In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Fabrication
2.2. Surface Wettability
2.3. Scanning Electron Microscopy and Confocal Scanning Microscopy
2.4. Bacterial Strains and Culture Conditions
2.5. Biofilm Development and Bacterial Viability Assay
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Hamad, K.Q.; Al Rashdan, B.A.; Al Omari, W.M.; Baba, N.Z. Comparison of the fit of lithium disilicate crowns made from conventional, digital, or conventional/digital techniques. J. Prosthodont. 2019, 28, e580–e586. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, H.; Yang, J.; Cai, Y.; Hu, X.; Yang, Y.; Li, B.; Li, H.; Li, H.; Li, C.; et al. Femtosecond Laser-Induced Micropattern and Ca/P Deposition on Ti Implant Surface and Its Acceleration on Early Osseointegration. ACS Appl. Mater. Interfaces 2013, 5, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Barao, V.A. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Femtosecond laser structuring of titanium implants. Appl. Surf. Sci. 2007, 253, 7272–7280. [Google Scholar] [CrossRef]
- Zeller, B.; Stöckli, S.; Zaugg, L.K.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Biofilm formation on metal alloys, zirconia and polyetherketoneketone as implant materials in vivo. Clin. Oral Implant. Res. 2020, 31, 1078–1086. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A.; Negri, B.; Fernández, M.P.R.; Gómez-Moreno, G. Histological and Histomorphometric Evaluation of Zirconia Dental Implants Modified by Femtosecond Laser versus Titanium Implants: An Experimental Study in Fox Hound Dogs. Clin. Implant. Dent. Relat. Res. 2015, 17, 525–532. [Google Scholar] [CrossRef]
- Cavalcanti, A.N.; Foxton, R.M.; Oliveira, T.F.W.M.T.; Giannini, M.; Marchi, G.M. Y–TZP Ceramics: Key Concepts for Clinical Application. Oper. Dent. 2009, 34, 344–351. [Google Scholar] [CrossRef]
- Delgado-Ruı, R.A.; Calvo-Guirado, J.L.; Moreno, P.; Guardia, J.; Gomez-Moreno, G.; Mate-Sa, J.E.; Ramirez-Ferna, P.; Chiva, F. Femtosecond laser microstructuring of zirconia dental implants. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96B, 91–100. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2010, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef]
