Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential
Abstract
1. Introduction
2. Materials and Methods
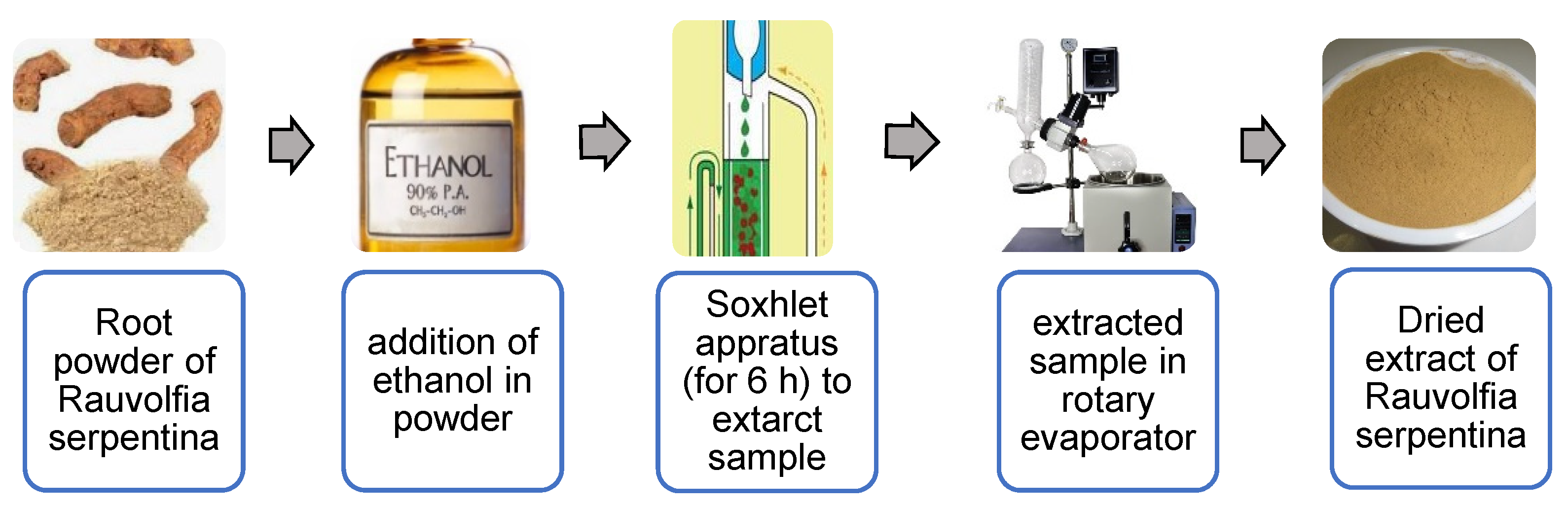
2.1. Extraction of Plant and Preparation of Nanosuspension
2.2. Charecterization of Nanosuspension
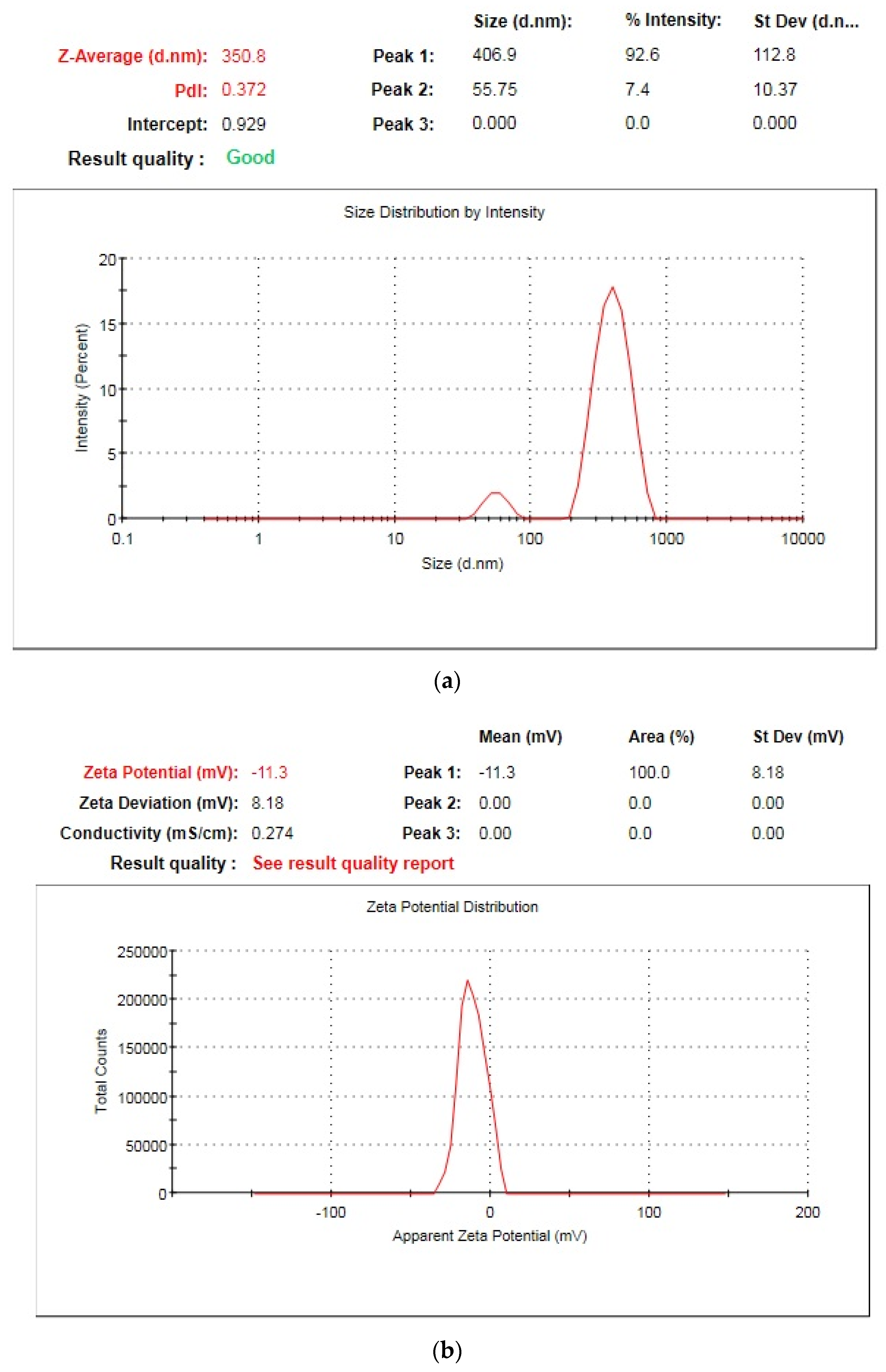
2.2.1. Particle Size and Zeta Potential
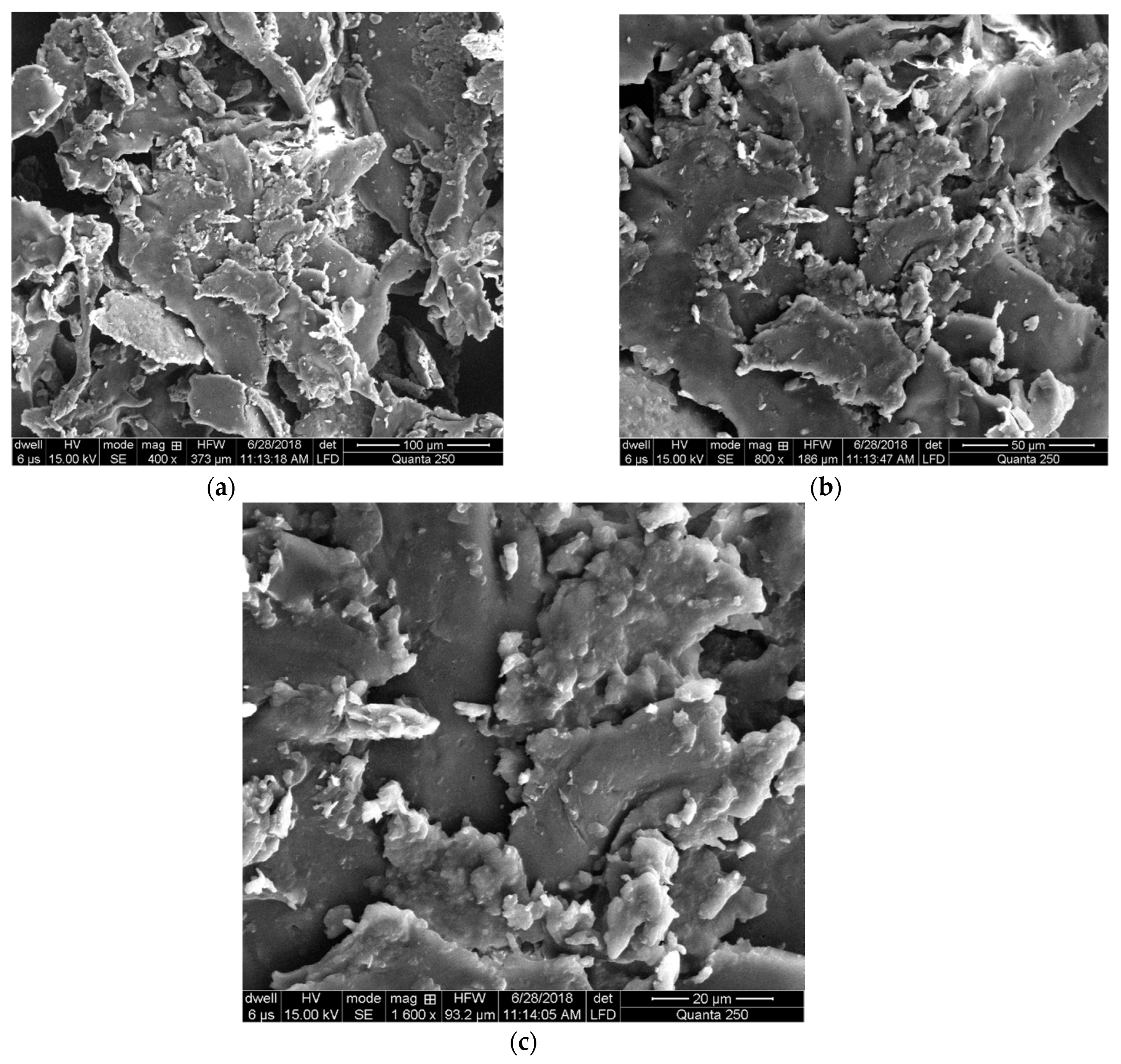
2.2.2. Scanning Electron Microscopy
2.3. Optimization of Rauvolfia serpentina Nanosuspension
2.4. In Vitro Biological Activities
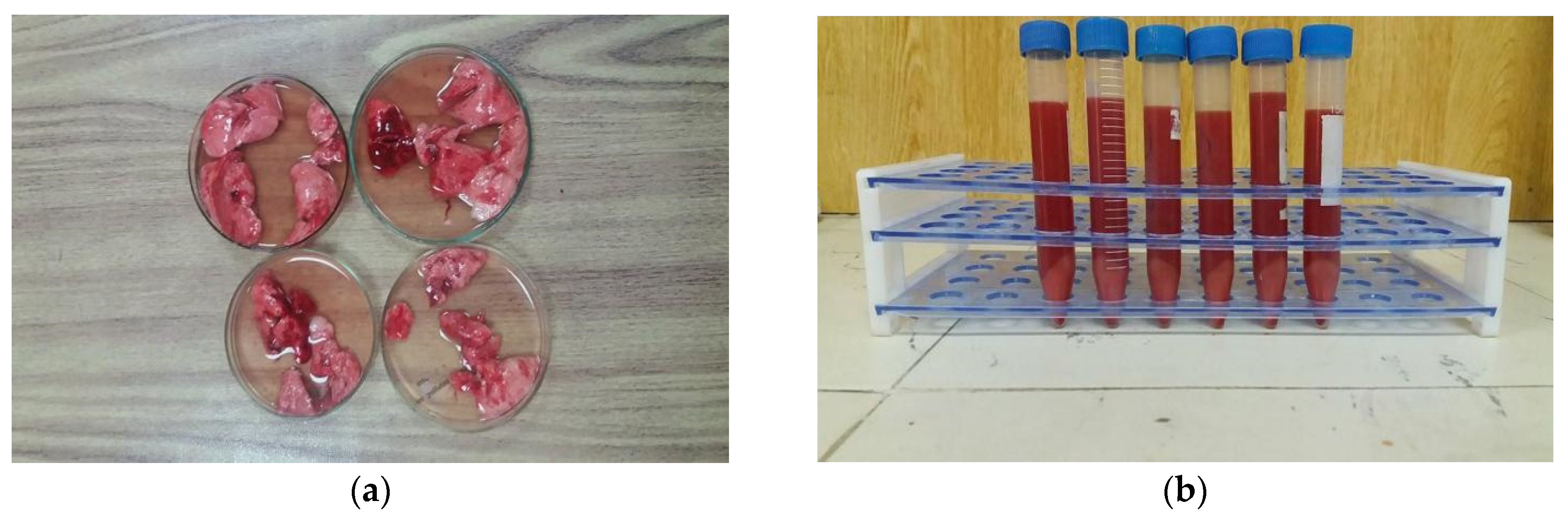
2.4.1. ACE Assay
2.4.2. Hemolytic Activity
3. Results and Discussion
3.1. Analysis of Data and Optimization
3.2. Effect of Independent Factors on Particle Size, Zeta Potential, and PdI
3.3. Scanning Electron Microscopy
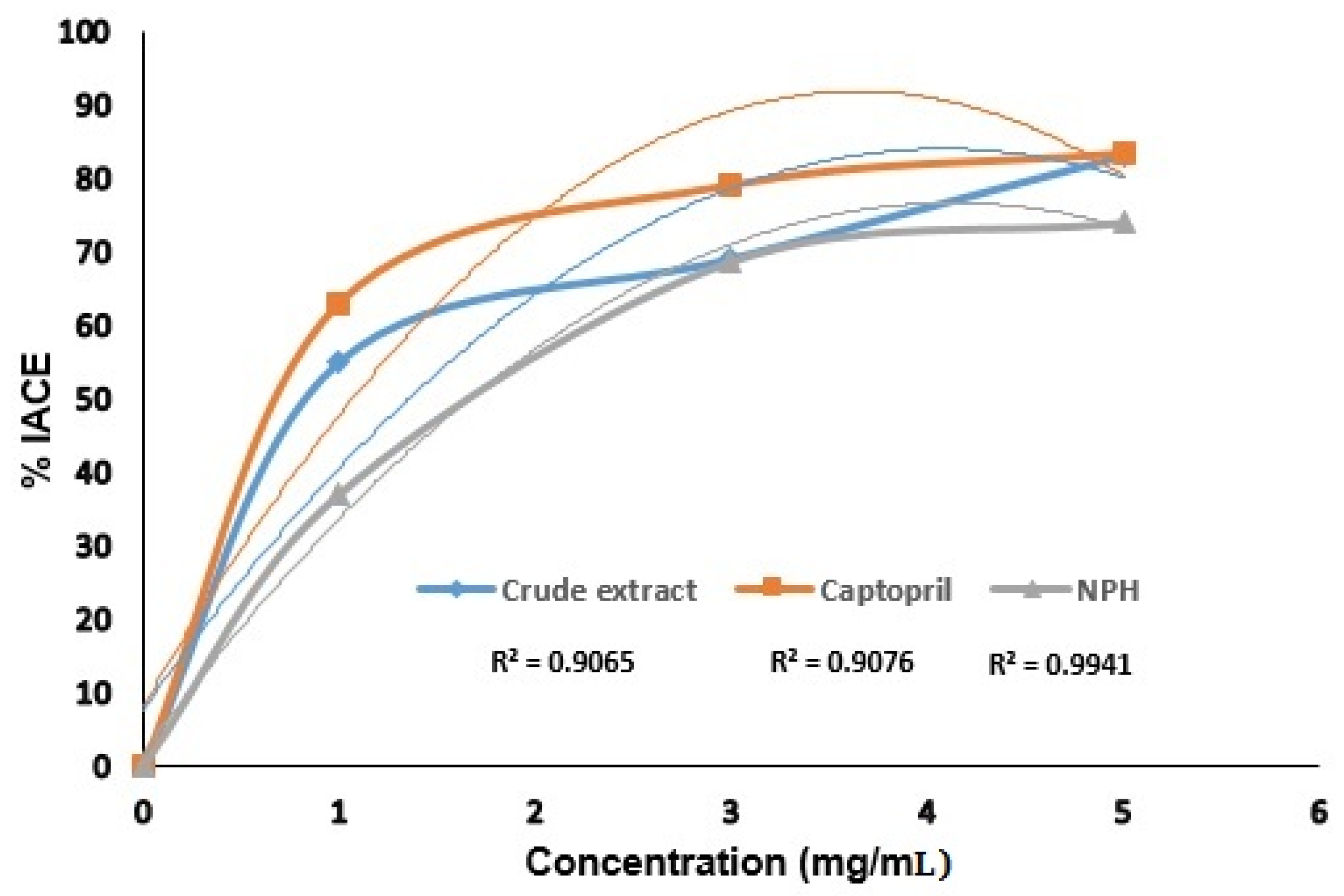
3.4. ACE Inhibitory Assay
3.5. Hemolytic Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ACE | Angiotensin converting enzymes |
| HPMC | Hydroxyl propyl methyl cellulose |
| SEM | Scanning electron microscopy |
| RAAS | Renin–angiotensin–aldosterone system |
| DLS | Dynamic light scattering zetasizer |
| DOE | Design of expert |
| RSM | Response surface methodology |
| NPH | Nanosuspension prepared with HPMC |
| ACEI | Angiotensin converting enzymes inhibition |
| PdI | Polydispersity index |
References
