Full Skin Equivalent Models for Simulation of Burn Wound Healing, Exploring Skin Regeneration and Cytokine Response
Abstract
:1. Introduction
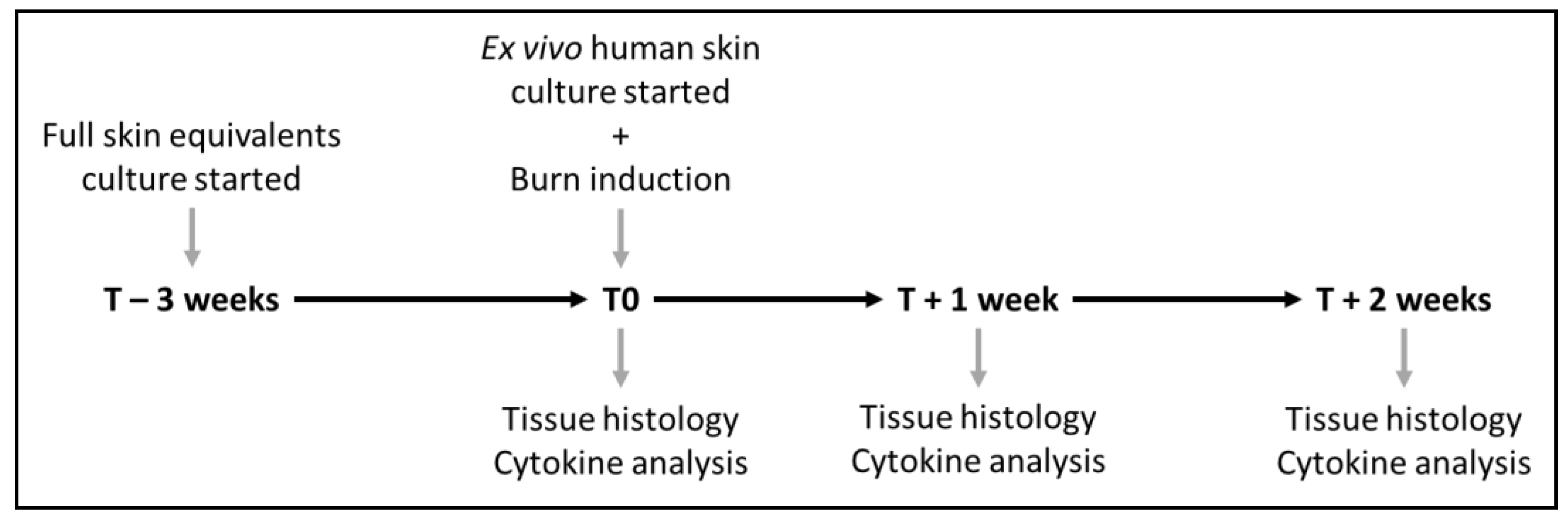
2. Materials and Methods
2.1. Isolation of Keratinocytes and Fibroblasts
2.2. Full Skin Equivalent Models
2.3. Ex Vivo Human Skin Model
2.4. Induction of Burn Injury
2.5. Immunohistochemistry
2.6. Microscopy
2.7. Re-Epithelization Rate
2.8. Immunoassay of Culture Medium
2.9. Statistical Analysis and Data Visualization
3. Results
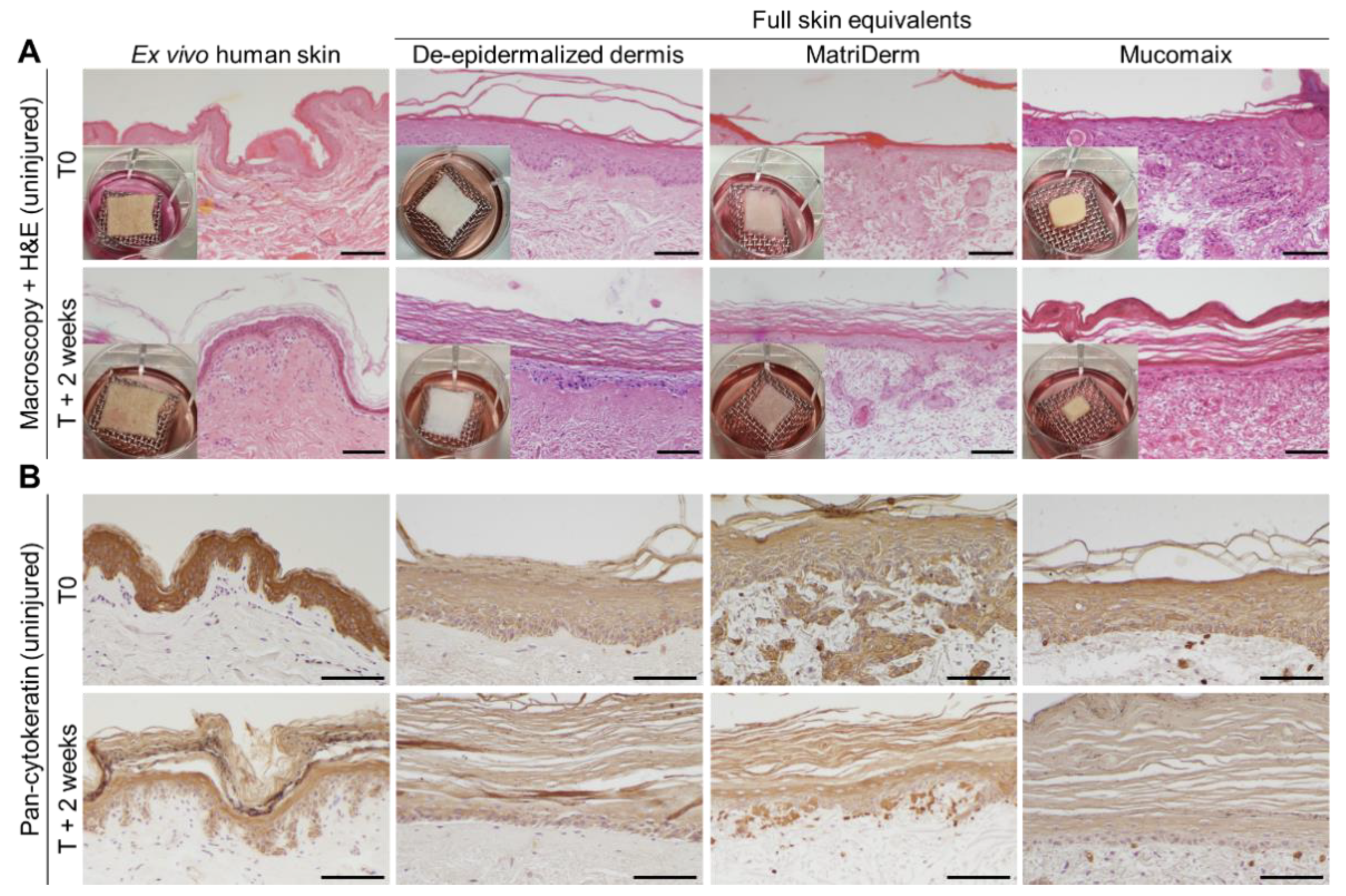
3.1. Skin Morphogenesis in Full Skin Equivalent Models Was Similar to Ex Vivo Human Skin
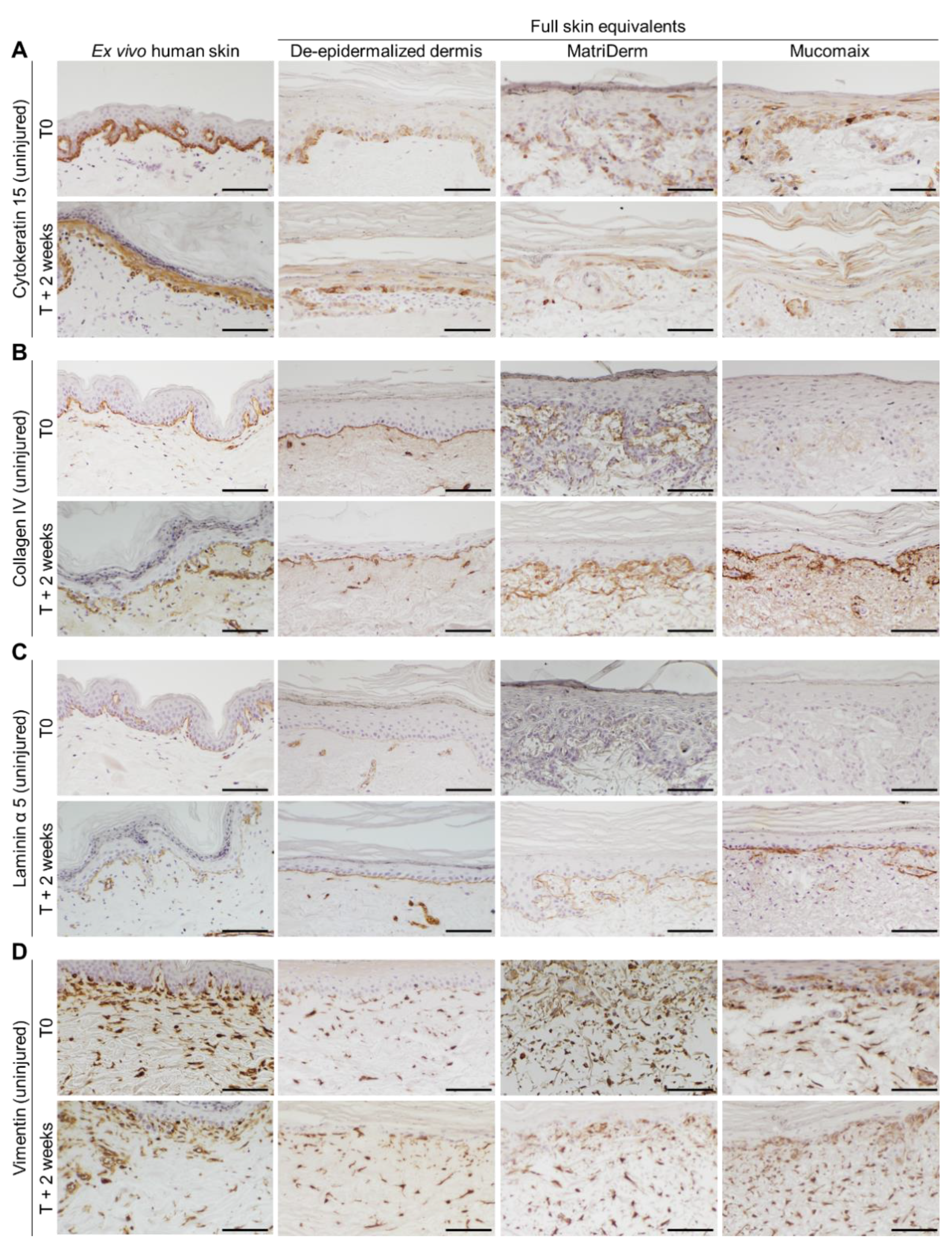
3.2. Epidermal and Dermal Structures Developed Completely in Full Skin Equivalents
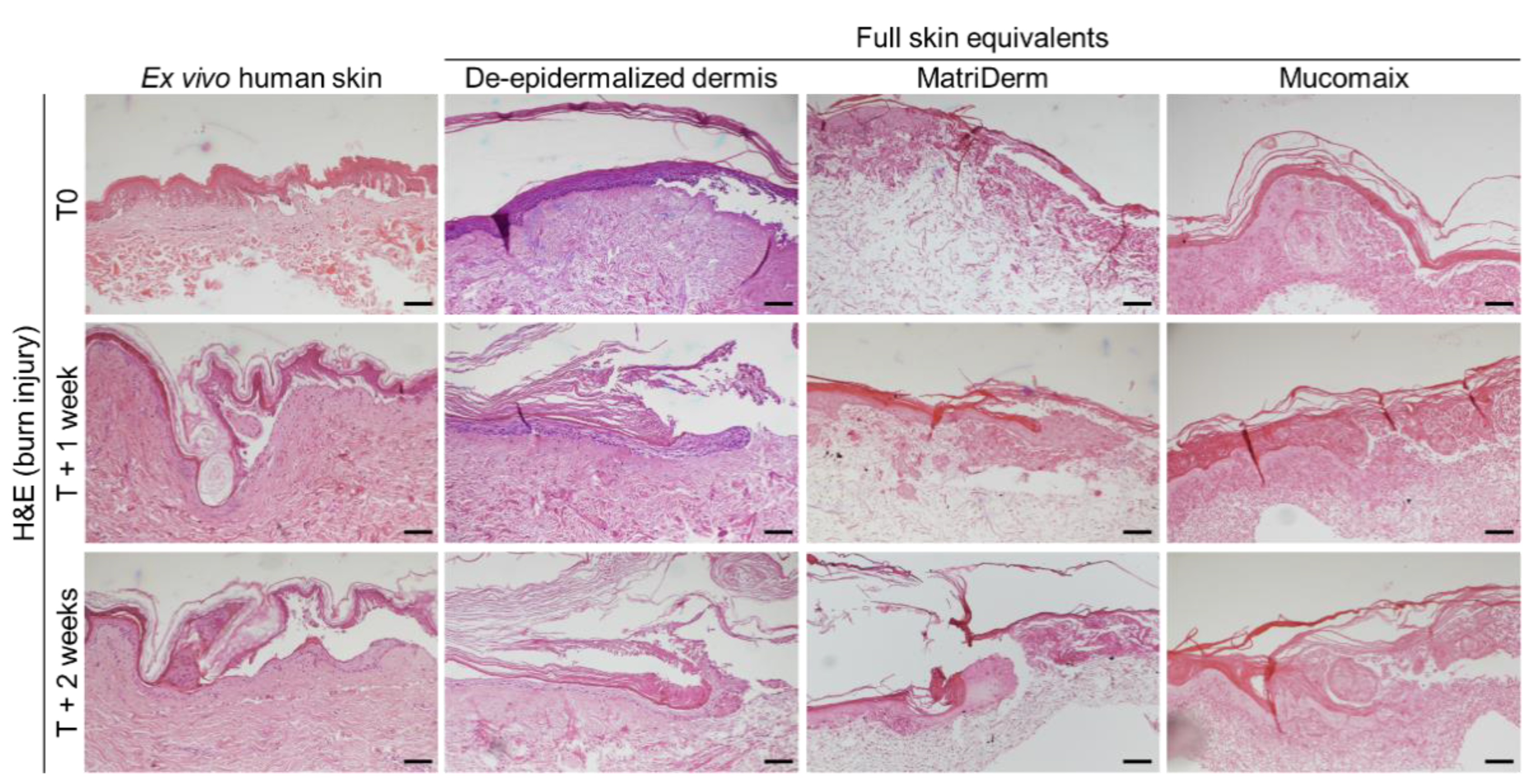
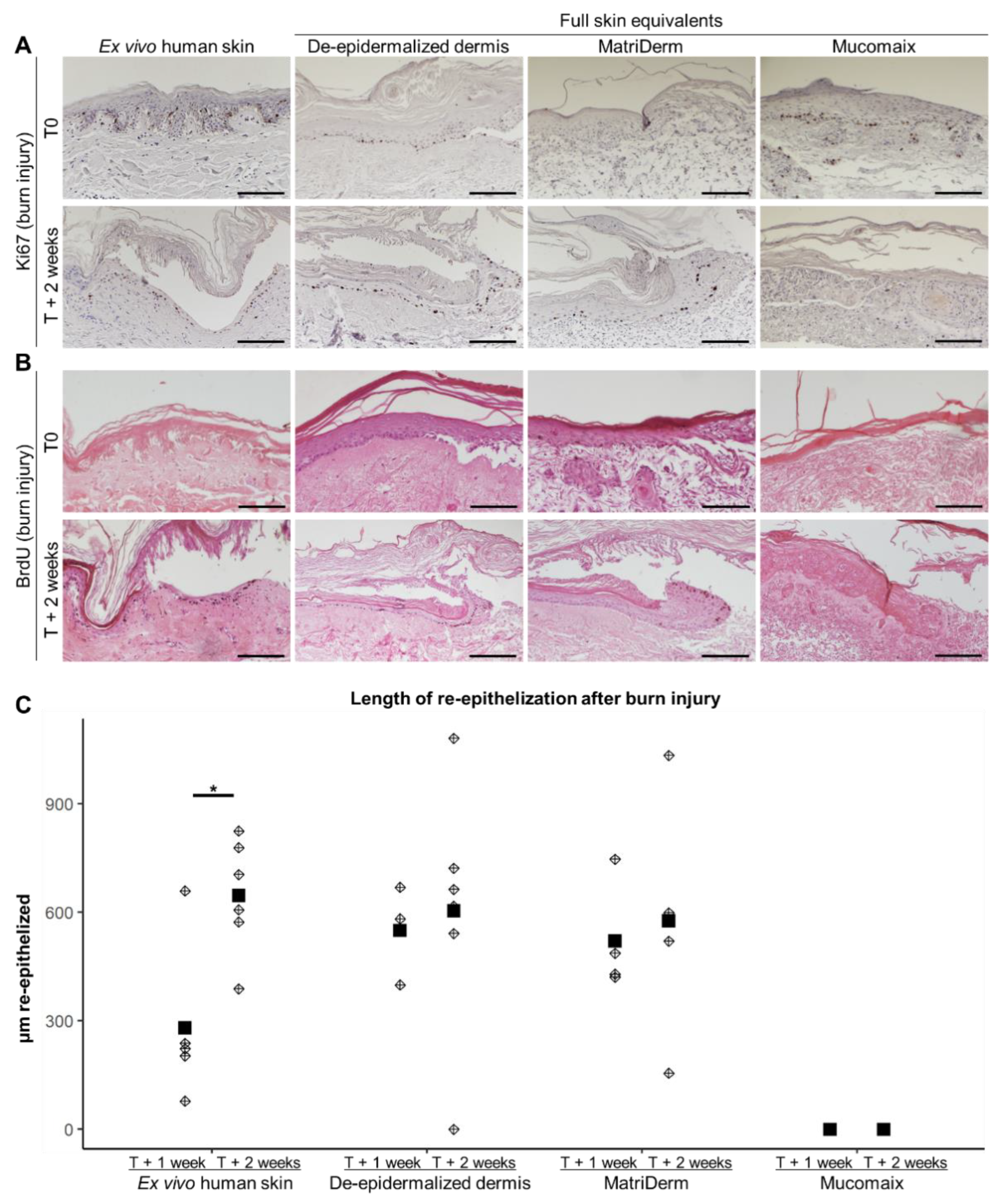
3.3. Regenerative Capacity of MatriDerm-Based FSEs Was Similar to DED-Based FSEs and Ex Vivo Human Skin at 2 Weeks after Burn Injury
3.4. Cytokine Response of Burn-Injured Full Skin Equivalent Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Comish, P.B.; Carlson, D.; Kang, R.; Tang, D. Damage-Associated Molecular Patterns and the Systemic Immune Consequences of Severe Thermal Injury. J. Immunol. 2020, 205, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.; Vlig, M.; Fasse, E.; Stoop, M.; Pijpe, P.; van Zuijlen, P.; Joosten, I.; Boekema, B.; Koenen, H. Burn-Injured Skin Is Marked by a Prolonged Local Acute Inflammatory Response of Innate Immune Cells and Pro- Inflammatory Cytokines. Front. Immunol. 2022, 13, 1034420. [Google Scholar] [CrossRef] [PubMed]
- Orgill, D.P. Excision and Skin Grafting of Thermal Burns. N. Engl. J. Med. 2009, 360, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The Use of Skin Models in Drug Development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar] [CrossRef]
