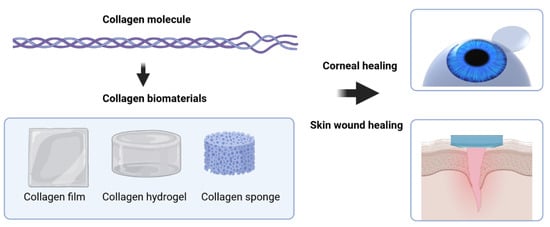
Collagen as a Biomaterial for Skin and Corneal Wound Healing
Abstract
1. Introduction
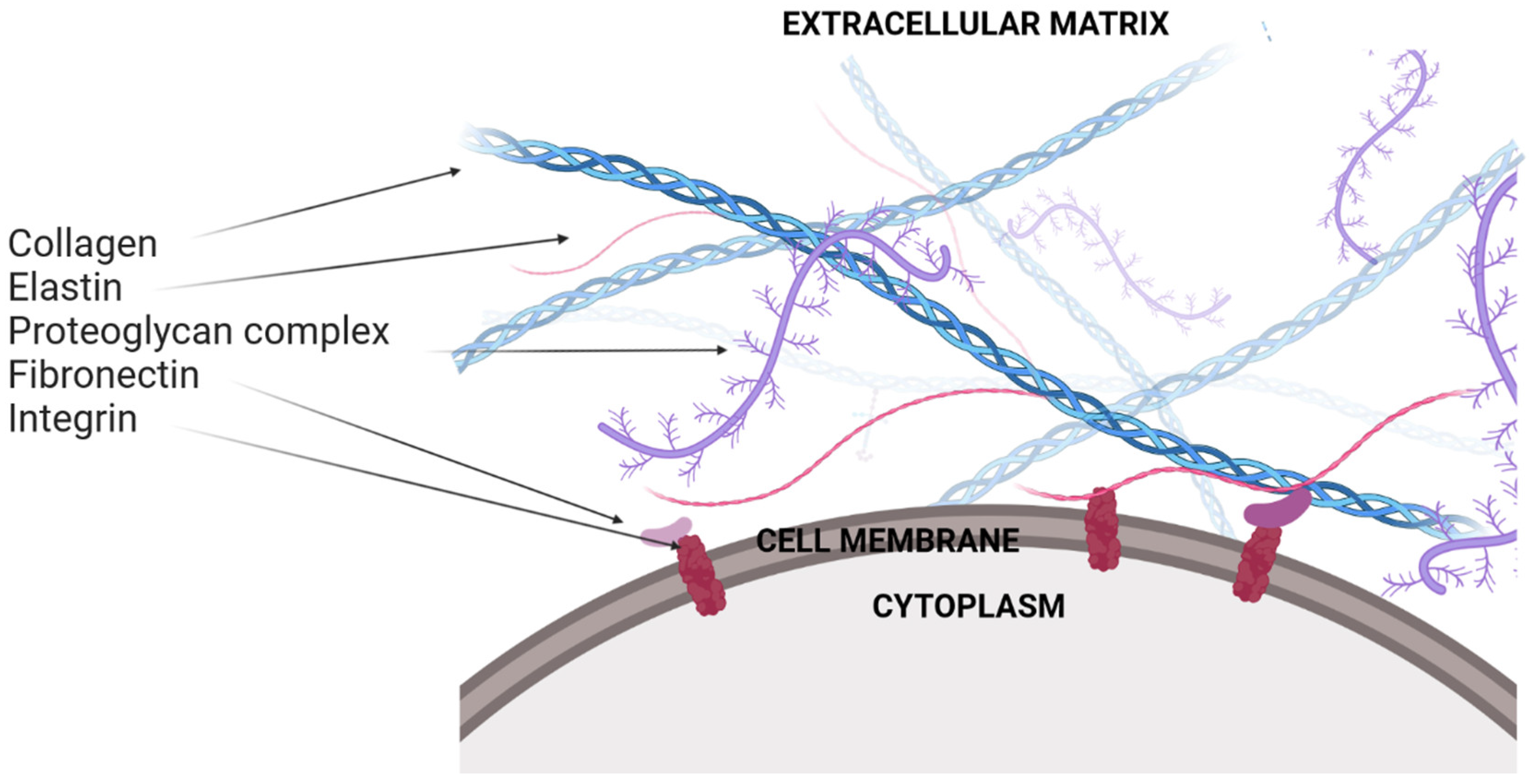
2. Extracellular Matrix
3. The Wound Healing Process
3.1. Skin Wound Healing
3.2. Corneal Wound Healing
3.2.1. Corneal Epithelial Healing
3.2.2. Corneal Stromal Healing
3.2.3. Corneal Endothelial Healing

4. Biomaterials
5. Collagen Biomaterials for Wound Healing
5.1. Collagen Sponges
5.2. Hydrogels
5.3. Other Applications: Films and Membranes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Son, Y.J.; Tse, J.W.; Zhou, Y.; Mao, W.; Yim, E.K.F.; Yoo, H.S. Biomaterials and controlled release strategy for epithelial wound healing. Biomater. Sci. 2019, 7, 4444–4471. [Google Scholar] [CrossRef] [PubMed]
- Netto, M.V.; Mohan, R.R.; Ambrósio, R.; Hutcheon, A.E.K.; Zieske, J.; Wilson, S. Wound Healing in the Cornea. Cornea 2005, 24, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Li, Y.; Jeong, J.; Song, W. Molecular Characteristics and Distribution of Adult Human Corneal Immune Cell Types. Front. Immunol. 2022, 13, 798346. [Google Scholar] [CrossRef]
- Nour, S.; Baheiraei, N.; Imani, R.; Khodaei, M.; Alizadeh, A.; Rabiee, N.; Moazzeni, S.M. A review of accelerated wound healing approaches: Biomaterial- assisted tissue remodeling. J. Mater. Sci. Mater. Med. 2019, 30, 120. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Kananavičiūtė, R.; Kvederavičiūtė, K.; Dabkevičienė, D.; Mackevičius, G.; Kuisienė, N. Collagen-like sequences encoded by extremophilic and extremotolerant bacteria. Genomics 2019, 112, 2271–2281. [Google Scholar] [CrossRef]
- Yamauchi, M.; Taga, Y.; Hattori, S.; Shiiba, M.; Terajima, M. Methods in Cell Biology. In Analysis of Collagen and Elastin Cross-Links; Academic Press: Cambridge, MA, USA, 2018; Volume 143, pp. 115–132. [Google Scholar] [CrossRef]
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A.M. Biomedical applications of collagens. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 665–675. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Werkmeister, J.A.; Dumsday, G.J. Bioengineered collagens. Bioengineered 2014, 5, 227–233. [Google Scholar] [CrossRef]
- Yu, X.; Tang, C.; Xiong, S.; Yuan, Q.; Gu, Z.P.; Li, Z.; Hu, Y. Modification of Collagen for Biomedical Applications: A Review of Physical and Chemical Methods. Curr. Org. Chem. 2016, 20, 1797–1812. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.-E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay between Extracellular Matrix and Cells in Tissues; Springer Nature: Berlin, Germany, 2019; Volume 1952, p. 485. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Karamanos, N.K. Extracellular matrix: Key structural and functional meshwork in health and disease. FEBS J. 2019, 286, 2826–2829. [Google Scholar] [CrossRef]
- Vindin, H.; Mithieux, S.M.; Weiss, A.S. Elastin architecture. Matrix Biol. 2019, 84, 4–16. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2019, 56, 50–55. [Google Scholar] [CrossRef]
- Sabatier, L.; Chen, D.; Fagotto-Kaufmann, C.; Hubmacher, D.; McKee, M.D.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Fibrillin Assembly Requires Fibronectin. Mol. Biol. Cell 2009, 20, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Köwitsch, A.; Zhou, G.; Groth, T. Medical application of glycosaminoglycans: A review. J. Tissue Eng. Regen. Med. 2017, 12, e23–e41. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef]
