Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review
Abstract
:1. Introduction
2. Bone Engineering
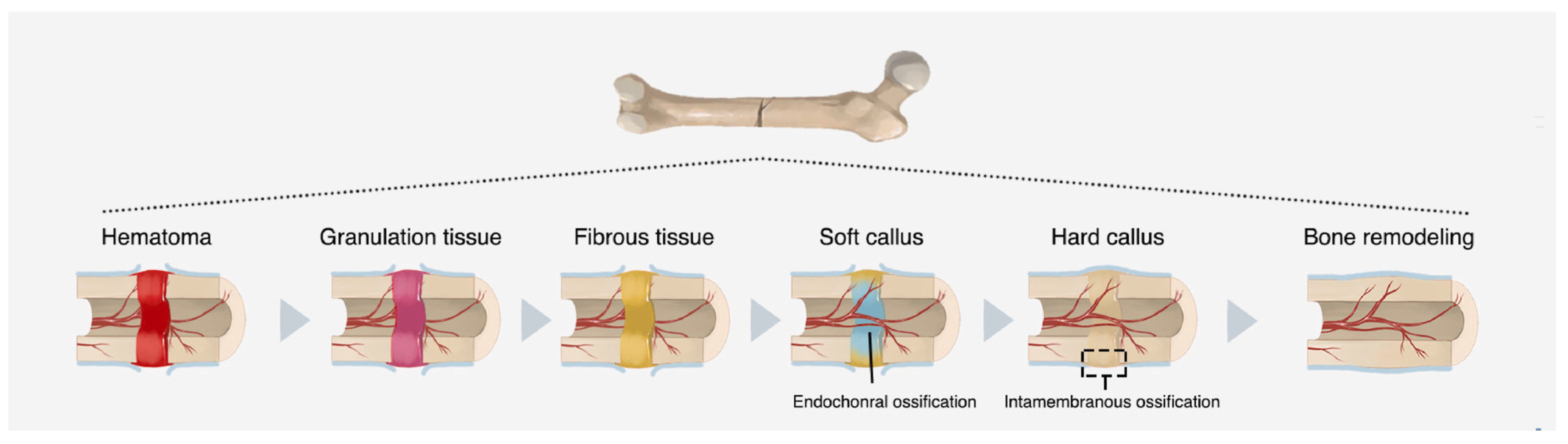
2.1. Bone Healing Mechanisms
2.2. Orthopedic Clinical Challenges
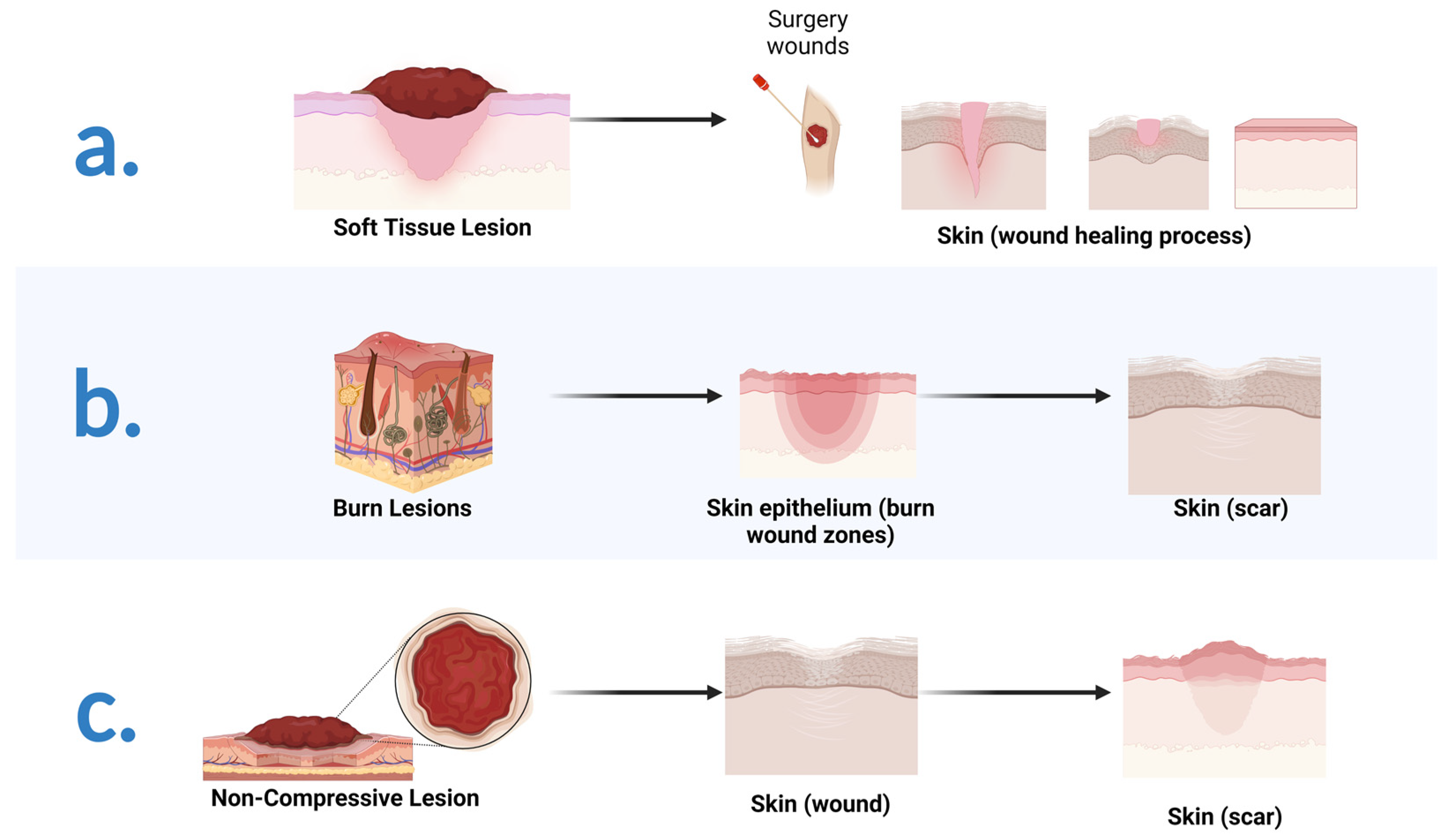
3. Soft Tissues Engineering
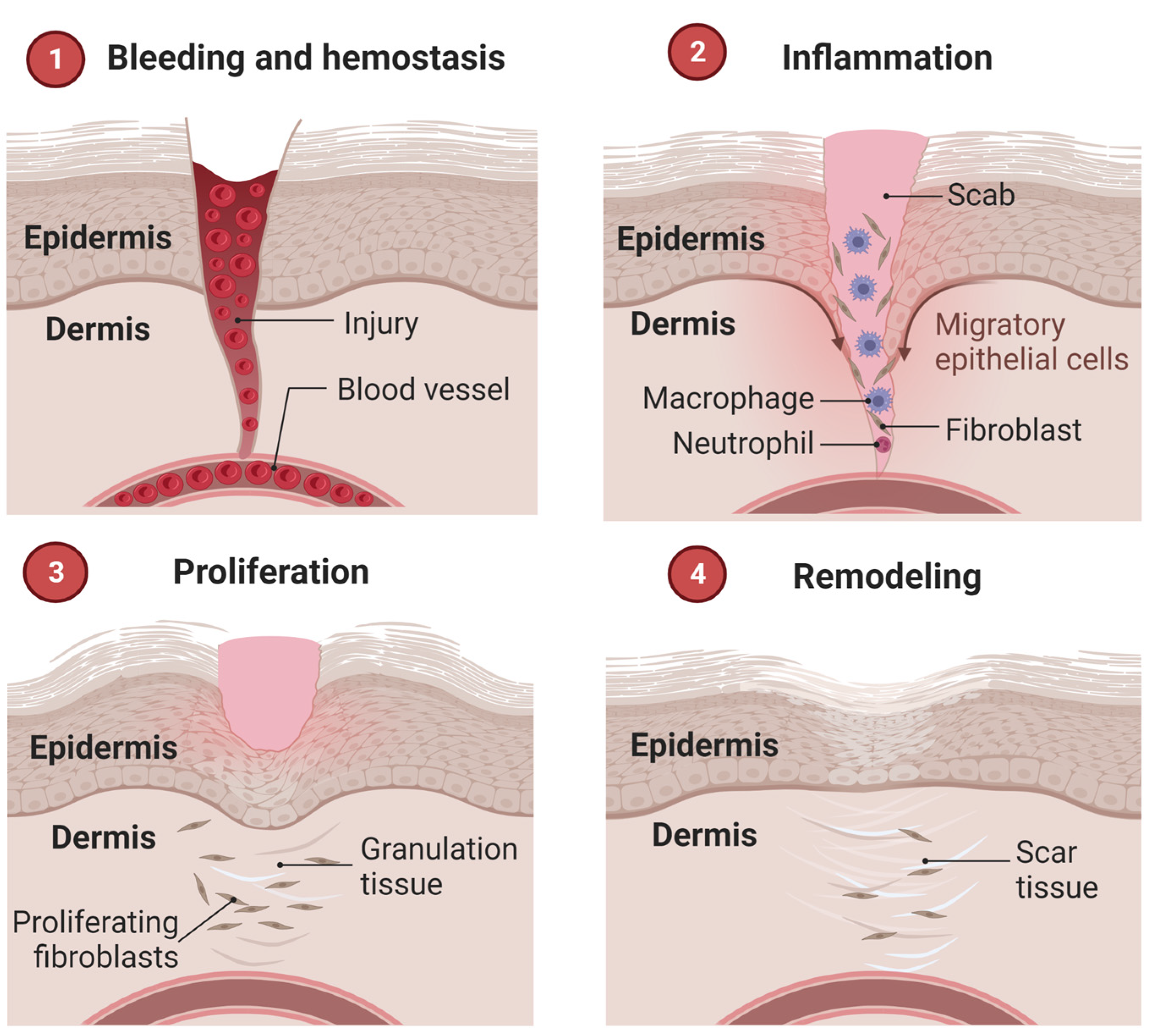
3.1. Wound Healing Mechanisms
3.2. Therapeutic Approaches in Wound Repair: A Brief Introduction
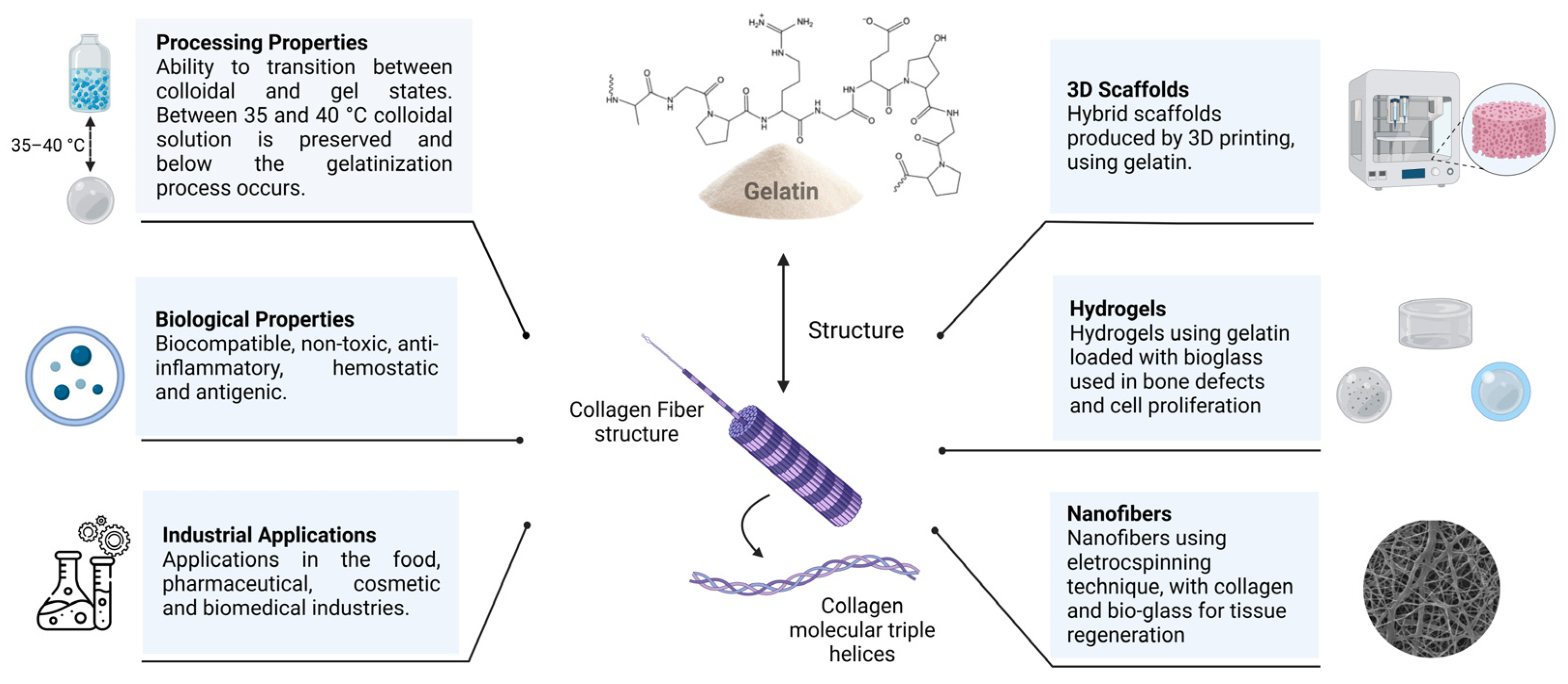
4. Gelatin
| Field | Properties | References |
|---|---|---|
| General | Biodegradability Biocompatibility Higher antigenicity than collagen Anti-inflammatory action Hemostatic action | [70,74,75,78,79,81] |
| Processing | Hydrophilicity Capability to form composites and hybrids Cross-linked to increase the stability Thermo-reversibility between colloidal and gel states Higher swelling and faster degradation than collagen Different configurations (hydrogels, microspheres, fibers, scaffolds, etc.) | [67,68,69,70,71,73,82] |
| Drug release | Combination between substances with distinct pH Rapid release of drugs and growth factors via electronic interaction | [76,77,78,79] |
| Immune system | Improved monitoring of the inflammatory process Detection of the pro-inflammatory phase of macrophages | [78,80] |
| Cancer therapy | Delivering growth factors directly to the tumor site Encapsulation of tumor cells present in the blood Optimization of the disease monitoring | [78,80,81,82,83] |
| 3D bioprinting | Matrix for cell culture Development of complex structures Control of the porosity | [70,78,81,82,84,85,86,87] |