- Hao, D.; Nourbakhsh, M. Recent Advances in Experimental Burn Models. Biology 2021, 10, 526. [Google Scholar] [CrossRef]
- Mulder, P.P.G.; Koenen, H.J.P.M.; Vlig, M.; Joosten, I.; de Vries, R.B.M.; Boekema, B.K.H.L. Burn-Induced Local and Systemic Immune Response: Systematic Review and Meta-Analysis of Animal Studies. J. Investig. Dermatol. 2022, 142, 3093–3109.e15. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal Models in Burn Research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [Green Version]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Zomer, H.D.; Trentin, A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef]
- Hubrecht; Carter The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [CrossRef] [PubMed] [Green Version]
- Ozdogan, C.Y.; Kenar, H.; Davun, K.E.; Yucel, D.; Doger, E.; Alagoz, S. An In Vitro 3D Diabetic Human Skin Model from Diabetic Primary Cells. Biomed. Mater. 2021, 16, 015027. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rinderknecht, H.; Histing, T.; Kolbenschlag, J.; Nussler, A.K.; Ehnert, S. Establishment of an In Vitro Scab Model for Investigating Different Phases of Wound Healing. Bioengineering 2022, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/Glycosaminoglycan-Based Matrices for Controlling Skin Cell Responses. Biol. Chem. 2021, 402, 1325–1335. [Google Scholar] [CrossRef]
- Hossian, A.K.M.N.; Mattheolabakis, G. Cellular Migration Assay: An In Vitro Technique to Simulate the Wound Repair Mechanism. In Wound Regeneration; Humana: New York, NY, USA, 2021; pp. 77–83. [Google Scholar]