- Pankov, R.; Yamada, K.M. Fibronectin at a glance. J. Cell Sci. 2002, 115, 3861–3863. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018, 75–76, 12–26. [Google Scholar] [CrossRef]
- Dhavalikar, P.; Robinson, A.; Lan, Z.; Jenkins, D.; Chwatko, M.; Salhadar, K.; Jose, A.; Kar, R.; Shoga, E.; Kannapiran, A.; et al. Review of Integrin-Targeting Biomaterials in Tissue Engineering. Adv. Health Mater. 2020, 9, 2000795. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2020, 89, 1619–1626. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection–a glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Heino, J. The collagen family members as cell adhesion proteins. BioEssays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Lorenzo-Martín, E.; Gallego-Muñoz, P.; Mar, S.; Fernández, I.; Cidad, P.; Martínez-García, M.C. Dynamic changes of the extracellular matrix during corneal wound healing. Exp. Eye Res. 2019, 186, 107704. [Google Scholar] [CrossRef]
- Torricelli, A.A.M.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The Corneal Epithelial Basement Membrane: Structure, Function, and Disease. Investig. Opthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef]
- Coupry, I.; Sibon, I.; Mortemousque, B.; Rouanet, F.; Miné, M.; Goizet, C. Ophthalmological Features Associated With COL4A1 Mutations. Arch. Ophthalmol. 2010, 128, 483–489. [Google Scholar] [CrossRef]
- Wiegand, C.; Schönfelder, U.; Abel, M.; Ruth, P.; Kaatz, M.; Hipler, U.-C. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch. Dermatol. Res. 2009, 302, 419–428. [Google Scholar] [CrossRef]
- Metzmacher, I.; Ruth, P.; Abel, M.; Friess, W. In vitro binding of matrix metalloproteinase-2 (MMP-2), MMP-9, and bacterial collagenase on collagenous wound dressings. Wound Repair Regen. 2007, 15, 549–555. [Google Scholar] [CrossRef]
- Schönfelder, U.; Abel, M.; Wiegand, C.; Klemm, D.; Elsner, P.; Hipler, U.-C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials 2005, 26, 6664–6673. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Svobodová, A.R. Effect of UVA radiation on the Nrf2 signalling pathway in human skin cells. J. Photochem. Photobiol. B Biol. 2020, 209, 111948. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sarveswaran, K.; Kurz, V.; Dong, Z.; Tanaka, T.; Penny, S.; Timp, G. Synthetic Capillaries to Control Microscopic Blood Flow. Sci. Rep. 2016, 6, 21885. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Mosier, D. Chapter 2-Vascular Disorders and Thrombosis1. In Pathologic Basis of Veterinary Disease, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 44–72.e1. [Google Scholar]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; De Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef]
- Schultz, G.S.; Davidson, J.M.; Kirsner, R.S.; Bornstein, P.; Herman, I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011, 19, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Demling, R. Nutrition, anabolism, and the wound healing process: An overview. Eplasty 2009, 9, e9. [Google Scholar]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Revilla, G.; Darwin, E.; Rantam, F. Effect of Allogeneic Bone Marrow-mesenchymal Stem Cells (BM-MSCs) to Accelerate Burn Healing of Rat on the Expression of Collagen Type I and Integrin α2β1. Pak. J. Biol. Sci. 2016, 19, 345–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Twardowski, T.; Fertala, A.; Orgel, J.; Antonio, J.S. Type I Collagen and Collagen Mimetics as Angiogenesis Promoting Superpolymers. Curr. Pharm. Des. 2007, 13, 3608–3621. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Fibroblast—Extracellular Matrix Interactions in Tissue Fibrosis. Curr. Pathobiol. Rep. 2016, 4, 11–18. [Google Scholar] [CrossRef]
