Nanohydroxyapatite/Titanate Nanotube Composites for Bone Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
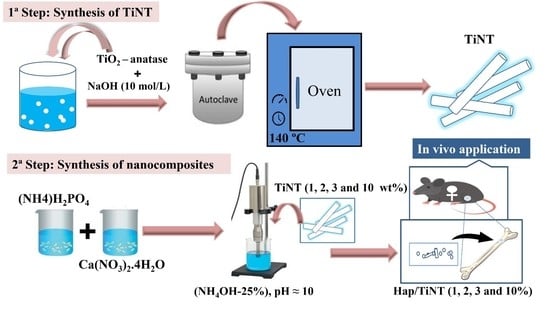
2.1. Synthesis of the TiNTs
2.2. Preparation of a nHAp/TiNT Nanocomposite
2.3. Characterization of nHAp, TiNTs, and nHAp/TiNTs
2.3.1. Raman Spectroscopy
2.3.2. X-ray Diffraction
2.3.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR–FTIR)
2.3.4. Particle Size Analysis and Zeta Potential
2.3.5. Scanning and Transmission Electron Microscopy
2.4. In Vitro Study
Cell Viability
2.5. In Vivo Study
2.5.1. Ethical Aspect
Animals
Surgical Procedure
2.5.2. Experimental Period
2.5.3. Raman Analysis of Osteogenesis
2.5.4. Histopathological Analysis
2.5.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armiento, A.R.; Hatt, L.P.; Rosenberg, G.S.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 30, 1909874. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuss, K.M.R.; von Rechenberg, B. Biocompatibility Issues with Modern Implants in Bone—A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Lahiri, D.; Ghosh, S.; Agarwal, A. Carbon Nanotube Reinforced Hydroxyapatite Composite for Orthopedic Application: A Review. Mater. Sci. Eng. C 2012, 32, 1727–1758. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Chen, Y.; Harimkar, S.P.; Dahotre, N.B.; Agarwal, A. Tribological Behavior of Plasma-Sprayed Carbon Nanotube-Reinforced Hydroxyapatite Coating in Physiological Solution. Acta Biomater. 2007, 3, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Yoo, J.-W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 3270–3275. [Google Scholar] [CrossRef] [Green Version]
- Zanin, H.; Rosa, C.M.R.; Eliaz, N.; May, P.W.; Marciano, F.R.; Lobo, A.O. Assisted Deposition of Nano-Hydroxyapatite onto Exfoliated Carbon Nanotube Oxide Scaffolds. Nanoscale 2015, 7, 10218–10232. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H.J. Titanium Alloys in Total Joint Replacement—a Materials Science Perspective Marc. Appl. Cogn. Psychol. 1994, 8, 597–611. [Google Scholar] [CrossRef]
- Niinomi, M. Recent Research and Development in Metallic Materials for Biomedical, Dental and Healthcare Products Applications. Mater. Sci. Forum 2007, 539–543, 193–200. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Rational Design, Bio-Functionalization and Biological Performance of Hybrid Additive Manufactured Titanium Implants for Orthopaedic Applications: A Review. J. Mech. Behav. Biomed. Mater. 2020, 105, 103671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Wen, C.; Li, Y. Processing and Characterization of SrTiO3–TiO2Nanoparticle–Nanotube Heterostructures on Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2015, 7, 16018–16026. [Google Scholar] [CrossRef] [PubMed]
- Zazakowny, K.; Lewandowska-Łańcucka, J.; Mastalska-Popławska, J.; Kamiński, K.; Kusior, A.; Radecka, M.; Nowakowska, M. Biopolymeric hydrogels—nanostructured TiO2 hybrid materials as potential injectable scaffolds for bone regeneration. Colloids Surf. B Biointerfaces 2016, 148, 607–614. [Google Scholar] [CrossRef]
- Sallem, F.; Boudon, J.; Heintz, O.; Séverin, I.; Megriche, A.; Millot, N. Synthesis and Characterization of Chitosan-Coated Titanate Nanotubes: Towards a New Safe Nanocarrier. Dalt. Trans. 2017, 46, 15386–15398. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Wang, T.; Ni, J. Highly Efficient Adsorption of Cr(VI) from Aqueous Solutions by Amino-Functionalized Titanate Nanotubes. Chem. Eng. J. 2013, 225, 153–163. [Google Scholar] [CrossRef]