- van Drongelen, V.; Haisma, E.M.; Out-Luiting, J.J.; Nibbering, P.H.; El Ghalbzouri, A. Reduced Filaggrin Expression Is Accompanied by Increased Staphylococcus Aureus Colonization of Epidermal Skin Models. Clin. Exp. Allergy 2014, 44, 1515–1524. [Google Scholar] [CrossRef]
- De Breij, A.; Haisma, E.M.; Rietveld, M.; El Ghalbzouri, A.; Van Den Broek, P.J.; Dijkshoorn, L.; Nibbering, P.H. Three-Dimensional Human Skin Equivalent as a Tool to Study Acinetobacter Baumannii Colonization. Antimicrob. Agents Chemother. 2012, 56, 2459–2464. [Google Scholar] [CrossRef] [Green Version]
- Haisma, E.M.; Rietveld, M.H.; Breij, A.; Van Dissel, J.T.; El Ghalbzouri, A.; Nibbering, P.H. Inflammatory and Antimicrobial Responses to Methicillin-Resistant Staphylococcus Aureus in an In Vitro Wound Infection Model. PLoS ONE 2013, 8, e82800. [Google Scholar] [CrossRef] [Green Version]
- Urciuolo, F.; Passariello, R.; Imparato, G.; Casale, C.; Netti, P.A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233. [Google Scholar] [CrossRef]
- Coolen, N.A.; Verkerk, M.; Reijnen, L.; Vlig, M.; Van Den Bogaerdt, A.J.; Breetveld, M.; Gibbs, S.; Middelkoop, E.; Ulrich, M.M.W. Culture of Keratinocytes for Transplantation without the Need of Feeder Layer Cells. Cell Transpl. 2007, 16, 649–661. [Google Scholar] [CrossRef]
- Waaijman, T.; Breetveld, M.; Ulrich, M.; Middelkoop, E.; Scheper, R.J.; Gibbs, S. Use of a Collagen-Elastin Matrix as Transport Carrier System to Transfer Proliferating Epidermal Cells to Human Dermis In Vitro. Cell Transpl. 2010, 19, 1339–1348. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, S.W.; Turin, S.Y.; Zielinski, E.R.; Galiano, R.D. Matrices and Dermal Substitutes for Wound Treatment; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 9783319669908. [Google Scholar]