- Ramasastry, S.S. Acute Wounds. Clin. Plast. Surg. 2005, 32, 195–208. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Reinke, J.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Moreno, A.; Baudouin, C.; Parsadaniantz, S.M.; Goazigo, A.R.-L. Morphological and Functional Changes of Corneal Nerves and Their Contribution to Peripheral and Central Sensory Abnormalities. Front. Cell. Neurosci. 2020, 14, 610342. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Azimzade, Y.; Hong, J.; Mashaghi, A. Immunophysical analysis of corneal neovascularization: Mechanistic insights and implications for pharmacotherapy. Sci. Rep. 2017, 7, 12220. [Google Scholar] [CrossRef] [PubMed]
- Clahsen, T.; Büttner, C.; Hatami, N.; Reis, A.; Cursiefen, C. Role of Endogenous Regulators of Hem- And Lymphangiogenesis in Corneal Transplantation. J. Clin. Med. 2020, 9, 479. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The Thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef]
- Dawson, D.W.; Volpert, O.V.; Gillis, P.; Crawford, S.E.; Xu, H.-J.; Benedict, W.; Bouck, N.P. Pigment Epithelium-Derived Factor: A Potent Inhibitor of Angiogenesis. Science 1999, 285, 245–248. [Google Scholar] [CrossRef]
- Mukwaya, A.; Jensen, L.; Lagali, N. Relapse of pathological angiogenesis: Functional role of the basement membrane and potential treatment strategies. Exp. Mol. Med. 2021, 53, 189–201. [Google Scholar] [CrossRef]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.-H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-H.; Huang, Y.-H.; Cunningham, C.M.; Han, K.-Y.; Chang, M.; Seiki, M.; Zhou, Z.; Azar, D.T. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv. Ophthalmol. 2015, 61, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Romanian J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A. Host immune cellular reactions in corneal neovascularization. Int. J. Ophthalmol. 2016, 9, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hadrian, K.; Willenborg, S.; Bock, F.; Cursiefen, C.; Eming, S.A.; Hos, D. Macrophage-Mediated Tissue Vascularization: Similarities and Differences Between Cornea and Skin. Front. Immunol. 2021, 12, 667830. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal Neovascularization: An Anti-VEGF Therapy Review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Shahriary, A.; Sabzevari, M.; Jadidi, K.; Yazdani, F.; Aghamollaei, H. The Role of Inflammatory Cytokines in Neovascularization of Chemical Ocular Injury. Ocul. Immunol. Inflamm. 2021, 30, 1149–1161. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, S.-M.; Lee, D.-I. Corneal Lymphangiogenesis: Current Pathophysiological Understandings and Its Functional Role in Ocular Surface Disease. Int. J. Mol. Sci. 2021, 22, 11628. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Atyabi, F.; Mojtabavi, N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology 2017, 24, 123–131. [Google Scholar] [CrossRef]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef]
- Hanna, C.; O’Brien, J.E. Cell Production and Migration in the Epithelial Layer of the Cornea. Arch. Ophthalmol. 1960, 64, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrósio, R.; Hong, J.; Lee, J. The Corneal Wound Healing Response: Cytokine-mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Lu, L.; Reinach, P.S.; Kao, W.W.-Y. Corneal Epithelial Wound Healing. Exp. Biol. Med. 2001, 226, 653–664. [Google Scholar] [CrossRef]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef]