- Yu, J.; Zhang, S.; Zhang, L. Amadori compounds as potent inhibitors of angiotensin-converting enzyme (ACE) and their effects on anti-ACE activity of bell peppers. J. Funct. Foods 2016, 27, 622–630. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Zhang, Y.; Ruan, X.; Zhang, R. Purification, characterization, synthesis, in vitro ACE inhibition and in vivo antihypertensive activity of bioactive peptides derived from oil palm kernel glutelin-2 hydrolysates. J. Funct. Foods 2017, 28, 48–58. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT-Food Sci. Technol. 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; Bennett, L.E. Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Crit. Rev. Food Sci. Nutr. 2016, 56, 181–214. [Google Scholar] [CrossRef]
- Dong, J.; Xu, X.; Liang, Y.; Head, R.; Bennett, L. Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011, 2, 310–319. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Thome, G.R.; Morsch, V.M.; Stefanello, N.; Goularte, G.F.; Oboh, A.B.G.; Schetinger, M.R.C. Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in L-NAME induced hypertensive rats. J. Funct. Foods 2015, 17, 792–801. [Google Scholar] [CrossRef]
- Balasuriya, N.; Rupasinghe, H.P.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Q.; Liu, H.J.; Li, S.Z.; Jiang, Z.O. Characterization of actinidin from Chinese kiwifruit cultivars and its applications in meat tenderization and production of angiotensin I-converting enzyme (ACE) inhibitory peptides. Food Sci. Technol. 2017, 78, 1–7. [Google Scholar] [CrossRef]
- Prakash, R.; Rajakani, R.; Gupta, V. Transcriptome-wide identification of Rauvolfia serpentina microRNAs and Prediction of their potential targets. Comput. Biol. Chem. 2016, 61, 62–74. [Google Scholar] [CrossRef]
- Panja, S.; Chaudhuri, I.; Khanra, K.; Bhattacharyya, N. Biological application of green silver nanoparticle synthesized from leaf extract of Rauvolfia serpentina Benth. Asian Pac. J. Trop. Dis. 2016, 6, 549–556. [Google Scholar] [CrossRef]
- Gantait, S.; Kundu, S.; Yeasmin, L.; Ali, M.N. Impact of differential levels of sodium alginate, calcium chloride and basal media on germination frequency of genetically true artificial seeds of Rauvolfia serpentina (L.) Benth. ex Kurz. J. Appl. Res. Med. Aromat. Plants 2017, 4, 75–81. [Google Scholar] [CrossRef]
- Shariare, M.H.; Sharmin, S.; Jahan, I.; Reza, H.M.; Mohsin, K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J. Drug Deliv. Sci. Technol. 2018, 43, 122–128. [Google Scholar] [CrossRef]
- Chavhan, S.; Joshi, G.; Petkar, K.; Sawant, K. Enhanced bioavailability and hypolipidemic activity of Simvastatin formulations by particle size engineering: Physicochemical aspects and in vivo investigations. Biochem. Eng. J. 2013, 79, 221–229. [Google Scholar] [CrossRef]