- Alrubaiy, L.; Al-Rubaiy, K.K. Skin Substitutes: A Brief Review of Types and Clinical Applications. Oman Med. J. 2009, 24, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, F.B.; Castro, J.C.D.; Almeida, I.R.; Farina-Junior, J.A.; Coltro, P.S. Evaluation of Contraction of the Split-Thickness Skin Graft Using Three Dermal Matrices in the Treatment of Burn Contractures: A Randomised Clinical Trial. Wound Repair Regen. 2022, 30, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Yun, I.S.; Lew, D.H.; Roh, T.S.; Lee, W.J. The Use of Matriderm and Autologous Skin Graft in the Treatment of Full Thickness Skin Defects. Arch. Plast. Surg. 2014, 41, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The Use of Dermal Substitutes in Burn Surgery: Acute Phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef]
- Udeabor, S.E.; Herrera-Vizcaíno, C.; Sader, R.; Kirkpatrick, C.J.; Al-Maawi, S.; Ghanaati, S. Characterization of the Cellular Reaction to a Collagen-Based Matrix: An In Vivo Histological and Histomorphometrical Analysis. Materials 2020, 13, 2730. [Google Scholar] [CrossRef]
- Bose, A.; Teh, M.T.; Mackenzie, I.C.; Waseem, A. Keratin K15 as a Biomarker of Epidermal Stem Cells. Int. J. Mol. Sci. 2013, 14, 19385–19398. [Google Scholar] [CrossRef] [Green Version]
- Amano, S.; Akutsu, N.; Matsunaga, Y.; Kadoya, K.; Nishiyama, T.; Champliaud, M.F.; Burgeson, R.E.; Adachi, E. Importance of Balance between Extracellular Matrix Synthesis and Degradation in Basement Membrane Formation. Exp. Cell Res. 2001, 271, 249–262. [Google Scholar] [CrossRef]
- Murphy, G.F.; Flynn, T.C.; Rice, R.H.; Pinkus, G.S. Involucrin Expression in Normal and Neoplastic Human Skin: A Marker for Keratinocyte Differentiation. J. Investig. Dermatol. 1984, 82, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zieman, A.; Coulombe, P.A. Skin Keratins. In Physiology & Behavior; Academic Press: Cambridge, MA, USA, 2016; Volume 176, pp. 303–350. ISBN 2163684814. [Google Scholar]