- Sugioka, K.; Fukuda, K.; Nishida, T.; Kusaka, S. The fibrinolytic system in the cornea: A key regulator of corneal wound healing and biological defense. Exp. Eye Res. 2021, 204, 108459. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekher, G.; Ma, X.; Lallier, T.; Bazan, H. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet-activating factor. Investig. Ophthalmol. V. Sci. 2002, 43, 1422–1428. [Google Scholar]
- Wilson, S.E. Fibrosis Is a Basement Membrane-Related Disease in the Cornea: Injury and Defective Regeneration of Basement Membranes May Underlie Fibrosis in Other Organs. Cells 2022, 11, 309. [Google Scholar] [CrossRef]
- Baratta, R.O.; Schlumpf, E.; Del Buono, B.J.; DeLorey, S.S.; Calkins, D.J. Corneal collagen as a potential therapeutic target in dry eye disease. Surv. Ophthalmol. 2021, 67, 60–67. [Google Scholar] [CrossRef]
- Wilson, S.E. Bowman’s layer in the cornea– structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef]
- Alberto, D.; Garello, R. Corneal Sublayers Thickness Estimation Obtained by High-Resolution FD-OCT. Int. J. Biomed. Imaging 2013, 2013, 11. [Google Scholar] [CrossRef]
- Wilson, S.E. Interleukin-1 and Transforming Growth Factor Beta: Commonly Opposing, but Sometimes Supporting, Master Regulators of the Corneal Wound Healing Response to Injury. Investig. Opthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.D. Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv. Ophthalmol. 1997, 41, 275–313. [Google Scholar] [CrossRef]
- Hong, J.; Liu, J.; Lee, J.; Mohan, R.; Mohan, R.; Woods, D.; He, Y.; Wilson, S. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest. Ophthalmol. Vis. Sci. 2001, 42, 2795–2803. [Google Scholar] [PubMed]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2012, 229, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Lewis, P.; Islam, M.M.; Doutch, J.; Sorensen, T.; White, T.; Griffith, M.; Meek, K.M. The structural and optical properties of type III human collagen biosynthetic corneal substitutes. Acta Biomater. 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Massoudi, D.; Malecaze, F.; Galiacy, S.D. Collagens and proteoglycans of the cornea: Importance in transparency and visual disorders. Cell Tissue Res. 2015, 363, 337–349. [Google Scholar] [CrossRef]
- Ishizaki, M.; Shimoda, M.; Wakamatsu, K.; Ogro, T.; Yamanaka, N.; Kao, C.W.-C.; Kao, W.W.-Y. Stromal fibroblasts are associated with collagen IV in scar tissues of alkali-burned and lacerated corneas. Curr. Eye Res. 1997, 16, 339–348. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mohan, R.R. Autophagy in Extracellular Matrix and Wound Healing Modulation in the Cornea. Biomedicines 2022, 10, 339. [Google Scholar] [CrossRef]
- Chameettachal, S.; Prasad, D.; Parekh, Y.; Basu, S.; Singh, V.; Bokara, K.K.; Pati, F. Prevention of Corneal Myofibroblastic Differentiation In Vitro Using a Biomimetic ECM Hydrogel for Corneal Tissue Regeneration. ACS Appl. Bio Mater. 2020, 4, 533–544. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef]
- Hussain, N.A.; Figueiredo, F.C.; Connon, C.J. Use of biomaterials in corneal endothelial repair. Ther. Adv. Ophthalmol. 2021, 13, 25158414211058249. [Google Scholar] [CrossRef] [PubMed]