- Han, M.; Liu, X.; Guo, Y.; Wang, Y.; Wang, X. Preparation, characterization, biodistribution and antitumor efficacy of hydroxycamptothecin nanosuspensions. Int. J. Pharm. 2013, 455, 85–92. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhao, J.; Li, C.; Zhou, Y.; Du, J.; Wang, Y. In vitro and in vivoevaluation of targeting tumor with folate-based amphiphilic multifunctional stabilizer for resveratrol nanosuspensions. Colloids Surf. B Biointerfaces 2017, 160, 462–472. [Google Scholar] [CrossRef]
- Lai, F.; Schlich, M.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Sinico, C. Production of Nanosuspensions as a tool to improve drug bioavailability: Focus on topical delivery. Curr. Pharm. Des. 2015, 21, 6089–6103. [Google Scholar] [CrossRef] [PubMed]
- Corrias, F.; Schlich, M.; Sinico, C.; Pireddu, R.; Valenti, D.; Fadda, A.M.; Marceddu, S.; Lai, F. Nile red nanosuspensions as investigative model to study the follicular targeting of drug nanocrystals. Int. J. Pharm. 2017, 524, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Formulation and process optimization of naproxen nanosuspensions stabilized by hydroxyl propyl methylcellulose. Carbohydr. Polym. 2015, 127, 300–308. [Google Scholar] [CrossRef]