- Marques, T.M.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Filho, E.C.S.; Viana, B.C.; et al. Amino-functionalized titanate nanotubes for highly efficient removal of anionic dye from aqueous solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Barbosa, M.C.; Messmer, N.R.; Brazil, T.R.; Marciano, F.R.; Lobo, A.O. The Effect of Ultrasonic Irradiation on the Crystallinity of Nano-Hydroxyapatite Produced via the Wet Chemical Method. Mater. Sci. Eng. C 2013, 33, 2620–2625. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Carvalho, J.O.; Gusmão, S.B.S.; Gonçalves, L.D.S.; Mendes, L.M.S.; Freitas, S.A.P.; Gusmão, G.; Viana, B.C.; Marciano, F.R.; Lobo, A.O. High loads of nano-hydroxyapatite/graphene nanoribbon composites guided bone regeneration using an osteoporotic animal model. Int. J. Nanomed. 2019, 14, 865–874. [Google Scholar] [CrossRef]
- Renaudin, G.; Laquerrière, P.; Filinchuk, Y.; Jallot, E.; Nedelec, J.M. Structural Characterization of Sol-Gel Derived Sr-Substituted Calcium Phosphates with Anti-Osteoporotic and Anti-Inflammatory Properties. J. Mater. Chem. 2008, 18, 3593–3600. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS). Alamos Natl. Lab. LAUR 2004, 748, 55. [Google Scholar]
- Poralan, G.M.; Gambe, J.E.; Alcantara, E.M.; Vequizo, R.M. X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. IOP Conf. Ser. Mater. Sci. Eng. 2015, 79, 012028. [Google Scholar] [CrossRef] [Green Version]
- Czekanska, E.M. Assessment of Cell Proliferation with Resazurin-Based Fluorescent Dye. Methods Mol. Biol. 2011, 740, 27–32. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993–5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Alves, A.M.M.; Fortaleza, L.M.D.M.; Filho, A.L.M.M.; Ferreira, D.C.L.; da Costa, C.L.S.; Viana, V.G.F.; Santos, J.Z.L.V.; de Oliveira, R.A.; Gusmão, G.O.D.M.; Soares, L.E.S. Evaluation of bone repair after application of a norbixin membrane scaffold with and without laser photobiomodulation (λ 780 nm). Lasers Med. Sci. 2018, 33, 1493–1504. [Google Scholar] [CrossRef]
- Monzem, S.; Sônego, D.A.; Martini, A.D.C.; Moura, A.P.B.D.; da Silva, F.G.; de Faria, J.L.B.; de Souza, R.L. Raman spectroscopic of osteoporosis model in mouse tibia in vivo. Vib. Spectrosc. 2018, 98, 88–91. [Google Scholar] [CrossRef]
- Hedner, E.; Linde, A. Efficacy of Bone Morphogenetic Protein (BMP) with Osteopromotive Membranes—an Experimental Study in Rat Mandibular Defects. Eur. J. Oral Sci. 1995, 103, 236–241. [Google Scholar] [CrossRef]
- Gusmão, S.B.; Ghosh, A.; Marques, T.M.; Vieira, L.H.S.; Ferreira, O.P.; Silva-Filho, E.C.; Lobo, A.O.; Osajima, J.A.; Viana, B.C. Titanate-based one-dimensional nano-heterostructure: Study of hydrothermal reaction parameters for improved photocatalytic application. Solid State Sci. 2019, 98, 106043. [Google Scholar] [CrossRef]
- Vieira, E.G.; Sousa, P.A.A.; Matos, J.M.E.; Santos, M.R.M.C.; Ininga, B. Síntese Pelo Método Da Coprecipitação e Caracterização Estrutural Do Tungstato de Cálcio Com Estrutura Tipo Scheelita (Synthesis by the Coprecipitation Method and Structural Characterization of Calcium Tungstate with Scheelite Type Structure). Cerâmica 2013, 59, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, L.S.; Longo, V.M.; Sczancoski, J.C.; Almeida, M.A.P.; Batista, A.A.; Varela, J.A.; Orlandi, M.O.; Longo, E.; Li, M.S. Electronic Structure, Growth Mechanism and Photoluminescence of CaWO 4 Crystals. CrystEngComm 2012, 14, 853–868. [Google Scholar] [CrossRef]
- Vieira, E.; Silva, M.; Maia-Filho, A.; Ferreira, D.; Figuerêdo-Silva, J.; Rovaris, K.; Fialho, A.; Leite-Oliveira, A.; de Oliveira, A.M.; da Fonseca, M.; et al. Effect of Cerium-Containing Hydroxyapatite in Bone Repair in Female Rats with Osteoporosis Induced by Ovariectomy. Minerals 2021, 11, 377. [Google Scholar] [CrossRef]
- Cho, J.S.; Um, S.-H.; Yoo, D.S.; Chung, Y.-C.; Chung, S.H.; Lee, J.-C.; Rhee, S.-H. Enhanced osteoconductivity of sodium-substituted hydroxyapatite by system instability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 102, 1046–1062. [Google Scholar] [CrossRef]