- Kocluk, Y.; Burcu, A.; Sukgen, E.A. Demonstration of cornea Dua’s layer at a deep anterior lamellar keratoplasty surgery. Oman J. Ophthalmol. 2016, 9, 179–181. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s membrane development, structure, function and regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Overmass, M.; Fan, J.; Hodge, C.; Sutton, G.; Lovicu, F.J.; You, J. Application of Collagen I and IV in Bioengineering Transparent Ocular Tissues. Front. Surg. 2021, 8, 639500. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, H.; Miron, A.; Oellerich, S.; Melles, G.R.; Dhubhghaill, S.N.; Koppen, C.; Bogerd, B.V.D. Corneal endothelial wound healing: Understanding the regenerative capacity of the innermost layer of the cornea. Transl. Res. 2022, 248, 111–127. [Google Scholar] [CrossRef]
- Miyamoto, T.; Sumioka, T.; Saika, S. Endothelial Mesenchymal Transition: A Therapeutic Target in Retrocorneal Membrane. Cornea 2010, 29, S52–S56. [Google Scholar] [CrossRef]
- Ishizaki, M.; Zhu, G.; Haseba, T.; Shafer, S.; Kao, W. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest. Ophthalmol. Vis. Sci. 1993, 32, 3320–3328. [Google Scholar]
- Tartaglia, G.; Cao, Q.; Padron, Z.; South, A. Impaired Wound Healing, Fibrosis, and Cancer: The Paradigm of Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2021, 22, 5104. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Naomi, R.; Bahari, H.; Ridzuan, P.; Othman, F. Natural-Based Biomaterial for Skin Wound Healing (Gelatin vs. Collagen): Expert Review. Polymers 2021, 13, 2319. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2019, 103, 24–51. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.; Roy, S.; Sen, C. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.A.T.; Almeida, M.C.; Avanzi, I.; Parisi, J.; Sales, A.F.S.; Na, Y.; Renno, A. Collagen membranes for skin wound repair: A systematic review. J. Biomater. Appl. 2020, 36, 95–112. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Best of Both Hydrogel Worlds: Harnessing Bioactivity and Tunability by Incorporating Glycosaminoglycans in Collagen Hydrogels. Bioengineering 2020, 7, 156. [Google Scholar] [CrossRef]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2021, 290, 120096. [Google Scholar] [CrossRef]
- Cziperle, D. Avitene™ Microfibrillar Collagen Hemostat for Adjunctive Hemostasis in Surgical Procedures: A Systematic Literature Review. Med. Dev. 2021, 14, 155–163. [Google Scholar] [CrossRef]
- Schimmer, C.; Gross, J.; Ramm, E.; Morfeld, B.-C.; Hoffmann, G.; Panholzer, B.; Hedderich, J.; Leyh, R.; Cremer, J.; Petzina, R. Prevention of surgical site sternal infections in cardiac surgery: A two-centre prospective randomized controlled study. Eur. J. Cardio-Thoracic Surg. 2016, 51, 67–72. [Google Scholar] [CrossRef]
- Jones, K.; Williams, C.; Yuan, T.; Bs, A.M.D.-F.; Bs, R.C.W.; Burton, T.; Hamlin, N.; Martinez, L. Comparative in vitro study of commercially available products for alveolar ridge preservation. J. Periodontol. 2021, 93, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.L.K.; Shelat, V.G.; Low, W.; George, S.; Rao, J. The Use of Collatamp G, Local Gentamicin-Collagen Sponge, in Reducing Wound Infection. Int. Surg. 2014, 99, 565–570. [Google Scholar] [CrossRef]
- Santhanam, R.; Rameli, M.A.P.; Al Jeffri, A.; Ismail, W.I.W. Bovine Based Collagen Dressings in Wound Care Management. J. Pharm. Res. Int. 2020, 32, 48–63. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; de Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef]