5. Bioactive Glasses (BGs)
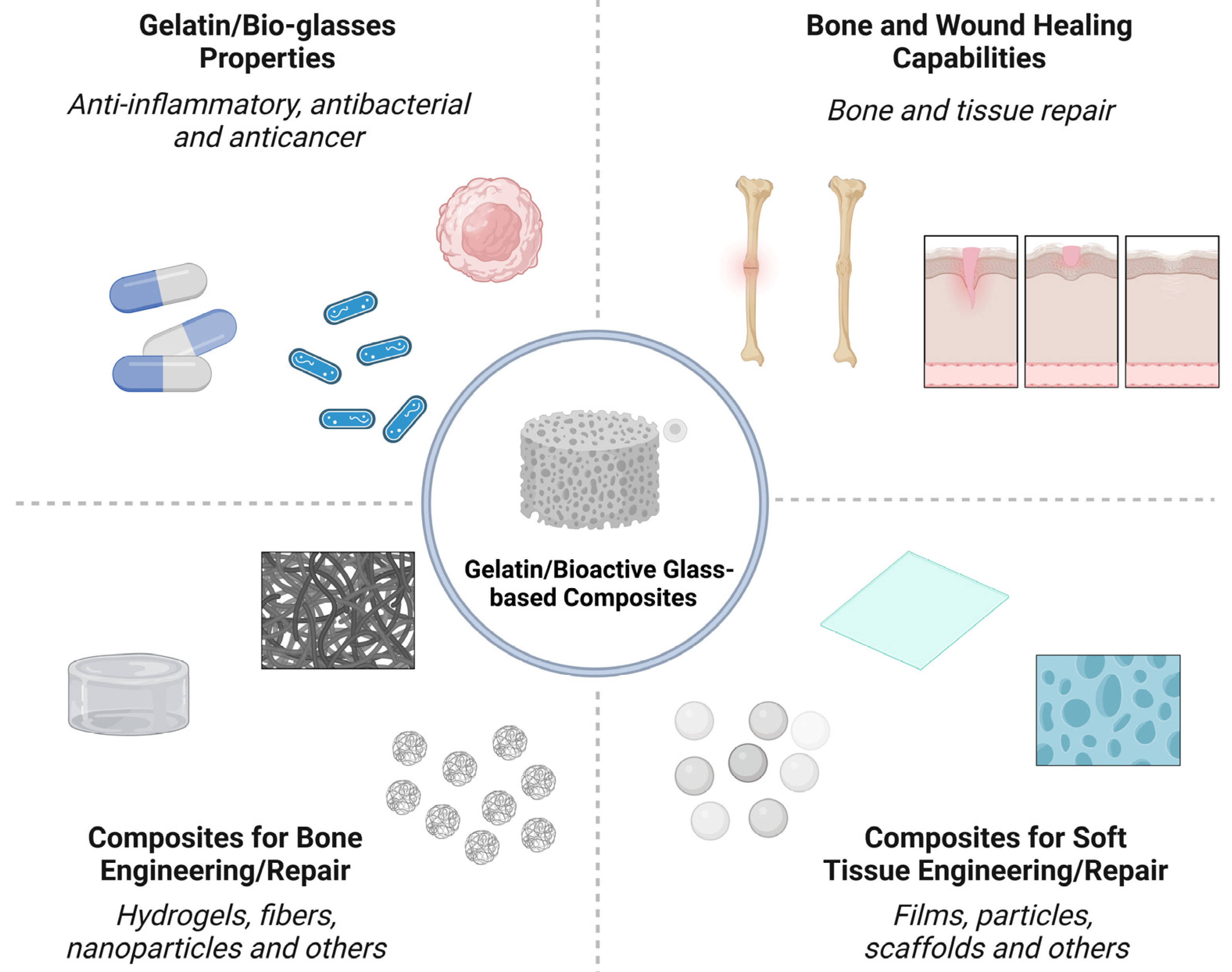
6. Composites of Gelatin/BG for Tissue Engineering
6.1. Bone Engineering/Repair
6.2. Soft Tissue Engineering/Repair
7. Challenges and Opportunities
8. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sonatkar, J.; Kandasubramanian, B. Bioactive glass with biocompatible polymers for bone applications. Eur. Polym. J. 2021, 160, 110801. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Ghenaatgar-Kasbi, M.; Baino, F. Bioactive glasses and glass/polymer composites for neuroregeneration: Should we be hopeful? Appl. Sci. 2020, 10, 3421. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A review of bioactive glass/natural polymer composites: State of the art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Van Damme, L.; Blondeel, P.; Van Vlierberghe, S. Injectable biomaterials as minimal invasive strategy towards soft tissue regeneration—An overview. J. Phys. Mater. 2021, 4, 022001. [Google Scholar] [CrossRef]
- Newman, H.; Shih, Y.V.; Varghese, S. Resolution of inflammation in bone regeneration: From understandings to therapeutic applications. Biomaterials 2021, 277, 121114. [Google Scholar] [CrossRef]
- Leong, C.; Gouliouris, T. Skin and soft tissue infections. Medicine 2021, 49, 699–705. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde (DATASUS). Available online: http://www2.datasus.gov.br/DATASUS/index.php?area=0203&id=6926&VObj=http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sih/cnv/ni (accessed on 30 January 2022).