- Manor, Y.; Oubaid, S.; Mardinger, O.; Chaushu, G.; Nissan, J. Characteristics of early versus late implant failure: A retrospective study. J. Oral Maxillofac. Surg. 2009, 67, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212. [Google Scholar] [CrossRef] [PubMed]
- Gnilitskyi, I.; Pogorielov, M.; Viter, R.; Ferraria, A.M.; Carapeto, A.P.; Oleshko, O.; Orazi, L.; Mishchenko, O. Cell and tissue response to nanotextured Ti6Al4V and Zr implants using high-speed femtosecond laser-induced periodic surface structures. Nanomedicine 2019, 21, 1020–1036. [Google Scholar] [CrossRef]
- Jaeggi, M.; Gyr, S.; AstasovFrauenhoffer, M.; Zitzmann, N.U.; Fischer, J.; Rohr, N. Influence of different zirconia surface treatments on biofilm formation in vitro and in situ. Clin. Oral Implant. Res. 2022, 33, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Gaggl, G.; Schultes, W.D.; Müller, H.; Kärcher, H. Scanning electron microscopical analysis of laser-treated titanium implant surfaces—A comparative study. Biomaterials 2000, 21, 1067. [Google Scholar] [CrossRef] [PubMed]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.-C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Chen, P.; Aso, T.; Sasaki, R.; Ashida, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Adhesion and differentiation behaviors of mesenchymal stem cells on titanium with micrometer and nanometer-scale grid patterns produced by femtosecond laser irradiation. J. Biomed. Mater. Res. A 2018, 106, 2735–2743. [Google Scholar] [CrossRef]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef]
- Lee, B.E.J.; Exir, H.; Weck, A.; Grandfield, K. Characterization and evaluation of femtosecond laser-induced sub-micron periodic structures generated on titanium to improve osseointegration of implants. Appl. Surf. Sci. 2018, 441, 1034–1042. [Google Scholar] [CrossRef]