- Karr, J.C.; Taddei, A.R.; Picchietti, S.; Gambellini, G.; Fausto, A.M.; Giorgi, F. A Morphological and Biochemical Analysis Comparative Study of the Collagen Products Biopad, Promogram, Puracol, and Colactive. Adv. Ski. Wound Care 2011, 24, 208–216. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M. Current Update of Collagen Nanomaterials—Fabrication, Characterisation and Its Applications: A Review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Kaur, J. Osteo-odonto keratoprosthesis: Innovative dental and ophthalmic blending. J. Indian Prosthodont. Soc. 2018, 18, 89–95. [Google Scholar] [CrossRef]
- Matthyssen, S.; Van den Bogerd, B.; Dhubhghaill, S.N.; Koppen, C.; Zakaria, N. Corneal regeneration: A review of stromal replacements. Acta Biomater. 2018, 69, 31–41. [Google Scholar] [CrossRef]
- Polisetti, N.; Islam, M.M.; Griffith, M. The Artificial Cornea. Corneal Regen. Med. 2013, 1014, 45–52. [Google Scholar] [CrossRef]
- Simpson, F.C.; McTiernan, C.D.; Islam, M.M.; Buznyk, O.; Lewis, P.N.; Meek, K.M.; Haagdorens, M.; Audiger, C.; Lesage, S.; Gueriot, F.-X.; et al. Collagen analogs with phosphorylcholine are inflammation-suppressing scaffolds for corneal regeneration from alkali burns in mini-pigs. Commun. Biol. 2021, 4, 608. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yan, Y.; Ji, Q.; Dai, Y.; Jin, S.; Liu, Y.; Chen, J.; Teng, L. A Sponge-Like Double-Layer Wound Dressing with Chitosan and Decellularized Bovine Amniotic Membrane for Promoting Diabetic Wound Healing. Polymers 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Si, Y.; Wang, X.; Deng, H.; Sheng, Z.; Li, Y.; Liu, J.; Zhao, J. A novel gene recombinant collagen hemostatic sponge with excellent biocompatibility and hemostatic effect. Int. J. Biol. Macromol. 2021, 178, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J. Mater. Sci. Mater. Med. 2021, 32, 12. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Christianson, D.J.; Hansbrough, J.F. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J. Biomed. Mater. Res. 1988, 22, 939–957. [Google Scholar] [CrossRef]
- Pozzolini, M.; Gallus, L.; Ghignone, S.; Ferrando, S.; Candiani, S.; Bozzo, M.; Bertolino, M.; Costa, G.; Bavestrello, G.; Scarfì, S. Insights into the evolution of metazoan regenerative mechanisms: TGF superfamily member roles in tissue regeneration of the marine sponge Chondrosia reniformis Nardo, 1847. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef]
- Chang, P.; Guo, B.; Hui, Q.; Liu, X.; Tao, K. A bioartificial dermal regeneration template promotes skin cell proliferation in vitro and enhances large skin wound healing in vivo. Oncotarget 2017, 8, 25226–25241. [Google Scholar] [CrossRef][Green Version]
- Jinno, C.; Morimoto, N.; Ito, R.; Sakamoto, M.; Ogino, S.; Taira, T.; Suzuki, S. A Comparison of Conventional Collagen Sponge and Collagen-Gelatin Sponge in Wound Healing. BioMed Res. Int. 2016, 2016, 4567146. [Google Scholar] [CrossRef]
- Borene, M.L.; Barocas, V.H.; Hubel, A. Mechanical and cellular changes during compaction of a collagen-sponge-based corneal stromal equivalent. Ann. Biomed. Eng. 2004, 32, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Orwin, E.J.; Hubel, A. In Vitro Culture Characteristics of Corneal Epithelial, Endothelial, and Keratocyte Cells in a Native Collagen Matrix. Tissue Eng. 2000, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Mude, L.; Sanapalli, B.K.R.; Narayanan, A.; Singh, S.K.; Karri, V.V.S.R. Overview of in situ gelling injectable hydrogels for diabetic wounds. Drug Dev. Res. 2021, 82, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Bonnesœur, S.; Morin-Grognet, S.; Thoumire, O.; Le Cerf, D.; Boyer, O.; Vannier, J.; Labat, B. Hyaluronan-based hydrogels as versatile tumor-like models: Tunable ECM and stiffness with genipin-crosslinking. J. Biomed. Mater. Res. Part A 2020, 108, 1256–1268. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Tripathi, D.; Sharma, A.; Tyagi, P.; Beniwal, C.S.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Fabrication of Three-Dimensional Bioactive Composite Scaffolds for Hemostasis and Wound Healing. AAPS PharmSciTech 2021, 22, 138. [Google Scholar] [CrossRef]
- Liu, L.; Wen, H.; Rao, Z.; Zhu, C.; Liu, M.; Min, L.; Fan, L.; Tao, S. Preparation and characterization of chitosan–collagen peptide/oxidized konjac glucomannan hydrogel. Int. J. Biol. Macromol. 2018, 108, 376–382. [Google Scholar] [CrossRef]
- Tripathi, D.; Rastogi, K.; Tyagi, P.; Rawat, H.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Comparative Analysis of Collagen and Chitosan-based Dressing for Haemostatic and Wound Healing Application. AAPS PharmSciTech 2021, 22, 76. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a recombinant collagen-peptide (RHC)-conjugated chitosan thermosensitive hydrogel for wound healing. Mater. Sci. Eng. C 2020, 119, 111555. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Dearman, B.L.; Boyce, S.T.; Greenwood, J.E. Advances in Skin Tissue Bioengineering and the Challenges of Clinical Translation. Front. Surg. 2021, 8, 640879. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef]
- McCoy, M.G.; Seo, B.R.; Choi, S.; Fischbach, C. Collagen I hydrogel microstructure and composition conjointly regulate vascular network formation. Acta Biomater. 2016, 44, 200–208. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen–gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2018, 126, 620–632. [Google Scholar] [CrossRef]
- Chen, Z.; You, J.; Liu, X.; Cooper, S.; Hodge, C.; Sutton, G.; Crook, J.M.; Wallace, G.G. Biomaterials for corneal bioengineering. Biomed. Mater. 2018, 13, 032002. [Google Scholar] [CrossRef]
- Jangamreddy, J.R.; Haagdorens, M.K.; Islam, M.M.; Lewis, P.; Samanta, A.; Fagerholm, P.; Liszka, A.; Ljunggren, M.K.; Buznyk, O.; Alarcon, E.I.; et al. Short peptide analogs as alternatives to collagen in pro-regenerative corneal implants. Acta Biomater. 2018, 69, 120–130. [Google Scholar] [CrossRef]
- Islam, M.M.; Ravichandran, R.; Olsen, D.; Ljunggren, M.K.; Fagerholm, P.; Lee, C.J.; Griffith, M.; Phopase, J. Self-assembled collagen-like-peptide implants as alternatives to human donor corneal transplantation. RSC Adv. 2016, 6, 55745–55749. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef] [PubMed]
- Na, K.-S.; Fernandes-Cunha, G.M.; Varela, I.B.; Lee, H.J.; Seo, Y.A.; Myung, D. Effect of mesenchymal stromal cells encapsulated within polyethylene glycol-collagen hydrogels formed in situ on alkali-burned corneas in an ex vivo organ culture model. Cytotherapy 2021, 23, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Cėpla, V.; He, C.; Edin, J.; Rakickas, T.; Kobuch, K.; Ruželė, Z.; Jackson, W.B.; Rafat, M.; Lohmann, C.P.; et al. Functional fabrication of recombinant human collagen–phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015, 12, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.; Richards, C.; Walker, T. Allergic reactions to oral, surgical and topical bovine collagen: Anaphylactic risk for surgeons. Aust. New Zealand J. Ophthalmol. 1996, 24, 257–260. [Google Scholar] [CrossRef]