- Araujo, T.A.T.; Almeida, M.C.; Avanzi, I.; Parisi, J.; Simon Sales, A.F.; Na, Y.; Renno, A. Collagen membranes for skin wound repair: A systematic review. J. Biomater. Appl. 2020, 36, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Pratim Das, P.; Aditya Bachchan, A.; Sahu, R.; Chaudhary, V. Whole body vibration: Effects on human body and role of biomaterials in repairing fracture joints and tissues. Mater. Today Proc. 2021, 43, 141–147. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Chen, X.; Zhao, Y. Preparation of the porous scaffolds of chitosan gelatin/APTES modified bioglass. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2009, 26, 47–52. [Google Scholar]
- Peter, M.; Binulal, N.S.; Nair, S.V.; Selvamurugan, N.; Tamura, H.; Jayakumar, R. Novel biodegradable chitosan-gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem. Eng. J. 2010, 158, 353–361. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Fooladi, A.A.I.; Mansoori, K.; Jamalpoor, Z.; Amiri, A.; Nourani, M.R. Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J. Mater. Sci. Mater. Med. 2014, 25, 363–373. [Google Scholar] [CrossRef]
- Liang, W.; Wu, X.; Dong, Y.; Shao, R.; Chen, X.; Zhou, P.; Xu, F. In vivo behavior of bioactive glass-based composites in animal models for bone regeneration. Biomater. Sci. 2021, 9, 1924–1944. [Google Scholar] [CrossRef]
- Lao, J.; Dieudonné, X.; Fayon, F.; Montouillout, V.; Jallot, E. Bioactive glass-gelatin hybrids: Building scaffolds with enhanced calcium incorporation and controlled porosity for bone regeneration. J. Mater. Chem. B 2016, 4, 2486–2497. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.M.; Abd El-Fattah, A.; El-Maghraby, A.; Ghareeb, D.A.; Kandil, S. Viscoelasticity, mechanical properties, and in vitro bioactivity of gelatin/borosilicate bioactive glass nanocomposite hydrogels as potential scaffolds for bone regeneration. Polymers 2021, 13, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Santhiya, D. In situ mineralization of bioactive glass in gelatin matrix. Mater. Lett. 2017, 188, 127–129. [Google Scholar] [CrossRef]
- Jain, S.; Gujjala, R.; Abdul Azeem, P.; Ojha, S.; Samudrala, R.K. A review on mechanical and In-vitro studies of polymer reinforced bioactive glass-scaffolds and their fabrication techniques. Ceram. Int. 2022, 48, 5908–5921. [Google Scholar] [CrossRef]
- Sharifi, E.; Sadati, S.A.; Yousefiasl, S.; Sartorius, R.; Zafari, M.; Rezakhani, L.; Alizadeh, M.; Nazarzadeh Zare, E.; Omidghaemi, S.; Ghanavatinejad, F.; et al. Cell loaded hydrogel containing Ag-doped bioactive glass–ceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng. Transl. Med. 2022, 7, e10386. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Gao, H.; Lin, Z.; Dai, Q.; Zhu, S.; Li, S.; Liu, C.; Feng, Q.; Li, Q.; Wang, G.; et al. A shape memory and antibacterial cryogel with rapid hemostasis for noncompressible hemorrhage and wound healing. Chem. Eng. J. 2022, 428, 131005. [Google Scholar] [CrossRef]
- Afghah, F.; Iyison, N.B.; Nadernezhad, A.; Midi, A.; Sen, O.; Saner Okan, B.; Culha, M.; Koc, B. 3D Fiber Reinforced Hydrogel Scaffolds by Melt Electrowriting and Gel Casting as a Hybrid Design for Wound Healing. Adv. Healthc. Mater. 2022, 11, 2102068. [Google Scholar] [CrossRef]
- Ma, W.; Yang, X.; Ma, L.; Wang, X.; Zhang, L.; Yang, G.; Han, C.; Gou, Z. Fabrication of bioactive glass-introduced nanofibrous membranes with multifunctions for potential wound dressing. RSC Adv. 2014, 4, 60114–60122. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Rao, Z.-F.; Liu, Y.-J.; Liu, X.-S.; Liu, Y.-F.; Xu, L.-J.; Wang, Z.-Q.; Guo, J.-Y.; Zhang, L.; Dong, Y.-S.; et al. Multifunctional Injectable Hydrogel Loaded with Cerium-Containing Bioactive Glass Nanoparticles for Diabetic Wound Healing. Biomolecules 2021, 11, 702. [Google Scholar] [CrossRef]
- Foroutan Koudehi, M.; Imani Fooladi, A.A.; Aghozbeni, E.A.H.; Nourani, M.R. Nano bioglass/gelatin scaffold enhanced by nanosilver as an antibacterial conduit for peripheral nerve regeneration. Mater. Technol. 2019, 34, 776–784. [Google Scholar] [CrossRef]
- Barabadi, Z.; Azami, M.; Sharifi, E.; Karimi, R.; Lotfibakhshaiesh, N.; Roozafzoon, R.; Joghataei, M.T.; Ai, J. Fabrication of hydrogel based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Mater. Sci. Eng. C 2016, 69, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Begines, B.; Arevalo, C.; Romero, C.; Hadzhieva, Z.; Boccaccini, A.R.; Torres, Y. Fabrication and Characterization of Bioactive Gelatin-Alginate-Bioactive Glass Composite Coatings on Porous Titanium Substrates. ACS Appl. Mater. Interfaces 2022, 14, 15008–15020. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, K.; Lin, S.; Xu, Z.; Sun, Y.; Shi, P.; Li, G.; Bian, L. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echave, M.C.; Saenz del Burgo, L.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Shearer, A.; Montazerian, M.; Sly, J.J.; Hill, R.G.; Mauro, J.C. Trends and Perspectives on the Commercialization of Bioactive Glasses. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4307825 (accessed on 20 December 2022).
- Junqueira, L.C.; Carneiro, J. Histologia Básica: Texto e Atlas, 13th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Tratado de Fisiologia Médica, 13th ed.; Elsevier: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Ross, M.H. Histologia: Texto e Atlas, 7th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/data/maternal-newborn-child-adolescent-ageing/advisorygroups/gama/gama-advisory-group-members (accessed on 15 October 2021).
- Li, L.; Lu, H.; Zhao, Y.; Luo, J.; Yang, L.; Liu, W.; He, Q. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: A review and new perspectives. Mater. Sci. Eng. C 2019, 98, 1241–1251. [Google Scholar] [CrossRef]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Udduttula, A.; Li, J.; Zhao, P.-Y.; Wang, G.-C.; Zhang, J.V.; Ren, P.-G. Sol-gel derived nanosized Sr5(PO4)2SiO4 powder with enhanced in vitro osteogenesis and angiogenesis for bone regeneration applications. Ceram. Int. 2019, 45, 3148–3158. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/news-room/factsheets/detail/road-traffic-injuries (accessed on 15 October 2021).
- Ramlee, S.N.L.; Sharifulden, N.S.A.N.; Mohamad, H.; Noor, S.N.F.M. Sol-gel derived bioactive glass scaffolds incorporated with polyvinyl-alcohol and pluronic P123 polymers using sponge replication technique. Mater. Today Proc. 2019, 17, 966–975. [Google Scholar] [CrossRef]
- Liao, X.; Wang, F.; Wang, G. Progress and challenges of bone tissue engineering scaffolds. Chin. J. Tissue Eng. Res. 2021, 25, 4553–4560. [Google Scholar] [CrossRef]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef]
- Kashte, S.; Jaiswal, A.K.; Kadam, S. Artificial Bone via Bone Tissue Engineering: Current Scenario and Challenges. Tissue Eng. Regen. Med. 2017, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, R.; Ganesh, S.S.; Harini, G.; Vatsala, K.; Anushikaa, R.; Aravind, S.; Abinaya, S.; Selvamurugan, N. Chitosan-based scaffolds as drug delivery systems in bone tissue engineering. Int. J. Biol. Macromol. 2022, 222, 132–153. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, W.; Zhu, M.; Wu, C.; Zhu, Y. Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review. Bioact. Mater. 2022, 18, 383–398. [Google Scholar] [CrossRef]
- Mohaghegh, S.; Hosseini, S.F.; Rad, M.R.; Khojasteh, A. 3D Printed Composite Scaffolds in Bone Tissue Engineering: A Systematic Review. Curr. Stem Cell Res. Ther. 2022, 17, 648–709. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Wells, J.A.; Gibbs, D.M.R.; Marshall, K.M.; Tang, D.K.O.; Oreffo, R.O.C. Chapter 50—Bone tissue engineering and bone regeneration. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 917–935. [Google Scholar]
- Diba, M.; Camargo, W.A.; Brindisi, M.; Farbod, K.; Klymov, A.; Schmidt, S.; Harrington, M.J.; Draghi, L.; Boccaccini, A.R.; Jansen, J.A.; et al. Composite Colloidal Gels Made of Bisphosphonate-Functionalized Gelatin and Bioactive Glass Particles for Regeneration of Osteoporotic Bone Defects. Adv. Funct. Mater. 2017, 27, 1703438. [Google Scholar] [CrossRef]