- Lee, J.; Koehler, K.R. Skin Organoids: A New Human Model for Developmental and Translational Research. Exp. Dermatol. 2021, 30, 613–620. [Google Scholar] [CrossRef]
- Jevtić, M.; Löwa, A.; Nováčková, A.; Kováčik, A.; Kaessmeyer, S.; Erdmann, G.; Vávrová, K.; Hedtrich, S. Impact of Intercellular Crosstalk between Epidermal Keratinocytes and Dermal Fibroblasts on Skin Homeostasis. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118722. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Lamme, E.; Ponec, M. Crucial Role of Fibroblasts in Regulating Epidermal Morphogenesis. Cell Tissue Res. 2002, 310, 189–199. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Hensbergen, P.; Gibbs, S.; Kempenaar, J.; Van Der Schors, R.; Ponec, M. Fibroblasts Facilitate Re-Epithelialization in Wounded Human Skin Equivalents. Lab. Investig. 2004, 84, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; Van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H.J.P.M. Crosstalk between Keratinocytes and T Cells in a 3D Microenvironment: A Model to Study Inflammatory Skin Diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Cho, K.H. The Effects of Epidermal Keratinocytes and Dermal Fibroblasts on the Formation of Cutaneous Basement Membrane in Three-Dimensional Culture Systems. Arch. Dermatol. Res. 2005, 296, 296–302. [Google Scholar] [CrossRef]
- Breetveld, M.; Richters, C.D.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Comparison of Wound Closure after Burn and Cold Injury in Human Skin Equivalents. J. Investig. Dermatol. 2006, 126, 1918–1921. [Google Scholar] [CrossRef] [Green Version]