- Pekar, J.E.; Magee, A.; Parker, E.; Moshiri, N.; Izhikevich, K.; Havens, J.L.; Gangavarapu, K.; Serrano, L.M.M.; Crits-Christoph, A.; Matteson, N.L.; et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science 2022, 377, 960–966. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Mazur, O. Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials 2020, 13, 3641. [Google Scholar] [CrossRef]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymers 2019, 11, 2094. [Google Scholar] [CrossRef]
- Socrates, R.; Prymak, O.; Loza, K.; Sakthivel, N.; Rajaram, A.; Epple, M.; Kalkura, S.N. Biomimetic fabrication of mineralized composite films of nanosilver loaded native fibrillar collagen and chitosan. Mater. Sci. Eng. C 2019, 99, 357–366. [Google Scholar] [CrossRef]

| Wound Dressing Materials/Collagen Form | Product | References |
|---|---|---|
| Collagen sponges | Avitene™ Ultrafoam™ Collagen Sponge | [122,123] |
| GENTA-COLL® resorb | [124] | |
| Helistat® | [122,125] | |
| Microfibrillar Dressing Instat™ Mch Collagen | [122] | |
| Collatamp®-G | [126,127] | |
| Collagen films and membranes | SkinTemp® II | [126,128] |
| Fibracol® | [128,129,130] | |
| Promogran™/Promogran Prisma® | [126,128,130,131] | |
| CollaSorb® | [129,130] | |
| BIOPAD™ | [131] | |
| Puracol® Plus/Puracol® Plus Ag | [128,131] | |
| ColActive® Plus/ColActive® Plus Ag | [131] | |
| DermaCol™ | [128] | |
| Collagen hydrogels | HYCOL® | [128] |
| Collatek® | [128] | |
| CellerateRX® | [128,129] |
| Wound Dressing Materials/Collagen Form | Non-Commercial Product | References |
|---|---|---|
| Collagen sponges | Collagen sponge form jellyfish | [138] |
| Recombinant collagen hemostatic sponge | [139] | |
| Collagen sponge form porcine, bovine and human skin | [140] | |
| Platelet-rich plasma–collagen sponge | [143] | |
| Carboxymethyl chitosan–collagen peptides sponge | [144] | |
| Collagen I sponge | [145,146] | |
| Collagen hydrogels | Collagen–hyaluronan hydrogels | [150,151] |
| Collagen–chitosan hydrogels | [152,153,154,155,156] | |
| Collagen hydrogels with incorporated cells | [157,158] | |
| Collagen hydrogels (type I and III) | [159,160,161,162,163] | |
| Collagen-like peptides hydrogels | [136,164,165,166,167] | |
| Recombinant human collagen type III hydrogels | [168] | |
| Collagen hydrogels (form porcine) | [169] | |
| Collagen films and membranes | Collagen membranes | [119,171] |
| Collagen–chitosan films | [172,173,174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklenářová, R.; Akla, N.; Latorre, M.J.; Ulrichová, J.; Franková, J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. J. Funct. Biomater. 2022, 13, 249. https://doi.org/10.3390/jfb13040249
Sklenářová R, Akla N, Latorre MJ, Ulrichová J, Franková J. Collagen as a Biomaterial for Skin and Corneal Wound Healing. Journal of Functional Biomaterials. 2022; 13(4):249. https://doi.org/10.3390/jfb13040249
Chicago/Turabian StyleSklenářová, Renáta, Naoufal Akla, Meagan Jade Latorre, Jitka Ulrichová, and Jana Franková. 2022. "Collagen as a Biomaterial for Skin and Corneal Wound Healing" Journal of Functional Biomaterials 13, no. 4: 249. https://doi.org/10.3390/jfb13040249
APA StyleSklenářová, R., Akla, N., Latorre, M. J., Ulrichová, J., & Franková, J. (2022). Collagen as a Biomaterial for Skin and Corneal Wound Healing. Journal of Functional Biomaterials, 13(4), 249. https://doi.org/10.3390/jfb13040249