- Gilarska, A.; Hinz, A.; Bzowska, M.; Dyduch, G.; Kamiński, K.; Nowakowska, M.; Lewandowska-Łańcucka, J. Addressing the Osteoporosis Problem—Multifunctional Injectable Hybrid Materials for Controlling Local Bone Tissue Remodeling. ACS Appl. Mater. Interfaces 2021, 13, 49762–49779. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, G.; Song, G.; Liu, T.; Cao, C.; Yang, Y.; Zhang, Y.; Hong, W. Incorporation of ZnO/Bioactive Glass Nanoparticles into Alginate/Chitosan Composite Hydrogels for Wound Closure. ACS Appl. Bio Mater. 2019, 2, 5042–5052. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Tsepkolenko, A.; Tsepkolenko, V.; Dash, S.; Mishra, A.; Bader, A.; Melerzanov, A.; Giri, S. The regenerative potential of skin and the immune system. Clin. Cosmet. Investig. Dermatol. 2019, 12, 519. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass Activated Skin Tissue Engineering Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef]
- Su, L.; Zheng, J.; Wang, Y.; Zhang, W.; Hu, D. Emerging progress on the mechanism and technology in wound repair. Biomed. Pharmacother. 2019, 117, 109191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory Microenvironment of Skin Wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef]
- Demmer, W.; Sorg, H.; Steiert, A.; Hauser, J.; Tilkorn, D.J. Wound Healing and Therapy in Soft Tissue Defects of the Hand and Foot from a Surgical Point of View. Med. Sci. 2021, 9, 71. [Google Scholar] [CrossRef]
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [Green Version]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Gelatin structure and composition linked to hard capsule dissolution: A review. Food Hydrocoll. 2015, 43, 360–376. [Google Scholar] [CrossRef]
- Alves, A.L.; Costa-Gouveia, J.; Vieira de Castro, J.; Sotelo, C.G.; Vázquez, J.A.; Pérez-Martín, R.I.; Torrado, E.; Neves, N.; Reis, R.L.; Castro, A.G.; et al. Study of the immunologic response of marine-derived collagen and gelatin extracts for tissue engineering applications. Acta Biomater. 2022, 141, 123–131. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, F.; Cui, Y.; Ren, H.; Xie, Y.; Li, A.; Ji, L.; Qu, X.; Qiu, D.; Yang, Z. An easy-to-use wound dressing gelatin-bioactive nanoparticle gel and its preliminary in vivo study. J. Mater. Sci. Mater. Med. 2016, 28, 10. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Hafidz, R.M.R.N.; Yaakob, C.M.; Amin, I.; Noorfaizan, A. Chemical and functional properties of bovine and porcine skin gelatin. Int. Food Res. J. 2011, 18, 787–791. [Google Scholar]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and its derivatives: From structure and properties to their applications in food industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012, 29, 144–151. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef]
- Yamada, K.; Tabata, Y.; Yamamoto, K.; Miyamoto, S.; Nagata, I.; Kikuchi, H.; Ikada, Y. Potential efficacy of basic fibroblast growth factor incorporated in biodegradable hydrogels for skull bone regeneration. J. Neurosurg. 1997, 86, 871–875. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef]
- Nii, T. Strategies Using Gelatin Microparticles for Regenerative Therapy and Drug Screening Applications. Molecules 2021, 26, 6795. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Jo, J.-i.; Tabata, Y. Preparation of antibody-immobilized gelatin nanospheres incorporating a molecular beacon to visualize the biological function of macrophages. Regen. Ther. 2020, 14, 11–18. [Google Scholar] [CrossRef]
- Echave, M.C.; Hernáez-Moya, R.; Iturriaga, L.; Pedraz, J.L.; Lakshminarayanan, R.; Dolatshahi-Pirouz, A.; Taebnia, N.; Orive, G. Recent advances in gelatin-based therapeutics. Expert Opin. Biol. Ther. 2019, 19, 773–779. [Google Scholar] [CrossRef]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Brancato, V.; Comunanza, V.; Imparato, G.; Corà, D.; Urciuolo, F.; Noghero, A.; Bussolino, F.; Netti, P.A. Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017, 49, 152–166. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Serpooshan, V.; Tong, X.; Venkatraman, S.; Lee, M.; Lee, J.; Chirikian, O.; Wu, J.C.; Wu, S.M.; Yang, F. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 2017, 131, 111–120. [Google Scholar] [CrossRef]
- Mallick, M.; Are, R.P.; Babu, A.R. An overview of collagen/bioceramic and synthetic collagen for bone tissue engineering. Materialia 2022, 22, 101391. [Google Scholar] [CrossRef]
- Borrego-González, S.; Becerra, J.; Díaz-Cuenca, A. Fabrication of gelatin/bioactive glass hybrid scaffolds for bone tissue-engineering. In Proceedings of the XIII Mediterranean Conference on Medical and Biological Engineering and Computing 2013, IFMBE Proceedings, Seville, Spain, 25–28 September 2013; pp. 1630–1633. [Google Scholar] [CrossRef] [Green Version]
- Dieudonné, X.; Montouillout, V.; Jallot, É.; Fayon, F.; Lao, J. Bioactive glass hybrids: A simple route towards the gelatin–SiO2–CaO system. Chem. Commun. 2014, 50, 8701–8704. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Boccafoschi, F.; Baino, F.; Vitale-Brovarone, C.; Vernè, E.; Barbani, N.; Ciardelli, G. Composite films of gelatin and hydroxyapatite/bioactive glass for tissue-engineering applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Li, S.; Page, S.J.; Shi, X.; Lee, P.D.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. 3D printed silica-gelatin hybrid scaffolds of specific channel sizes promote collagen Type II, Sox9 and Aggrecan production from chondrocytes. Mater. Sci. Eng. C 2021, 123, 111964. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Kim, D.; Kang, D.; Yang, G.H.; Jung, B.; Yeo, M.; Park, M.-J.; An, S.; Lee, K.; Kim, J.S.; et al. 3D-printed gelatin methacrylate (GelMA)/silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen. Biomater. 2021, 8, rbab001. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Venkatesan, R.; Karuppiah, P.S.; Kumar, G.; Paliwal, P.; Krishnamurthy, S.; Kadalmani, B.; Mahto, S.K.; Misra, N. Silica Release from Silane Cross-Linked Gelatin Based Hybrid Scaffold Affects Cell Proliferation. ACS Appl. Bio Mater. 2020, 3, 197–207. [Google Scholar] [CrossRef]
- Qin, X.; Zheng, J.; Yang, X.; Gong, W.; Luo, L.; Ji, L. Bioactivity of a gelatin/organic-inorganic hybrid biomaterial fibre enhanced by metronidazole release. Mater. Lett. 2022, 325, 132803. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.D.; Beidokhti, S.M.; Najaran, Z.T.; Sahebian Saghi, S. Highly improved biological and mechanical features of bioglass-ceramic/ gelatin composite scaffolds using a novel silica coverage. Ceram. Int. 2021, 47, 14048–14061. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E. Bioactive Glass-ceramics: Processing, Properties and Applications. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D., Hupa, L., Eds.; Royal Society of Chemistry: London, UK, 2016; pp. 27–60. [Google Scholar]
- Montazerian, M.; Zanotto, E.D. The Glassy State. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 448–461. [Google Scholar]
- Karadjian, M.; Essers, C.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Biological properties of calcium phosphate bioactive glass composite bone substitutes: Current experimental evidence. Int. J. Mol. Sci. 2019, 20, 305. [Google Scholar] [CrossRef] [Green Version]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef]
- Farooq, I.; Imran, Z.; Farooq, U.; Leghari, M.A.; Ali, H. Bioactive Glass: A Material for the Future. World J. Dent. 2012, 3, 199–201. [Google Scholar] [CrossRef]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Shah, F.A.; Czechowska, J. 9—Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. In Bioactive Glasses, 2nd ed.; Ylänen, H., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 201–233. [Google Scholar]
- Mouriño, V.; Vidotto, R.; Cattalini, J.P.; Boccaccini, A.R. Enhancing biological activity of bioactive glass scaffolds by inorganic ion delivery for bone tissue engineering. Curr. Opin. Biomed. Eng. 2019, 10, 23–34. [Google Scholar] [CrossRef]
- Rahmani, M.; Moghanian, A.; Yazdi, M.S. The effect of Ag substitution on physicochemical and biological properties of sol-gel derived 60%SiO2–31%CaO–4%P2O5–5%Li2O (mol%) quaternary bioactive glass. Ceram. Int. 2021, 47, 15985–15994. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef]
- Taye, M.B. Biomedical applications of ion-doped bioactive glass: A review. Appl. Nanosci. 2022, 12, 3797–3812. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef]