- Griffoni, C.; Neidhart, B.; Yang, K.; Groeber-Becker, F.; Maniura-Weber, K.; Dandekar, T.; Walles, H.; Rottmar, M. In Vitro Skin Culture Media Influence the Viability and Inflammatory Response of Primary Macrophages. Sci. Rep. 2021, 11, 7070. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, Y.; Li, M.; Yan, Y.; Cheng, R.; Zhao, Y.; Shao, Q.; Wang, J.; Sang, S. Construction of Tissue-Engineered Skin with Rete Ridges Using Co-Network Hydrogels of Gelatin Methacrylated and Poly(Ethylene Glycol) Diacrylate. Mater. Sci. Eng. C 2021, 129, 112360. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Zhang, M.; Song, F.; Feng, C.; Liu, H. Simple and Robust 3D Bioprinting of Full-Thickness Human Skin Tissue. Bioengineered 2022, 13, 10087–10097. [Google Scholar] [CrossRef]
- Thakoersing, V.S.; Gooris, G.S.; Mulder, A.; Rietveld, M.; El Ghalbzouri, A.; Bouwstra, J.A. Unraveling Barrier Properties of Three Different In-House Human Skin Equivalents. Tissue Eng.-Part C Methods 2012, 18, 1–11. [Google Scholar] [CrossRef]
- Boehnke, K.; Mirancea, N.; Pavesio, A.; Fusenig, N.E.; Boukamp, P.; Stark, H.J. Effects of Fibroblasts and Microenvironment on Epidermal Regeneration and Tissue Function in Long-Term Skin Equivalents. Eur. J. Cell Biol. 2007, 86, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Erikss, J.E. Vimentin Coordinates Fibroblast Proliferation and Keratinocyte Differentiation in Wound Healing via TGF-β-Slug Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middelkoop, E.; de Vries, H.J.C.; Ruuls, L.; Everts, V.; Wildevuur, C.H.R.; Westerhof, W. Adherence, Proliferation and Collagen Turnover by Human Fibroblasts Seeded into Different Types of Collagen Sponges. Cell Tissue Res. 1995, 280, 447–453. [Google Scholar] [CrossRef]