- Kurella, N.B.; Dahotre, A. Review paper: Surface Modification for Bioimplants: The Role of Laser Surface Engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef]
- Joób-Fancsaly, T.; Divinyi, A.; Fazekas, A.; Daroczi, C.; Karacs, A.; Petõ, G. Pulsed laser-induced micro-and nanosized morphology and composition of titanium dental implants. Smart Mater. Struct. 2002, 11, 819. [Google Scholar] [CrossRef]
- Stangl, R.; Rinne, B.; Kastl, S.; Hendrich, C. The influence of pore geometry in cp Ti-implants—A cell culture investigation. Eur. Cells Mater. 2001, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.L.; Alexander, H. Laser microtexturing of implant surfaces for enhanced tissue integration. Key Eng. Mater. 2001, 179, 198–199. [Google Scholar] [CrossRef]
- Trtica, M.; Gakovic, B.; Batani, D.; Desai, T.; Panjan, P.; Radak, B. Surface modifications of a titanium implant by a picosecond Nd: YAG laser operating at 1064 and 532 nm. Appl. Surf. Sci. 2006, 253, 2551. [Google Scholar] [CrossRef]
- Bereznai, M.; Pelsöczi, I.; Tóth, Z.; Turzó, K.; Radnai, M.; Bor, Z.; Fazekas, A. Surface modifications induced by ns and sub-ps excimer laser pulses on titanium implant material. Biomaterials 2003, 24, 4197. [Google Scholar] [CrossRef]
- Simões, I.G.; Dos Reis, A.C.; da Costa Valente, M.L. Analysis of the influence of surface treatment by high-power laser irradiation on the surface properties of titanium dental implants: A systematic review. J. Prosthet. Dent. 2021, 129, 863–870. [Google Scholar] [CrossRef]
- Sadid-Zadeh, R.; Willis, J.; Forgo, G.; Haraszthy, V. Comparative analysis of biofilm formation on materials used for the fabrication of implant-supported prostheses. Braz. Dent. J. 2020, 31, 380–384. [Google Scholar] [CrossRef]
- Seo, B.Y.; Son, K.; Son, Y.T.; Dahal, R.H.; Kim, S.; Kim, J.; Kim, J.W. Influence of Dental Titanium Implants with Different Surface Treatments Using Femtosecond and Nanosecond Lasers on Biofilm Formation. J. Funct. Biomater. 2023, 14, 297. [Google Scholar] [CrossRef]