- Vafa, E.; Bazargan-Lari, R.; Bahrololoom, M.E. Synthesis of 45S5 bioactive glass-ceramic using the sol-gel method, catalyzed by low concentration acetic acid extracted from homemade vinegar. J. Mater. Res. Technol. 2021, 10, 1427–1436. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Chapter 13—Bioactive glass–based composites in bone tissue engineering: Synthesis, processing, and cellular responses. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–439. [Google Scholar]
- Pope, E.J.A.; Sakka, S.; Klein, L.C. Sol–Gel Science and Technology; American Ceramic Society: Westerville, OH, USA, 1995; Volume 55. [Google Scholar]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses: Promising platforms for antibacterial strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. A guided walk through Larry Hench’s monumental discoveries. J. Mater. Sci. 2017, 52, 8695–8732. [Google Scholar] [CrossRef]
- Moghanian, A.; Zohourfazeli, M.; Tajer, M.H.M. The effect of zirconium content on in vitro bioactivity, biological behavior and antibacterial activity of sol-gel derived 58S bioactive glass. J. Non-Cryst. Solids 2020, 546, 120262. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T. Bioceramics and Their Clinical Applications; Woodhead Publishing: Sawston, UK, 2008. [Google Scholar]
- Montazerian, M.; Zanotto, E.D.; Mauro, J.C. Model-driven design of bioactive glasses: From molecular dynamics through machine learning. Int. Mater. Rev. 2020, 65, 297–321. [Google Scholar] [CrossRef]
- Kim, D.; Shim, Y.S.; An, S.Y.; Lee, M.J. Role of zinc-doped bioactive glass encapsulated with microspherical gelatin in localized supplementation for tissue regeneration: A contemporary review. Molecules 2021, 26, 1823. [Google Scholar] [CrossRef] [PubMed]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Ur Rehman, M.A. Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Wagley, Y.; Maduka, C.V.; Youngstrom, D.W.; Yeger, A.; Badylak, S.F.; Hammer, N.D.; Hankenson, K.; Chatzistavrou, X. Silver-doped bioactive glass particles for in vivo bone tissue regeneration and enhanced methicillin-resistant Staphylococcus aureus (MRSA) inhibition. Mater. Sci. Eng. C 2021, 120, 111693. [Google Scholar] [CrossRef]
- Da Silva Buriti, J.; Barreto, M.E.V.; Barbosa, F.C.; de Brito Buriti, B.M.A.; de Lima Souza, J.W.; de Vasconcelos Pina, H.; de Luna Rodrigues, P.; Fook, M.V.L. Synthesis and characterization of Ag-doped 45S5 bioglass and chitosan/45S5-Ag biocomposites for biomedical applications. J. Therm. Anal. Calorim. 2021, 145, 39–50. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Büttner, T.; Miguez Pacheco, V.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Wu, C.; Miron, R.; Sculean, A.; Kaskel, S.; Doert, T.; Schulze, R.; Zhang, Y. Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds. Biomaterials 2011, 32, 7068–7078. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Pazarçeviren, E.; Ece Akgün, E.; Evis, Z.; Keskin, D.; Şahin, S.; Tezcaner, A. In vitro performance of a nanobiocomposite scaffold containing boron-modified bioactive glass nanoparticles for dentin regeneration. J. Biomater. Appl. 2018, 33, 834–853. [Google Scholar] [CrossRef]
- Majumdar, S.; Hira, S.K.; Tripathi, H.; Kumar, A.S.; Manna, P.P.; Singh, S.P.; Krishnamurthy, S. Synthesis and characterization of barium-doped bioactive glass with potential anti-inflammatory activity. Ceram. Int. 2021, 47, 7143–7158. [Google Scholar] [CrossRef]
- Paliwal, P.; Kumar, A.S.; Tripathi, H.; Singh, S.P.; Patne, S.C.U.; Krishnamurthy, S. Pharmacological application of barium containing bioactive glass in gastro-duodenal ulcers. Mater. Sci. Eng. C 2018, 92, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, M.; Gonçalves, G.V.S.; Barreto, M.E.V.; Lima, E.P.N.; Cerqueira, G.R.C.; Sousa, J.A.; Malek Khachatourian, A.; Souza, M.K.S.; Silva, S.M.L.; Fook, M.V.L.; et al. Radiopaque Crystalline, Non-Crystalline and Nanostructured Bioceramics. Materials 2022, 15, 7477. [Google Scholar] [CrossRef]
- Chen, X.; Karpukhina, N.; Brauer, D.S.; Hill, R.G. Novel Highly Degradable Chloride Containing Bioactive Glasses. Biomed. Glas. 2015, 1, 108–118. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Pedone, A.; Apperley, D.; Hill, R.G.; Karpukhina, N. New Insight into Mixing Fluoride and Chloride in Bioactive Silicate Glasses. Sci. Rep. 2018, 8, 1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
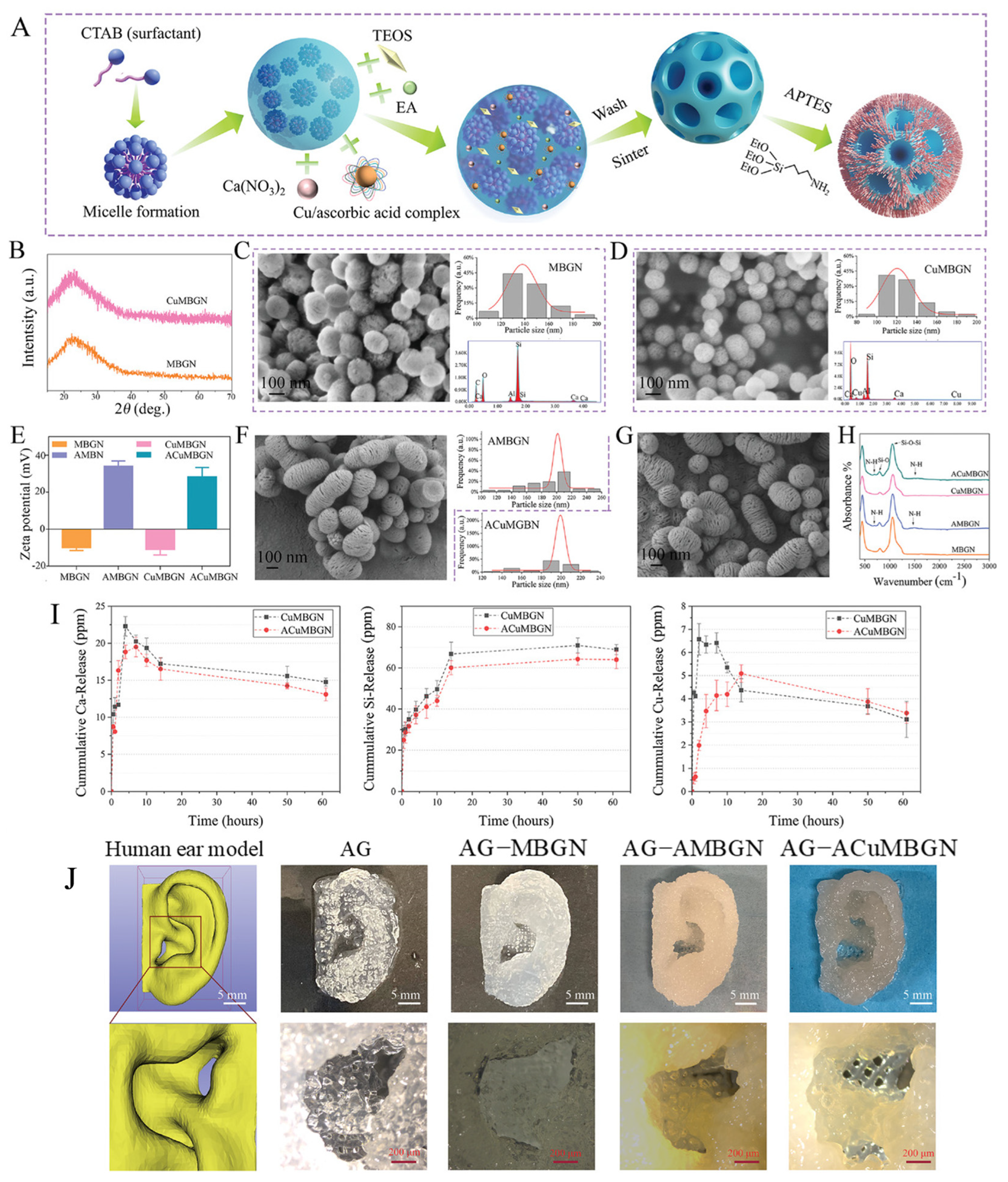
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1a and TNF-a pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, J.; Li, W.; Dippold, D.; Wan, Y.; Boccaccini, A.R. Incorporation of Cu-Containing Bioactive Glass Nanoparticles in Gelatin-Coated Scaffolds Enhances Bioactivity and Osteogenic Activity. ACS Biomater. Sci. Eng. 2018, 4, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Filip, G.A.; Achim, M.; Mihalte, P.; Miclaus, M.O.; Cristea, C.; Melinte, G.; Gheban, B.; Munteanu, D.M.; Cadar, O.; Simon, I.; et al. Design, in vitro bioactivity and in vivo influence on oxidative stress and matrix metalloproteinases of bioglasses in experimental skin wound. J. Trace Elem. Med. Biol. 2021, 68, 126846. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, H.-W. Iron ions-releasing mesoporous bioactive glass ultrasmall nanoparticles designed as ferroptosis-based bone cancer nanotherapeutics: Ultrasonic-coupled sol–gel synthesis, properties and iron ions release. Mater. Lett. 2021, 294, 129759. [Google Scholar] [CrossRef]
- Kermani, F.; Vojdani-Saghir, A.; Mollazadeh Beidokhti, S.; Nazarnezhad, S.; Mollaei, Z.; Hamzehlou, S.; El-Fiqi, A.; Baino, F.; Kargozar, S. Iron (Fe)-doped mesoporous 45S5 bioactive glasses: Implications for cancer therapy. Transl. Oncol. 2022, 20, 101397. [Google Scholar] [CrossRef]
- Sedighi, O.; Alaghmandfard, A.; Montazerian, M.; Baino, F. A critical review of bioceramics for magnetic hyperthermia. J. Am. Ceram. Soc. 2022, 105, 1723–1747. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Synthesis and characterization of cerium- and gallium-containing borate bioactive glass scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 67. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.S.; Souza, L.P.d.; Isaacs, M.A.; Raja, F.N.S.; Morrell, A.P.; Martin, R.A. Development and Characterization of Gallium-Doped Bioactive Glasses for Potential Bone Cancer Applications. ACS Biomater. Sci. Eng. 2017, 3, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Kurtuldu, F.; Mutlu, N.; Michálek, M.; Zheng, K.; Masar, M.; Liverani, L.; Chen, S.; Galusek, D.; Boccaccini, A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: Bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2021, 124, 112050. [Google Scholar] [CrossRef]
- Ye, J.; Wen, C.; Wu, J.; Wen, N.; Sa, B.; Zhang, T. Mechanical and bioactive properties of lithium disilicate glass-ceramic mixtures synthesized by two different methods. J. Non-Cryst. Solids 2019, 509, 1–9. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Moztarzadeh, F.; Arabyazdi, S. A heat-generating lithium-ferrite doped bioactive glass for cancer hyperthermia. Phys. B Condens. Matter 2020, 593, 412298. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Wang, Y.; Lin, K.; Liu, J. Lithium-containing bioactive glasses enhanced 3D-printed PLGA scaffolds for bone regeneration in diabetes. Compos. Part B Eng. 2022, 230, 109550. [Google Scholar] [CrossRef]
- Raz, M.; Moztarzadeh, F.; Kordestani, S.S. Sol-gel Based Fabrication and Properties of Mg-Zn Doped Bioactive Glass/Gelatin Composite Scaffold for Bone Tissue Engineering. Silicon 2018, 10, 667–674. [Google Scholar] [CrossRef]
- Sohrabi, M.; Yekta, B.E.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. The effect of magnesium on bioactivity, rheology and biology behaviors of injectable bioactive glass-gelatin-3-glycidyloxypropyl trimethoxysilane nanocomposite-paste for small bone defects repair. Ceram. Int. 2021, 47, 12526–12536. [Google Scholar] [CrossRef]
- Shamosi, A.; Mehrabani, D.; Azami, M.; Ebrahimi-Barough, S.; Siavashi, V.; Ghanbari, H.; Sharifi, E.; Roozafzoon, R.; Ai, J. Differentiation of human endometrial stem cells into endothelial-like cells on gelatin/chitosan/bioglass nanofibrous scaffolds. Artif. Cells Nanomed. Biotechnol. 2016, 45, 163–173. [Google Scholar] [CrossRef]