- Iljas, J.D.; Röhl, J.; Parker, T.J.; McGovern, J.A.; Moromizato, K.H.; Cuttle, L. A Human Skin Equivalent Burn Model to Study the Effect of a Nanocrystalline Silver Dressing on Wound Healing. Burns 2021, 47, 417–429. [Google Scholar] [CrossRef]
- Schneider, V.; Kruse, D.; de Mattos, I.B.; Zöphel, S.; Tiltmann, K.K.; Reigl, A.; Khan, S.; Funk, M.; Bodenschatz, K.; Groeber-Becker, F. A 3D In Vitro Model for Burn Wounds: Monitoring of Regeneration on the Epidermal Level. Biomedicines 2021, 9, 1153. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair. Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- van den Broek, L.J.; Bergers, L.I.J.C.; Reijnders, C.M.A.; Gibbs, S. Progress and Future Prospectives in Skin-on-Chip Development with Emphasis on the Use of Different Cell Types and Technical Challenges. Stem Cell Rev. Rep. 2017, 13, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, M.N.; Jeschke, M.G.; Amini-Nik, S. Cellularized Bilayer Pullulan-Gelatin Hydrogel for Skin Regeneration. Tissue Eng.-Part A 2016, 22, 754–764. [Google Scholar] [CrossRef] [Green Version]
- Pontiggia, L.; Van Hengel, I.A.J.; Klar, A.; Rütsche, D.; Nanni, M.; Scheidegger, A.; Figi, S.; Reichmann, E.; Moehrlen, U.; Biedermann, T. Bioprinting and Plastic Compression of Large Pigmented and Vascularized Human Dermo-Epidermal Skin Substitutes by Means of a New Robotic Platform. J. Tissue Eng. 2022, 13, 20417314221088513. [Google Scholar] [CrossRef]
- Hosseini, M.; Koehler, K.R.; Shafiee, A. Biofabrication of Human Skin with Its Appendages. Adv. Healthc. Mater. 2022, 11, 2201626. [Google Scholar] [CrossRef]