- He, S.; Yang, H.; Zhang, R.; Li, Y.; Duan, L. Preparation and in vitro–in vivo evaluation of teniposide nanosuspensions. Int. J. Pharm. 2015, 478, 131–137. [Google Scholar] [CrossRef]
- Belovic, M.M.; Llic, N.M.; Tepic, A.N.; Sumic, Z.M. Selection of conditions for angiotensin-converting enzyme inhibition assay: Influence of sample preparation and buffer. Food Feed. Res. 2013, 40, 11–15. [Google Scholar]
- Ven, V.H.; Paulussen, C.; Feijens, P.B.; Matheeussen, A.; Rambaut, P.; Kayaert, P.; Mooter, G.V.D.; Weyenberg, W.; Cos, P.; Maes, L.; et al. PLGA nanoparticles and nanosuspensions with amphotericin B: Potent in vitro and in vivo alternatives to Fungizone and AmBisome. J. Control. Release 2012, 161, 795–803. [Google Scholar] [PubMed]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Zimmerman, A.; Millqvist-Fureby, A.; Elema, M.R.; Hansen, T.; Mullertz, A.; Hovgaard, L. Adsorption of pharmaceutical excipients onto microcrystals of siramesine hydrochloride: Effects on physicochemical properties. Eur. J. Pharm. Biopharm. 2009, 71, 109–116. [Google Scholar] [CrossRef]
- Mishra, B.; Sahoo, J.; Dixit, P.K. Enhanced bioavailability of cinnarizine nanosuspensions by particle size engineering: Optimization and physicochemical investigations. Mater. Sci. Eng. C 2016, 63, 62–69. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ghosh, I. Identifying the correlation between drug/stabilizer properties and critical qualiy attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur. J. Pharm. Sci. 2013, 48, 142–152. [Google Scholar] [CrossRef]
- Ammeeduzzafar, S.; Imam, S.; Bukhari, S.N.A.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Frias, J.; Penas, E.; Zielinski, H.; Giemenz-Bastida, J.A.; Wizkowski, W.; Zielinska, D.; Martinez-Villaluenga, C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J. Funct. Foods 2015, 18, 319–332. [Google Scholar] [CrossRef]
- Hansen, K.; Adsersen, A.; Smitt, U.V.; Nyman, U.; Christensen, S.B.; Schwartner, C.; Hagner, H. Angiotensin Converting Enzyme (ACE) inhibitory flavonoids from Erthyroxylum laurifolium. Phytomedicine 1996, 2, 313–317. [Google Scholar] [CrossRef]



| Sr. No. | Concentration of Stabilizer (g) | Volume of Antisolvent (mL) | Particle Size (nm) | Zeta Potential (mV) | PdI |
|---|---|---|---|---|---|
| NPH1 | 0.20 | 50 | 1275 | −3.41 | 0.322 |
| NPH2 | 0.30 | 50 | 1642 | −5.47 | 0.781 |
| NPH3 | 0.18 | 100 | 623.8 | −2.55 | 0.438 |
| NPH4 | 0.25 | 171.71 | 990.2 | −15.2 | 0.139 |
| NPH5 | 0.20 | 150 | 1528 | −10.4 | 0.572 |
| NPH6 | 0.32 | 100 | 1624 | −8.93 | 0.622 |
| NPH7 | 0.30 | 150 | 1529 | −19.2 | 0.724 |
| NPH8 | 0.25 | 29.29 | 1154 | −0.362 | 0.444 |
