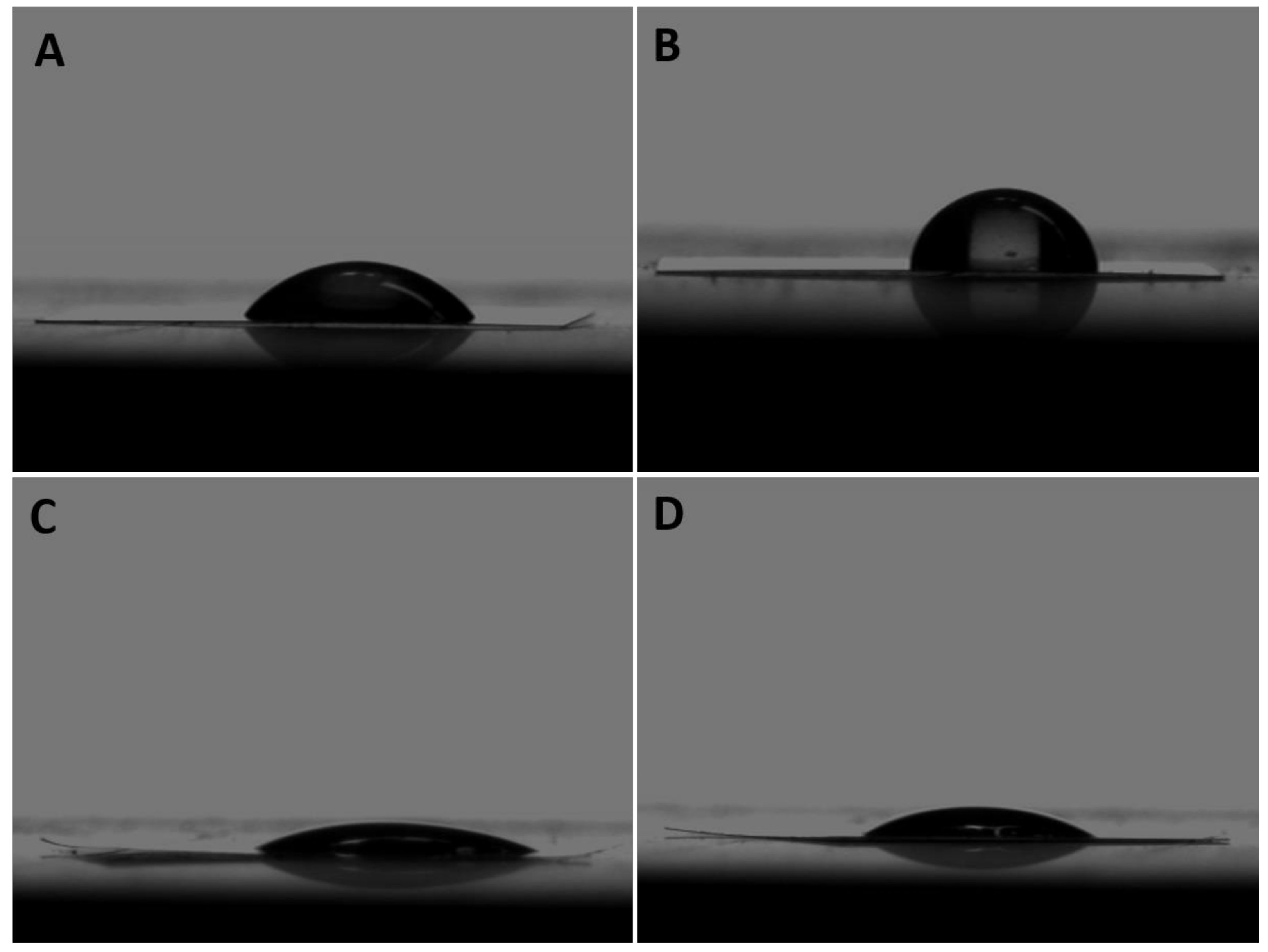




| Group | Mean Contact Angle ±SD |
|---|---|
| Non-coated 50 nm | 43.7 ± 4.6° |
| Non-coted 100 nm | 64.4 ± 0.3° |
| Coated 50 nm | 23.5 ± 3.8° |
| Coated 100 nm | 29.2 ± 4.8° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderon, P.d.S.; Rocha, F.R.G.; Xia, X.; Camargo, S.E.A.; Pascoal, A.L.d.B.; Chiu, C.-W.; Ren, F.; Ghivizzani, S.; Esquivel-Upshaw, J.F. Effect of Silicon Carbide Coating on Osteoblast Mineralization of Anodized Titanium Surfaces. J. Funct. Biomater. 2022, 13, 247. https://doi.org/10.3390/jfb13040247
Calderon PdS, Rocha FRG, Xia X, Camargo SEA, Pascoal ALdB, Chiu C-W, Ren F, Ghivizzani S, Esquivel-Upshaw JF. Effect of Silicon Carbide Coating on Osteoblast Mineralization of Anodized Titanium Surfaces. Journal of Functional Biomaterials. 2022; 13(4):247. https://doi.org/10.3390/jfb13040247
Chicago/Turabian StyleCalderon, Patricia dos Santos, Fernanda Regina Godoy Rocha, Xinyi Xia, Samira Esteves Afonso Camargo, Ana Luisa de Barros Pascoal, Chan-Wen Chiu, Fan Ren, Steve Ghivizzani, and Josephine F. Esquivel-Upshaw. 2022. "Effect of Silicon Carbide Coating on Osteoblast Mineralization of Anodized Titanium Surfaces" Journal of Functional Biomaterials 13, no. 4: 247. https://doi.org/10.3390/jfb13040247
APA StyleCalderon, P. d. S., Rocha, F. R. G., Xia, X., Camargo, S. E. A., Pascoal, A. L. d. B., Chiu, C.-W., Ren, F., Ghivizzani, S., & Esquivel-Upshaw, J. F. (2022). Effect of Silicon Carbide Coating on Osteoblast Mineralization of Anodized Titanium Surfaces. Journal of Functional Biomaterials, 13(4), 247. https://doi.org/10.3390/jfb13040247