- Abaci, H.E.; Guo, Z.; Doucet, Y.; Jacków, J.; Christiano, A. Next Generation Human Skin Constructs as Advanced Tools for Drug Development. Exp. Biol. Med. 2017, 242, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, L.J.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Development, Validation, and Testing of a Human Tissue Engineered Hypertrophic Scar Model. ALTEX 2012, 29, 389–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergers, L.I.J.C.; Reijnders, C.M.A.; van den Broek, L.J.; Spiekstra, S.W.; de Gruijl, T.D.; Weijers, E.M.; Gibbs, S. Immune-Competent Human Skin Disease Models. Drug Discov. Today 2016, 21, 1479–1488. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulder, P.P.G.; Raktoe, R.S.; Vlig, M.; Elgersma, A.; Middelkoop, E.; Boekema, B.K.H.L. Full Skin Equivalent Models for Simulation of Burn Wound Healing, Exploring Skin Regeneration and Cytokine Response. J. Funct. Biomater. 2023, 14, 29. https://doi.org/10.3390/jfb14010029
Mulder PPG, Raktoe RS, Vlig M, Elgersma A, Middelkoop E, Boekema BKHL. Full Skin Equivalent Models for Simulation of Burn Wound Healing, Exploring Skin Regeneration and Cytokine Response. Journal of Functional Biomaterials. 2023; 14(1):29. https://doi.org/10.3390/jfb14010029
Chicago/Turabian StyleMulder, Patrick P. G., Rajiv S. Raktoe, Marcel Vlig, Anouk Elgersma, Esther Middelkoop, and Bouke K. H. L. Boekema. 2023. "Full Skin Equivalent Models for Simulation of Burn Wound Healing, Exploring Skin Regeneration and Cytokine Response" Journal of Functional Biomaterials 14, no. 1: 29. https://doi.org/10.3390/jfb14010029
APA StyleMulder, P. P. G., Raktoe, R. S., Vlig, M., Elgersma, A., Middelkoop, E., & Boekema, B. K. H. L. (2023). Full Skin Equivalent Models for Simulation of Burn Wound Healing, Exploring Skin Regeneration and Cytokine Response. Journal of Functional Biomaterials, 14(1), 29. https://doi.org/10.3390/jfb14010029