- Aguayo, S.; Donos, N.; Spratt, D.; Bozec, L. Nanoadhesion of Staphylococcus aureus onto titanium implant surfaces. J. Dent. Res. 2015, 94, 1078–1084. [Google Scholar] [CrossRef]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Topographic characterization of multispecies biofilms growing on dental implant surfaces: An in vitro model. Clin. Oral Implant. Res. 2019, 30, 229–241. [Google Scholar] [CrossRef]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Myrvik, Q.M.; Wagner, W.; Gristina, A.G. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials 1989, 10, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Okutan, Y.; Kandemir, B.; Gundogdu, Y.; Kilic, H.S.; Yucel, M.T. Combined application of femtosecond laser and air-abrasion protocols to monolithic zirconia at different sintering stages: Effects on surface roughness and resin bond strength. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Kobayashi, T.; Yaeshima, T.; Iwatsuki, K.; Yoshie, H. Inhibitory Effects of Lactoferrin on Growth and Biofilm Formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents Chemother. 2009, 53, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Dhein, J.; Haller, C.; Reichl, F.X.; Milz, S.; Hickel, R.; Kollmuss, M.; Högg, C. Intranuclear cell uptake and toxicity of titanium dioxide and zirconia particles as well as bacterial adhesion on dental titanium-and zirconia-implants. Dent. Mater. 2022, 38, 517–528. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Perumal, G.; Arora, H.S.; Popat, K.C. Enhanced antibacterial properties on superhydrophobic micro-nano structured titanium surface. J. Biomed. Mater. Res. A 2022, 110, 1314–1328. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Rabaey, K. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, T.; Lei, Y.; Luo, Y. Metal surface wettability modification by nanosecond laser surface texturing: A review. Biosurf. Biotribol. 2022, 8, 95–120. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Shao, T.; Xie, Q.; Xu, J.; Yang, W. Hydrophobic Treatment on Polymethylmethacrylate Surface by Nanosecond-Pulse DBDs in CF4 at Atmospheric Pressure. Appl. Surf. Sci. 2014, 311, 468–477. [Google Scholar] [CrossRef]