- Nawaz, Q.; Ur Rehman, M.A.; Roether, J.A.; Yufei, L.; Grünewald, A.; Detsch, R.; Boccaccini, A.R. Bioactive glass based scaffolds incorporating gelatin/manganese doped mesoporous bioactive glass nanoparticle coating. Ceram. Int. 2019, 45, 14608–14613. [Google Scholar] [CrossRef]
- Barrioni, B.R.; Norris, E.; Li, S.; Naruphontjirakul, P.; Jones, J.R.; Pereira, M.d.M. Osteogenic potential of sol–gel bioactive glasses containing manganese. J. Mater. Sci. Mater. Med. 2019, 30, 86. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-F.; Fei, Y.-C.; Chou, Y.-J. Investigation of in vitro bioactivity and antibacterial activity of manganese-doped spray pyrolyzed bioactive glasses. J. Non-Cryst. Solids 2020, 549, 120336. [Google Scholar] [CrossRef]
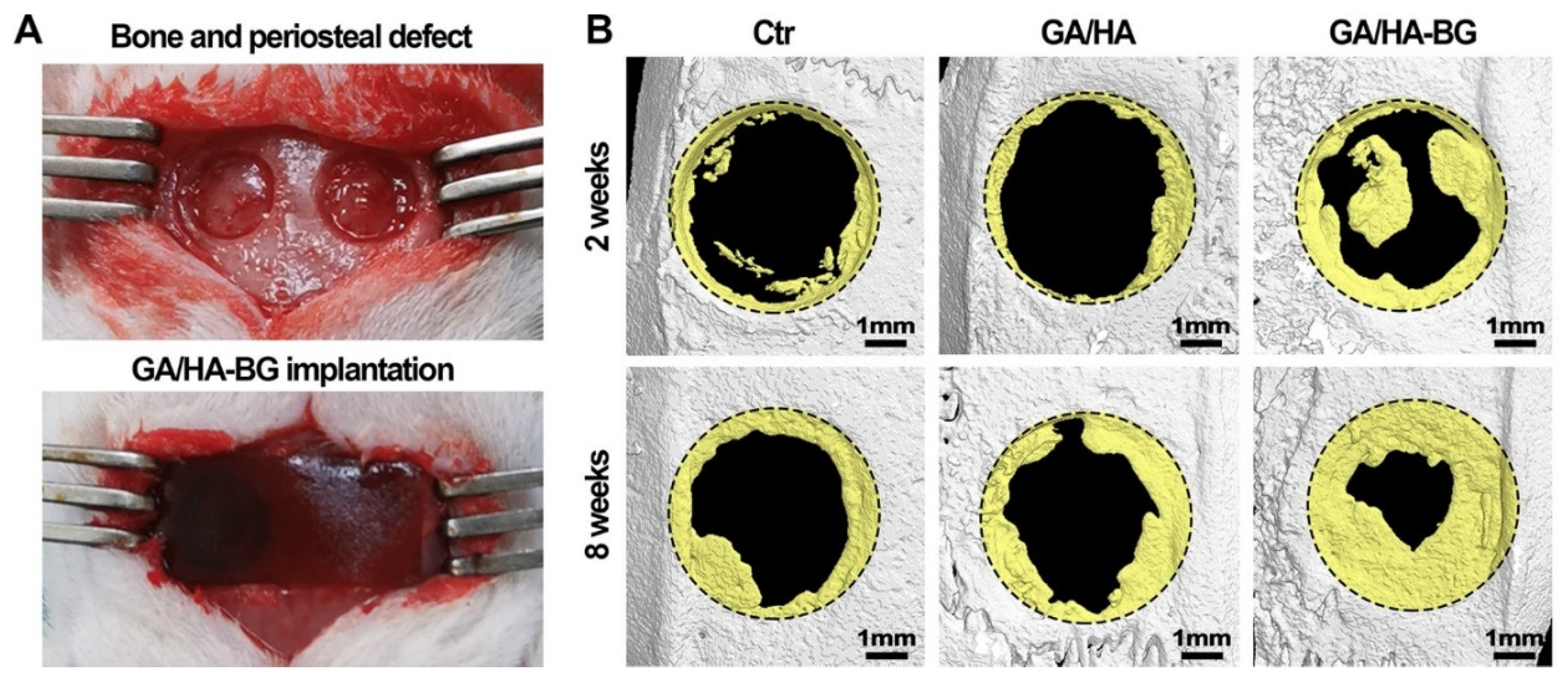
- Zare Jalise, S.; Baheiraei, N.; Bagheri, F. The effects of strontium incorporation on a novel gelatin/bioactive glass bone graft: In vitro and in vivo characterization. Ceram. Int. 2018, 44, 14217–14227. [Google Scholar] [CrossRef]
- Govindan, R.; Gu, F.; Karthi, S.; Girija, E. Effect of phosphate glass reinforcement on the mechanical and biological properties of freeze-dried gelatin composite scaffolds for bone tissue engineering applications. Mater. Today Commun. 2020, 22, 100765. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhao, F.; Wang, Y.; Tang, J.; Chen, X. Characterization of the mechanical behaviors and bioactivity of tetrapod ZnO whiskers reinforced bioactive glass/gelatin composite scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 68, 8–15. [Google Scholar] [CrossRef]
- Montazerian, M.; Schneider, J.F.; Yekta, B.E.; Marghussian, V.K.; Rodrigues, A.M.; Zanotto, E.D. Sol–gel synthesis, structure, sintering and properties of bioactive and inert nano-apatite–zirconia glass–ceramics. Ceram. Int. 2015, 41, 11024–11045. [Google Scholar] [CrossRef]
- Montazerian, M.; Yekta, B.E.; Marghussian, V.K.; Bellani, C.F.; Siqueira, R.L.; Zanotto, E.D. Bioactivity and cell proliferation in radiopaque gel-derived CaO–P2O5–SiO2–ZrO2 glass and glass–ceramic powders. Mater. Sci. Eng. C 2015, 55, 436–447. [Google Scholar] [CrossRef]
- Fu, Q. Chapter 15—Bioactive Glass Scaffolds for Bone Tissue Engineering. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Kaur, G., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 417–442. [Google Scholar]
- Montazerian, M.; Baino, F.; Fiume, E.; Migneco, C.; Alaghmandfard, A.; Sedighi, O.; DeCeanne, A.V.; Wilkinson, C.J.; Mauro, J.C. Glass-ceramics in dentistry: Fundamentals, technologies, experimental techniques, applications, and open issues. Prog. Mater. Sci. 2023, 132, 101023. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 Bioglass® by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Barberi, J.; Baino, F.; Fiume, E.; Orlygsson, G.; Nommeots-Nomm, A.; Massera, J.; Verné, E. Robocasting of SiO2-Based Bioactive Glass Scaffolds with Porosity Gradient for Bone Regeneration and Potential Load-Bearing Applications. Materials 2019, 12, 2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, W.; Lau, G.Y.; Huang, W.; Zhang, C.; Tomsia, A.P.; Fu, Q. Cellular Response to 3-D Printed Bioactive Silicate and Borosilicate Glass Scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Tulyaganov, D.U.; Fiume, E.; Akbarov, A.; Ziyadullaeva, N.; Murtazaev, S.; Rahdar, A.; Massera, J.; Verné, E.; Baino, F. In Vivo Evaluation of 3D-Printed Silica-Based Bioactive Glass Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 74. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Dukle, A.; Murugan, D.; Nathanael, A.J.; Rangasamy, L.; Oh, T.-H. Can 3D-Printed Bioactive Glasses Be the Future of Bone Tissue Engineering? Polymers 2022, 14, 1627. [Google Scholar] [CrossRef]
- Baino, F.; Tulyaganov, D.U.; Kahharov, Z.; Rahdar, A.; Verné, E. Foam-Replicated Diopside/Fluorapatite/Wollastonite-Based Glass–Ceramic Scaffolds. Ceramics 2022, 5, 120–130. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Moztarzadeh, F. Scanning electron microscope (SEM) investigations on gelatin-chitosan-bioactive glass (58S) scaffolds for bone tissue engineering. In Proceedings of the 2017 International Conference on Computational Biology and Bioinformatics, ACM International Conference Proceeding Series, Newark, NJ, USA, 18–20 October 2017; pp. 93–96. [Google Scholar]
- Zhou, L.; Fan, L.; Zhang, F.M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.A.; Tu, L.J.; Zhou, Z.N.; Li, X.J.; et al. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Zeimaran, E.; Pourshahrestani, S.; Djordjevic, I.; Pingguan-Murphy, B.; Kadri, N.A.; Towler, M.R. Bioactive glass reinforced elastomer composites for skeletal regeneration: A review. Mater. Sci. Eng. C 2015, 53, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef]
- Arabi, N.; Zamanian, A.; Rashvand, S.N.; Ghorbani, F. The Tunable Porous Structure of Gelatin–Bioglass Nanocomposite Scaffolds for Bone Tissue Engineering Applications: Physicochemical, Mechanical, and In Vitro Properties. Macromol. Mater. Eng. 2018, 303, 1700539. [Google Scholar] [CrossRef]
- Akturk, A.; Erol Taygun, M.; Goller, G. Optimization of the electrospinning process variables for gelatin/silver nanoparticles/bioactive glass nanocomposites for bone tissue engineering. Polym. Compos. 2020, 41, 2411–2425. [Google Scholar] [CrossRef]
- Nadeem, D.; Kiamehr, M.; Yang, X.; Su, B. Fabrication and in vitro evaluation of a sponge-like bioactive-glass/ gelatin composite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Wang, Z.; Yang, Z.; Tu, J.; Liu, Z.; Yao, F.; Xiong, G.; Wan, Y. Constructing three-dimensional nanofibrous bioglass/gelatin nanocomposite scaffold for enhanced mechanical and biological performance. Chem. Eng. J. 2017, 326, 210–221. [Google Scholar] [CrossRef]
- Heid, S.; Becker, K.; Byun, J.; Biermann, I.; Neščáková, Z.; Zhu, H.; Groll, J.; Boccaccini, A.R. Bioprinting with bioactive alginate dialdehyde-gelatin (ADA-GEL) composite bioinks: Time-dependent in-situ crosslinking via addition of calcium-silicate particles tunes in vitro stability of 3D bioprinted constructs. Bioprinting 2022, 26, e00200. [Google Scholar] [CrossRef]
- Lacroix, J.; Jallot, E.; Lao, J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem. Eng. J. 2014, 256, 9–13. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112470. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Miao, G.; Zheng, Z.; Li, Z.; Ren, W.; Wu, C.; Li, Y.; Huang, Z.; Yang, L.; Guo, L. 3D printing mesoporous bioactive glass/sodium alginate/gelatin sustained release scaffolds for bone repair. J. Biomater. Appl. 2018, 33, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhang, Y.; Dai, K.; Chen, X.; Read, H.M.; Zeng, L.; Hang, F. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J. Mater. Sci. Mater. Med. 2020, 31, 77. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Rabiee, M.; Azami, M.; Maleknia, S. Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl. Surf. Sci. 2010, 257, 1740–1749. [Google Scholar] [CrossRef]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. In vitro evaluation of electrospun gelatin-bioactive glass hybrid scaffolds for bone regeneration. J. Appl. Polym. Sci. 2013, 127, 2588–2599. [Google Scholar] [CrossRef]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Gao, C.; Rahaman, M.N.; Gao, Q.; Teramoto, A.; Abe, K. Robotic deposition and in vitro characterization of 3D gelatin-bioactive glass hybrid scaffolds for biomedical applications. J. Biomed. Mater. Res. Part A 2013, 101, 2027–2037. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, F.; Zhang, W.; Mo, Y.; Zeng, L.; Li, X.; Chen, X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng. C 2018, 89, 119–127. [Google Scholar] [CrossRef]
- Huang, G.; Xu, L.; Wu, J.; Wang, S.; Dong, Y. Gelatin/bioactive glass composite scaffold for promoting the migration and odontogenic differentiation of bone marrow mesenchymal stem cells. Polym. Test. 2021, 93, 106915. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Rabiee, M.; Azami, M.; Maleknia, S.; Tahriri, M.; Moztarzadeh, Z.; Nezafati, N. Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tissue engineering. Ceram. Int. 2010, 36, 2431–2439. [Google Scholar] [CrossRef]