| NPH9 | 0.25 | 100 | 350.8 | −11.3 | 0.372 |
| NPH10 | 0.25 | 100 | 441.1 | −10.99 | 0.3 |
| NPH11 | 0.25 | 100 | 405 | −11 | 0.5 |
| NPH12 | 0.25 | 100 | 355.8 | −8 | 0.125 |
| NPH13 | 0.25 | 100 | 380.9 | −10 | 0.29 |
| Samples | %Age Hemolysis |
|---|---|
| Crude extract | 4.27 ± 0.01 |
| HPMC | −11.84 ± 0.01 |
| NPH | −1.283 ± 0.001 |
| Triton X-100 | 57.91 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touqeer, S.I.; Jahan, N.; Abbas, N.; Ali, A. Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential. J. Funct. Biomater. 2022, 13, 268. https://doi.org/10.3390/jfb13040268
Touqeer SI, Jahan N, Abbas N, Ali A. Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential. Journal of Functional Biomaterials. 2022; 13(4):268. https://doi.org/10.3390/jfb13040268
Chicago/Turabian StyleTouqeer, Syeeda Iram, Nazish Jahan, Naseem Abbas, and Ahsan Ali. 2022. "Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential" Journal of Functional Biomaterials 13, no. 4: 268. https://doi.org/10.3390/jfb13040268
APA StyleTouqeer, S. I., Jahan, N., Abbas, N., & Ali, A. (2022). Formulation and Process Optimization of Rauvolfia serpentina Nanosuspension by HPMC and In Vitro Evaluation of ACE Inhibitory Potential. Journal of Functional Biomaterials, 13(4), 268. https://doi.org/10.3390/jfb13040268