- Carvalho, A.; Grenhod, L.; Fernandesd, M.H.; Daskalovaf, A.; Trifonovg, A.; Buchvarovg, I.; Monteiro, F.J. Femtosecond laser microstructuring of alumina toughened zirconia for surface functionalization of dental implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar] [CrossRef]
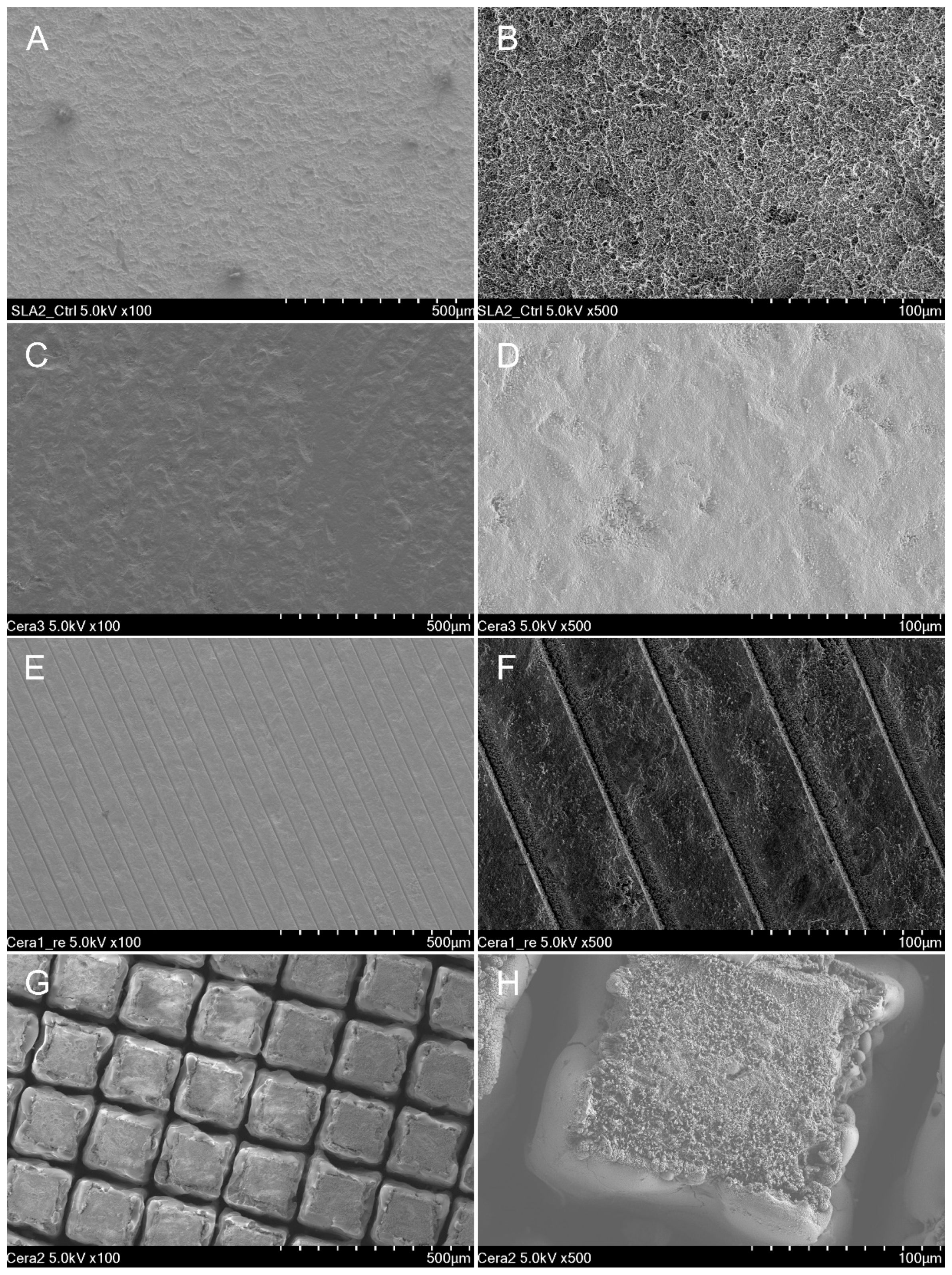
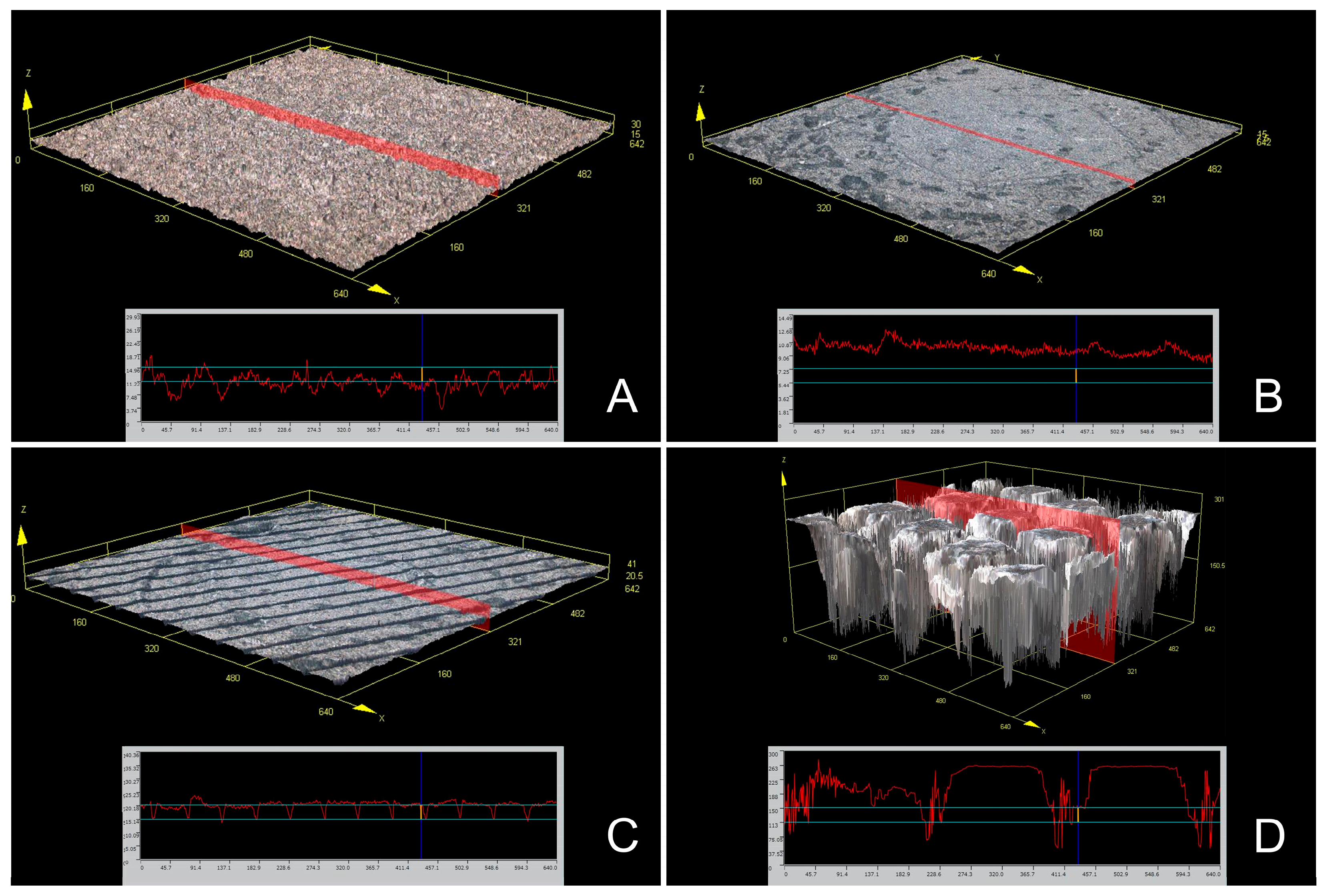
| Surface Treatment Type | Mean | SD | 95% Confidence Interval | p * | Comparison ** | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| T group | 67.528 | 3.453 | 65.058 | 69.998 | <0.001 | A |
| M group | 80.176 | 2.981 | 78.043 | 82.309 | B | |
| HF group | 64.652 | 2.867 | 62.601 | 66.703 | A | |
| HN group | 124.977 | 3.882 | 122.200 | 127.754 | C | |
| Roughness Type | Surface Treatment Type | Mean | SD | 95% Confidence Interval | p * | Comparison ** | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Ra | T group | 1.645 | 0.111 | 1.507 | 1.783 | <0.001 | AB |
| M group | 1.081 | 0.396 | 0.590 | 1.573 | A | ||
| HF group | 2.322 | 0.456 | 1.755 | 2.888 | B | ||
| HN group | 27.105 | 5.643 | 20.097 | 34.112 | C | ||
| Rz | T group | 10.452 | 0.430 | 9.918 | 10.986 | 0.001 | AB |
| M group | 4.437 | 0.310 | 4.053 | 4.822 | A | ||
| HF group | 11.721 | 1.458 | 9.911 | 13.532 | B | ||
| HN group | 133.379 | 20.770 | 107.590 | 159.169 | C | ||
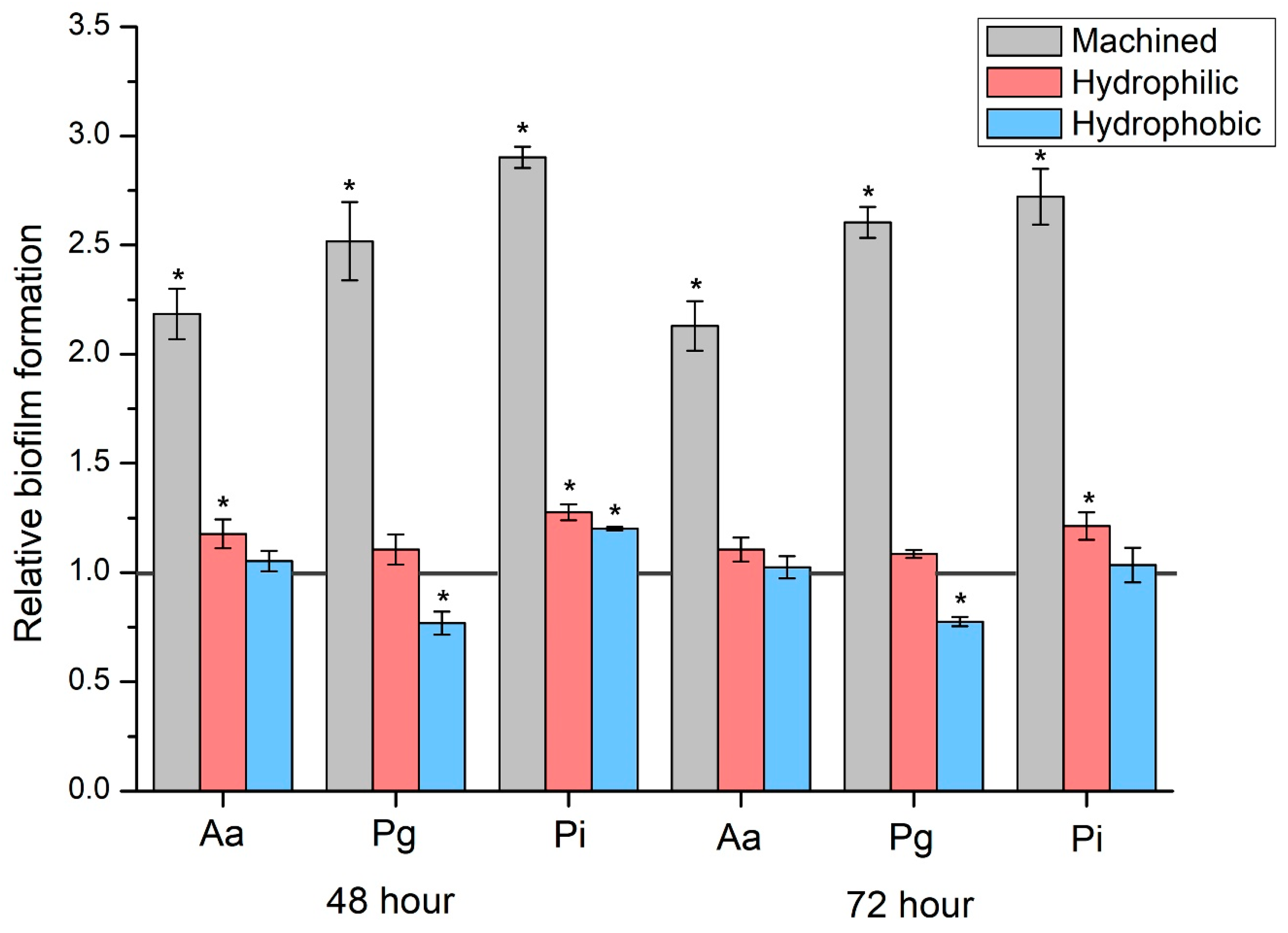
| Biofilm Type | Surface Treatment Type | Mean | SD | 95% Confidence Interval | p * | Comparison ** | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Aa | T group | 4770.0 | 228.0 | 4486.9 | 5053.1 | <0.001 | A |
| M group | 10,395.0 | 206.5 | 10,138.6 | 10,651.4 | B | ||
| HF group | 5600.0 | 176.8 | 5380.5 | 5819.5 | C | ||
| HN group | 5010.0 | 65.2 | 4929.1 | 5090.9 | A | ||
| Pg | T group | 3928.0 | 193.3 | 3688.0 | 4168.0 | <0.001 | A |
| M group | 9860.0 | 232.9 | 9570.8 | 10,149.2 | B | ||