- Mozafari, M.; Moztarzadeh, F.; Rabiee, M.; Azami, M.; Nezafati, N.; Moztarzadeh, Z.; Tahriri, M. Development of 3D bioactive nanocomposite scaffolds made from gelatin and nano bioactive glass for biomedical applications. Adv. Compos. Lett. 2010, 19, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kargozar, S.; Hashemian, S.J.; Soleimani, M.; Milan, P.B.; Askari, M.; Khalaj, V.; Samadikuchaksaraie, A.; Hamzehlou, S.; Katebi, A.R.; Latifi, N.; et al. Acceleration of bone regeneration in bioactive glass/gelatin composite scaffolds seeded with bone marrow-derived mesenchymal stem cells over-expressing bone morphogenetic protein-7. Mater. Sci. Eng. C 2017, 75, 688–698. [Google Scholar] [CrossRef]
- Zhu, H.; Monavari, M.; Zheng, K.; Distler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 2022, 18, 2104996. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Vashaee, D.; Assefa, S.; Walker, K.J.; Madihally, S.V.; Köhler, G.A.; Tayebi, L. Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 911–931. [Google Scholar] [CrossRef] [PubMed]
- Elkhouly, H.; Mamdouh, W.; El-Korashy, D.I. Electrospun nano-fibrous bilayer scaffold prepared from polycaprolactone/gelatin and bioactive glass for bone tissue engineering. J. Mater. Sci. Mater. Med. 2021, 32, 111. [Google Scholar] [CrossRef]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New generation of hybrid materials based on gelatin and bioactive glass particles for bone tissue regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef]
- Sadeghian, A.; Kharaziha, M.; Khoroushi, M. Osteoconductive visible light-crosslinkable nanocomposite for hard tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127761. [Google Scholar] [CrossRef]
- Kazemi, M.; Azami, M.; Johari, B.; Ahmadzadehzarajabad, M.; Nazari, B.; Kargozar, S.; Hajighasemlou, S.; Mozafari, M.; Soleimani, M.; Samadikuchaksaraei, A.; et al. Bone Regeneration in rat using a gelatin/bioactive glass nanocomposite scaffold along with endothelial cells (HUVECs). Int. J. Appl. Ceram. Technol. 2018, 15, 1427–1438. [Google Scholar] [CrossRef]
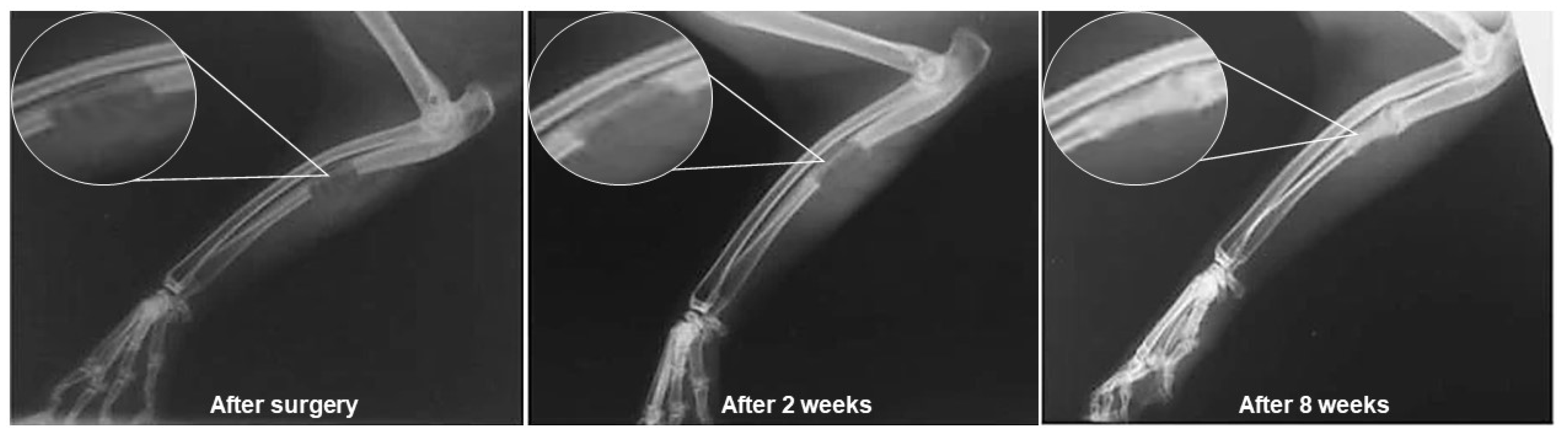
- Hafezi, F.; Hosseinnejad, F.; Fooladi, A.A.I.; Mohit Mafi, S.; Amiri, A.; Nourani, M.R. Transplantation of nano-bioglass/gelatin scaffold in a non-autogenous setting for bone regeneration in a rabbit ulna. J. Mater. Sci. Mater. Med. 2012, 23, 2783–2792. [Google Scholar] [CrossRef]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: A promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; von Marttens, A. Bionanocomposite scaffolds based on chitosan–gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ji, X.; Yan, Y.; Yang, Z.; Chen, X.; Ma, L. Fabrication and Characterization of Ta–GelMA–BG Scaffolds by Chemical Crosslinking Processing for Promotion Osteointegration. Front. Mater. 2021, 8, 701268. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, Y.; Pan, Y.; Yao, Q. Novel three-dimensional bioglass functionalized gelatin nanofibrous scaffolds for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Z.; Ding, L.; Zhang, P.; Liu, C.; Chen, D.; Zhao, F.; Wang, G.; Chen, X. Self-Adhesive Hydrogel Biomimetic Periosteum to Promote Critical-Size Bone Defect Repair via Synergistic Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2022, 14, 36395–36410. [Google Scholar] [CrossRef] [PubMed]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Osteoinductive fibrous scaffolds of biopolymer/mesoporous bioactive glass nanocarriers with excellent bioactivity and long-term delivery of osteogenic drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef]
- Jayalekshmi, A.C.; Sharma, C.P. Gold nanoparticle incorporated polymer/bioactive glass composite for controlled drug delivery application. Colloids Surf. B Biointerfaces 2015, 126, 280–287. [Google Scholar] [CrossRef]
- Gupta, N.; Goel, H.; Santhiya, D.; Srivastava, C.M.; Mishra, S.; Rai, P. Aqueous-Phased Electrospun Bioactive Glass Mineralized Gelatin-Pectin Hybrid Composite Fiber Matrix For 7-Dehydrocholesterol Delivery. ChemistrySelect 2020, 5, 4364–4370. [Google Scholar] [CrossRef]
- Borrego-González, S.; Romero-Sánchez, L.B.; Blázquez, J.; Díaz-Cuenca, A. Nanostructured hybrid device mimicking bone extracellular matrix as local and sustained antibiotic delivery system. Microporous Mesoporous Mater. 2018, 256, 165–176. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S.; Nam, H.Y.; Abd Razak, N.A.B.; Kalantari, K.; Kamarul, T.; Salamatinia, B.; Kadri, N.A. Engineering stiffness in highly porous biomimetic gelatin/tertiary bioactive glass hybrid scaffolds using graphene nanosheets. React. Funct. Polym. 2020, 154, 104668. [Google Scholar] [CrossRef]
- Zhou, Z.; Xiang, L.; Ou, B.; Huang, T.; Zhou, H.; Zeng, W.; Liu, L.; Liu, Q.; Zhao, Y.; He, S.; et al. Biological Assessment In-Vivo of Gel-HA Scaffold Materials Containing Nano-Bioactive Glass for Tissue Engineering. J. Macromol. Sci. Part A 2014, 51, 572–576. [Google Scholar] [CrossRef]
- Zhou, Z.; He, S.; Ou, B.; Huang, T.; Zeng, W.; Liu, L.; Liu, Q.; Chen, J.; Zhao, Y.; Yang, Z.; et al. Influence of Nano-Bioactive Glass (NBG) content on properties of gelatin-hyaluronic acid/NBG composite scaffolds. J. Macromol. Sci. Part B Phys. 2014, 53, 1145–1155. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Hu, Y.; Yang, Q. Preparation of Bioactive Glass/Modified Gelatin/Collagen Composite Scaffold and Its Effect on Repair of Sciatic Nerve Defect. Sci. Adv. Mater. 2020, 12, 1814–1823. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Di Virgilio, A.L.; Audisio, M.C.; Boccaccini, A.R.; Gorustovich, A.A. 45S5 Bioglass® concentrations modulate the release of vancomycin hydrochloride from gelatin–starch films: Evaluation of antibacterial and cytotoxic effects. J. Mater. Sci. 2017, 52, 9091–9102. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Semon, J.A.; Bromet, B.; Day, D.E.; Leu, M.C. Bioprinting with human stem cell-laden alginate-gelatin bioink and bioactive glass for tissue engineering. Int. J. Bioprinting 2019, 5, 204. [Google Scholar] [CrossRef] [PubMed]
- Kolan, K.C.R.; Semon, J.A.; Bindbeutel, A.T.; Day, D.E.; Leu, M.C. Bioprinting with bioactive glass loaded polylactic acid composite and human adipose stem cells. Bioprinting 2020, 18, e00075. [Google Scholar] [CrossRef]
- Bertuola, M.; Aráoz, B.; Gilabert, U.; Gonzalez-Wusener, A.; Pérez-Recalde, M.; Arregui, C.O.; Hermida, É.B. Gelatin–alginate–hyaluronic acid inks for 3D printing: Effects of bioglass addition on printability, rheology and scaffold tensile modulus. J. Mater. Sci. 2021, 56, 15327–15343. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Janmohammadi, M.; Arab, S.; Talebi, A.; Nooshabadi, V.T.; Koohsarian, P.; Nourbakhsh, M.S. Bone Scaffolds: An Incorporation of Biomaterials, Cells, and Biofactors. ACS Biomater. Sci. Eng. 2021, 7, 5397–5431. [Google Scholar] [CrossRef]
- Sohrabi, M.; Yekta, B.E.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. Enhancing mechanical properties and biological performances of injectable bioactive glass by gelatin and chitosan for bone small defect repair. Biomedicines 2020, 8, 616. [Google Scholar] [CrossRef]
- Nawaz, A.; Bano, S.; Yasir, M.; Wadood, A.; Ur Rehman, M.A. Ag and Mn-doped mesoporous bioactive glass nanoparticles incorporated into the chitosan/gelatin coatings deposited on PEEK/bioactive glass layers for favorable osteogenic differentiation and antibacterial activity. Mater. Adv. 2020, 1, 1273–1284. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Yang, X.; Han, C.; Gao, C.; Gou, Z. Bioactive glasses-incorporated, core–shell-structured polypeptide/polysaccharide nanofibrous hydrogels. Carbohydr. Polym. 2013, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Hassanzadeh Chinijani, T.; Malek Khachatourian, A.; Vinicius Lia Fook, M.; Baino, F.; Montazerian, M. A critical review of bioactive glasses and glass–ceramics in cancer therapy. Int. J. Appl. Glass Sci. 2022, 14, 69–87. [Google Scholar] [CrossRef]
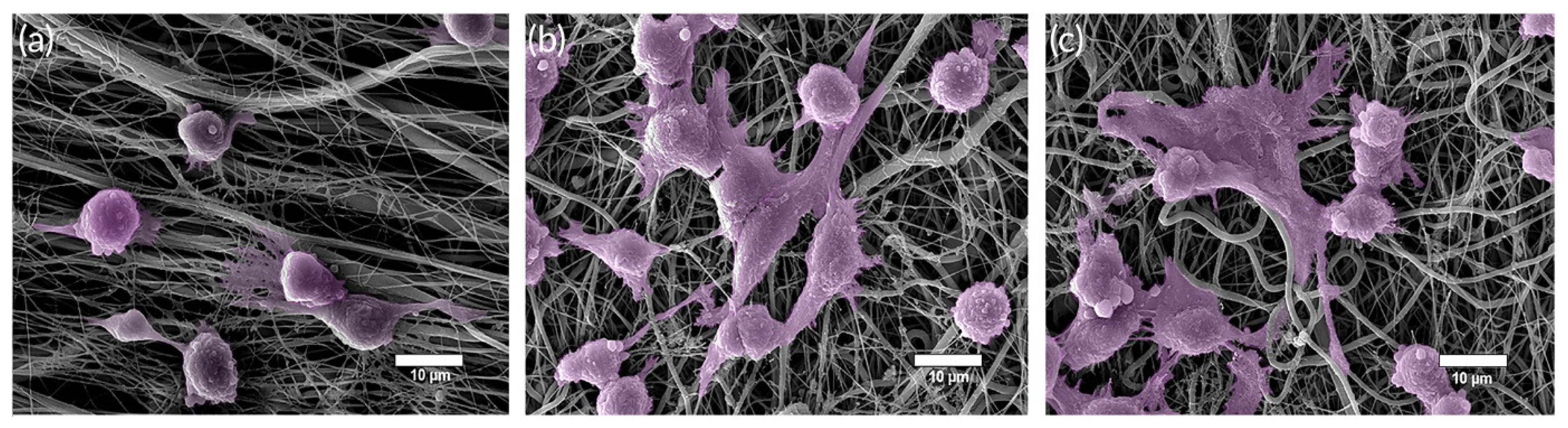


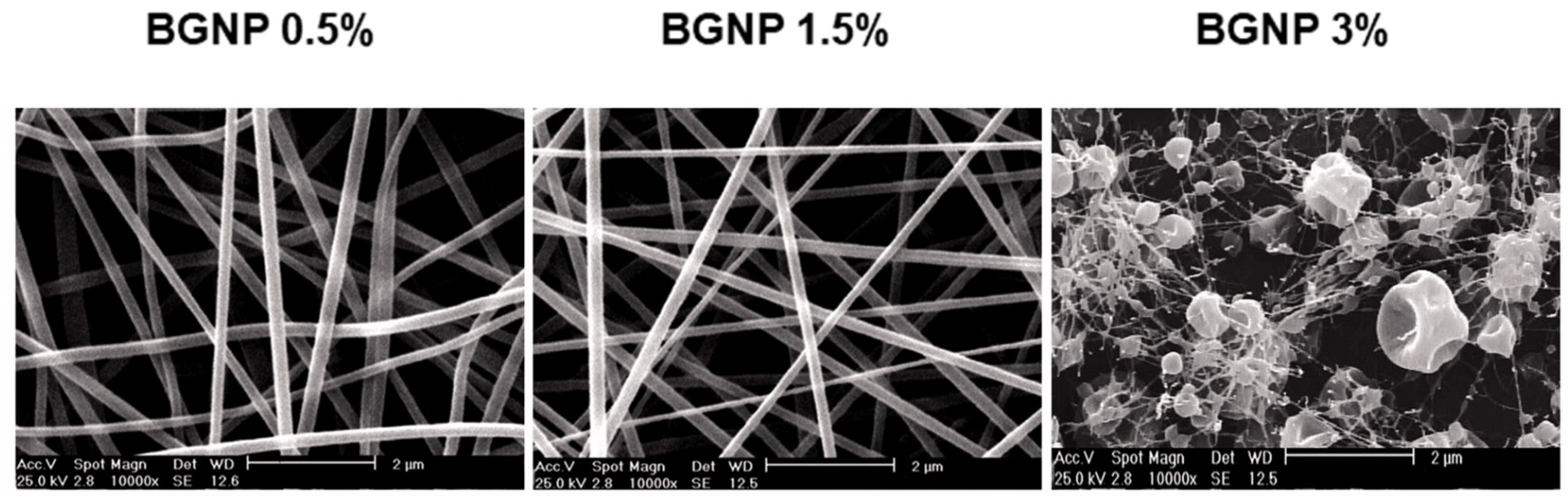
| Ion | Properties | References |
|---|---|---|
| Ag | Exhibits bactericidal and anti-inflammatory actions Promotes bone formation in vivo Enhances VEGF expression | [23,25,28,106,121,122,123] |
| B | Stimulates bone formation Increases collagen gene expression Induces angiogenesis | [119,124,125,126] |
| Ba | Exhibits anti-inflammatory effect Increases cell migration Effective healer of gastric ulcers Radiopacifier | [127,128,129] |