- Viana, B.C.; Ferreira, O.P.; Filho, A.G.S.; Hidalgo, A.A.; Filho, J.M.; Alves, O.L. Highlighting the mechanisms of the titanate nanotubes to titanate nanoribbons transformation. J. Nanoparticle Res. 2011, 13, 3259–3265. [Google Scholar] [CrossRef]
- Rodrigues, B.V.M.; Leite, N.C.S.; das Neves Cavalcanti, B.; da Silva, N.S.; Marciano, F.R.; Corat, E.J.; Webster, T.J.; Lobo, A.O. Graphene Oxide/Multi-Walled Carbon Nanotubes as Nanofeatured Scaffolds for the Assisted Deposition of Nanohydroxyapatite: Characterization and Biological Evaluation. Int. J. Nanomed. 2016, 11, 2569–2585. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, J.S.; Oliveira, A.M.; De Carvalho, J.O.; Ricci, R.; Martins, M.D.C.D.C.E.; Rodrigues, B.V.M.; Webster, T.J.; Viana, B.C.; Vasconcellos, L.M.R.; Canevari, R.; et al. Nanohydroxyapatite/Graphene Nanoribbons Nanocomposites Induce In Vitro Osteogenesis and Promote in Vivo Bone Neoformation. ACS Biomater. Sci. Eng. 2018, 4, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.P.; Filho, A.G.S.; Filho, J.M.; Alves, O.L. Unveiling the structure and composition of titanium oxide nanotubes through ion exchange chemical reactions and thermal decomposition processes. J. Braz. Chem. Soc. 2006, 17, 393–402. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casals, E.; Galán, A.M.; Escolar, G.; Gallardo, M.; Estelrich, J. Physical Stability of Liposomes Bearing Hemostatic Activity. Chem. Phys. Lipids 2003, 125, 139–146. [Google Scholar] [CrossRef]










| Lattice Parameters (Hydroxyapatite) | |||||
|---|---|---|---|---|---|
| a = b (Å) | c (Å) | α = β (◦) | g (◦) | V (Å3) | Ρ (g/cm3) |
| 9.436(1) | 6.8875(7) | 90 | 120 | 531.1 (1) | 3.141 |
| Samples | CIRaman (Average) | CIX-ray (Average) |
|---|---|---|
| nHAp | 0.71 | 0.42 |
| nHAp/TiNT1% | 0.62 | 0.20 |
| nHAp/TiNT2% | 0.63 | 0.22 |
| nHAp/TiNT3% | 0.50 | 0.19 |
| nHAp/TiNT10% | 0.49 | 0.18 |
| Samples | 2θ [°] Peak (002) | (2θ) FWHM | Crystallite Size XRD (nm) |
|---|---|---|---|
| nHAp | 25.87 | 0.208 | 54 |
| nHAp/TiNT1% | 25.88 | 0.270 | 37 |
| nHAp/TiNT2% | 25.89 | 0.225 | 48 |
| nHAp/TiNT3% | 25.88 | 0.273 | 36 |
| nHAp/TiNT10% | 25.89 | 0.286 | 34 |
| Samples | Zeta Potential (mV) | Hydrodynamic Diameter (nm) | PdI |
|---|---|---|---|
| TiNT | −27.40 | 300.4 | 0.552 |
| nHAp | −5.160 | 4888 | 0.274 |
| nHap/TiNT1% | −0.921 | 3824 | 0.266 |
| nHap/TiNT2% | −0.391 | 3416 | 0.215 |
| nHap/TiNT3% | −0.019 | 3606 | 0.363 |
| nHap/TiNT10% | −1.520 | 2247 | 0.586 |
| Samples | Ti % | Ca/P % |
|---|---|---|
| nHAp | - | 1.93 |
| nHAp/TiNT1% | 0.37 | 1.92 |
| nHAp/TiNT2% | 0.40 | 2.13 |
| nHAp/TiNT3% | 0.61 | 3.03 |
| nHAp/TiNT10% | 8.53 | 2.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusmão, S.B.S.; Ghosh, A.; de Menezes, A.S.; Pereira, A.F.M.; Lopes, M.T.P.; Souza, M.K.; Dittz, D.; Abreu, G.J.P.; Pinto, L.S.S.; Maia Filho, A.L.M.; et al. Nanohydroxyapatite/Titanate Nanotube Composites for Bone Tissue Regeneration. J. Funct. Biomater. 2022, 13, 306. https://doi.org/10.3390/jfb13040306
Gusmão SBS, Ghosh A, de Menezes AS, Pereira AFM, Lopes MTP, Souza MK, Dittz D, Abreu GJP, Pinto LSS, Maia Filho ALM, et al. Nanohydroxyapatite/Titanate Nanotube Composites for Bone Tissue Regeneration. Journal of Functional Biomaterials. 2022; 13(4):306. https://doi.org/10.3390/jfb13040306
Chicago/Turabian StyleGusmão, Suziete B. S., Anupama Ghosh, Alan S. de Menezes, Antônio F. M. Pereira, Miriam T. P. Lopes, Madaline K. Souza, Dalton Dittz, Guilherme J. P. Abreu, Lucielma S. S. Pinto, Antônio L. M. Maia Filho, and et al. 2022. "Nanohydroxyapatite/Titanate Nanotube Composites for Bone Tissue Regeneration" Journal of Functional Biomaterials 13, no. 4: 306. https://doi.org/10.3390/jfb13040306