| Ce | Promotes angiogenesis It has an antibacterial effect Increases collagen production and osteoblastic differentiation | [27,109,119] |
| Cl | Increases the degradation rate Stimulates the apatite formation | [130,131] |
| Cu | It has an antibacterial/antimicrobial effect It favors osteogenesis and angiogenesis | [119,132,133] |
| F | Exhibits antibacterial and anti-inflammatory action Stimulates osteoblast activity when inserted in moderate concentrations Favors the formation of fluorapatite (FAp) | [119,134] |
| Fe | It is promising for hyperthermia therapy Induces the ferroptosis of tumor cells Stimulates osteoblastic proliferation | [135,136,137] |
| Ga | Exhibits antibacterial and bacteriostatic action Effective against sarcoma cells Promotes apatite formation | [119,138,139,140] |
| Li | Induces osteoblastic cell activity Stimulates angiogenesis | [119,141,142,143] |
| Mg | Stimulates the expression of type I collagen Induces alkaline phosphatase (ALP) activity Increases angiogenesis | [17,144,145,146] |
| Mn | Increases in vitro bioactivity Promotes greater mechanical strength Exhibits antibacterial effect | [147,148,149] |
| Sr | Improves mechanical stability Increases in vitro bioactivity Increases bone cell adhesion and stability May optimize the treatment of osteoporosis Radiopacifier | [119,129,150,151,152,153] |
| Zn | Induces cell proliferation and bone formation It has antibacterial activity Increases mechanical stability It has an anti-inflammatory effect Radiopacifier | [119,129,144,154] |
| Zr | Produces greater mechanical strength Exhibits antibacterial effect Improves the proliferation of osteoblastic cells Radiopacifier | [116,129,155,156] |
| Composite | Type | Method | Main Results | Reference |
|---|---|---|---|---|
| Gelatin/BG 55SiO2–24CaO–6P2O5–15B2O3 | Hydrogels/ Scaffolds | Lyophilization | Notable increase in bioactivity and durability | [20] |
| Gelatin-chitosan-polyethylene oxide/ Ag-doped BG 45SiO2–24.5CaO–24.5Na2O–6P2O5 | Nanofibers | Electrospinning | Showed antibacterial activity against gram-positive and gram-negative species; induced a considerable reduction in wound area between 3 and 7 days | [23] |
| Gelatin- polycaprolactone/ BG 60SiO2–30CaO–8P2O5–2Ag2O | Hydrogels/ Scaffolds | Electrospinning | Induced tissue granulation and proliferation of fibroblast cells after the first week of implantation | [25] |
| Gelatin/BG SiO2–CaO–P2O5–CeO2 | Hydrogels | Mixing solution | Promoted a wound closing rate of 94.85 ± 2.33% and antibacterial effect against E. coli and S. aureuse | [27] |
| Gelatin/BG 64SiO2–5P2O5–26CaO–5MgO | Scaffolds | Freeze-drying | Confirmed antibacterial activity and proliferation of fibroblasts after 3 days | [28] |
| Gelatin-alginate/BG 45SiO2–24.5CaO–24.5Na2O–6P2O5 | Scaffolds | Lyophilization | Greater elastic recovery and in vitro degradation rate | [30] |
| Gelatin-chitosan /BG 50SiO2–10P2O5–34CaO–5SrO–1Ag2O | Coatings | Electrophoretic deposition | Increase in the bioactivity and antibacterial action against gram-negative species | [121] |
| Gelatin/Ag-doped BG 58SiO2–33CaO–9P2O5 | Scaffolds | Commercial sponges loaded with BG suspension | Greater filling of the defect with newly formed bone tissue and promising release of vancomycin | [122] |
| Gelatin-silk fibroin/Cu-doped BG | Hydrogels/ Scaffolds | 3D printing | Promotion of significant vascularization and osteogenesis | [132] |
| Gelatin/BG 95SiO2–2.5CaO–2.5CuO | Scaffolds | Foam replica | Increase in the osteogenic activity and the antibacterial effect | [133] |
| Gelatin/BG SiO2–CaO–P2O5–MgO–ZnO | Scaffolds | Freeze-drying | Promotion of antibacterial activity, stimulation of type I collagen expression and alkaline phosphatase (ALP) activity | [144] |
| Gelatin/BG 50SiO2–35CaO–10P2O5–5MnO | Scaffolds | Foam replica | Exhibited higher cell viability in vitro and five times higher compressive strength | [147] |
| Gelatin/Sr-modified BG SiO2–CaO–P2O5 | Scaffolds | Freeze-drying | Accelerated degradation rate, improved compressive strength and elastic modulus | [150] |
| Gelatin/BG 45P2O5–24CaO–21Na2O–5SrO–5Fe2O3 | Scaffolds | Freeze-drying | Sustained release of ciprofloxacin | [151] |
| Gelatin/BG SiO2–CaO–SrO–P2O5 | Scaffolds | Freeze-drying | Promoted bone tissue formation and the presence of mature collagen after 4 weeks | [152] |
| Gelatin-sodium alginate/BG 80SiO2–16CaO–4P2O5 mol | Scaffolds | 3D printing | Exhibited high biocompatibility, biodegradability and osteogenesis | [180] |
| GelMa/BG 40SiO2–45CaO–15P2O5 | Hydrogels/ Scaffolds | Lyophilization | Promotion of cell adhesion, proliferation and osteogenic differentiation | [187] |
| Gelatin/BG 54.2SiO2–35CaO–10.8P2O5 | Scaffolds | Casting and freeze-drying | Good in vitro mineralization and biocompatibility; stiffness of 50–60 kPa, suitable for dental pulp regeneration | [188] |
| Gelatin-dialdehyde alginate/Cu-doped BG 85SiO2–15CaO | Scaffolds | 3D printing | Increase in ALP activity and apatite deposition in vitro 3 days | [192] |
| Gelatin- polycaprolactone/ BG 45SiO2–24.5CaO–6P2O5 | Fibers | Electrospinning | Higher hydrophilic, swelling, tensile strength, elastic modulus and ductility; apatite deposition after 14 days | [194] |
| Gelatin-GPTMS/ BGs 54.6SiO2–22.1CaO–7.9K2O–7.7MgO–6Na2O–1.7P2O5 and 43.7SiO2–22.1CaO–7.9K2O–7.7MgO–6Na2O–1.7P2O5–10.9B2O3 | Scaffolds | Freeze-drying | Precipitation of hydroxyapatite after 2 weeks | [195] |
| GelMa/BG 45SiO2–24.5CaO–6P2O5 | Hydrogels | Mixing solution and sonication | Increase in the proliferation of osteoblastic cells, in vitro cell viability, ALP activity and mechanical properties | [196] |
| Gelatin/BG 64SiO2–31CaO–5P2O5 | Scaffolds | Lyophilization and lamination | Considerable cell viability and in vitro bioactivity; tissue growth increased between 4 and 12 weeks | [197] |
| Gelatin-chitosan/ BG 58SiO2–40CaO–5P2O5 | Scaffolds | Lyophilization | Increase of ~80% in the amount of new bone tissue formed after 8 weeks of implantation | [200] |
| Gelatin/BG 60SiO2–36CaO–4P2O5 | Scaffolds | Lyophilization | Significant osseointegration in the initial phase of implantation | [201] |
| Gelatin/BG 45SiO2–24.5CaO–24.5Na2O–6P2O5 | Scaffolds | Freeze-drying | Newly formed bone tissue after 6 weeks | [202] |
| Gelatin- hyaluronic acid/BG 60SiO2–36CaO–4P2O5 | Hydrogels | Mixing solution | Effective revascularization and bone regeneration within 8 weeks | [203] |
| Gelatin-GPTMS/ BG 58SiO2–33CaO–9P2O5 and graphene oxide | Scaffolds | Gas foam | Showed biocompatible and stimulated cell adhesion and proliferation in vitro | [208] |
| GelMa-collagen/BG | Scaffolds | Lyophilization | Induced biocompatibility, adhesion, proliferation, and cell differentiation, culminating in nerve fibers formation | [211] |
| Gelatin-alginate/ BG 53B2O3–20CaO–12K2O–6Na2O–5MgO–4P2O5 | Scaffolds | 3D printing | Improved mechanical properties and cell viability | [213] |
| Gelatin- alginate/PLA/ BG 53B2O3–20CaO–12K2O–6Na2O–5MgO–4P2O5 | Scaffolds | 3D printing | Demonstrated faster dissolution and bioactivity in 3D cell culture conditions | [214] |
| Gelatin-hyaluronic acid/BG 45SiO2–24.5CaO–24.5Na2O–6P2O5 | Scaffolds | 3D printing | Showed bioactivity and greater surface reactivity; increased the modulus of elasticity | [215] |
| Gelatin-chitosan/BG 64SiO2–27CaO–4MgO–5P2O5 and GPTMS | Injectable pastes | Mixing solution and air drying | Superior mechanical resistance; improved the metabolic activity of cells and supported stem cells’ osteogenic differentiation in a 3D model | [217] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2023, 14, 23. https://doi.org/10.3390/jfb14010023
Barreto MEV, Medeiros RP, Shearer A, Fook MVL, Montazerian M, Mauro JC. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. Journal of Functional Biomaterials. 2023; 14(1):23. https://doi.org/10.3390/jfb14010023
Chicago/Turabian StyleBarreto, Maria E. V., Rebeca P. Medeiros, Adam Shearer, Marcus V. L. Fook, Maziar Montazerian, and John C. Mauro. 2023. "Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review" Journal of Functional Biomaterials 14, no. 1: 23. https://doi.org/10.3390/jfb14010023
APA StyleBarreto, M. E. V., Medeiros, R. P., Shearer, A., Fook, M. V. L., Montazerian, M., & Mauro, J. C. (2023). Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. Journal of Functional Biomaterials, 14(1), 23. https://doi.org/10.3390/jfb14010023