| HF group | 4330.0 | 75.8 | 4235.8 | 4424.2 | C | ||
| HN group | 3010.0 | 82.2 | 2908.0 | 3112.0 | D | ||
| Pi | T group | 4500.0 | 45.3 | 4443.8 | 4556.2 | <0.001 | A |
| M group | 13,060.0 | 119.4 | 12,911.8 | 13,208.2 | B | ||
| HF group | 5740.0 | 147.5 | 5556.9 | 5923.1 | C | ||
| HN group | 5400.0 | 79.1 | 5301.8 | 5498.2 | D | ||
| Biofilm Type | Surface Treatment Type | Mean | SD | 95% Confidence Interval | p * | Comparison ** | Comparison with 48 h | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | p *** | ||||||
| Aa | T group | 5353.6 | 256.6 | 5034.9 | 5672.3 | <0.001 | A | 0.005 |
| M group | 11,380.0 | 336.5 | 10,962.1 | 11,797.9 | B | 0.001 | ||
| HF group | 5900.0 | 50.0 | 5837.9 | 5962.1 | C | 0.017 | ||
| HN group | 5470.0 | 115.1 | 5327.1 | 5612.9 | A | <0.001 | ||
| Pg | T group | 4119.6 | 73.2 | 4028.7 | 4210.5 | <0.001 | A | 0.092 |
| M group | 10,720.0 | 168.1 | 10,511.3 | 10,928.7 | B | <0.001 | ||
| HF group | 4470.0 | 57.0 | 4399.2 | 4540.8 | C | 0.012 | ||
| HN group | 3190.0 | 65.2 | 3109.1 | 3270.9 | D | 0.005 | ||
| Pi | T group | 5039.2 | 209.3 | 4779.3 | 5299.1 | <0.001 | A | 0.004 |
| M group | 13,700.0 | 434.5 | 13,160.6 | 14,239.4 | B | 0.028 | ||
| HF group | 6100.0 | 176.8 | 5880.5 | 6319.5 | C | 0.009 | ||
| HN group | 5200.0 | 259.8 | 4877.4 | 5522.6 | A | 0.164 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bihn, S.K.; Son, K.; Son, Y.-T.; Dahal, R.H.; Kim, S.; Kim, J.; Hwang, J.H.; Kwon, S.-M.; Lee, J.H.; Kim, H.D.; et al. In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers. J. Funct. Biomater. 2023, 14, 486. https://doi.org/10.3390/jfb14100486
Bihn SK, Son K, Son Y-T, Dahal RH, Kim S, Kim J, Hwang JH, Kwon S-M, Lee JH, Kim HD, et al. In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers. Journal of Functional Biomaterials. 2023; 14(10):486. https://doi.org/10.3390/jfb14100486
Chicago/Turabian StyleBihn, Soo Kyum, Keunbada Son, Young-Tak Son, Ram Hari Dahal, Shukho Kim, Jungmin Kim, Jun Ho Hwang, Sung-Min Kwon, Jong Hoon Lee, Hyun Deok Kim, and et al. 2023. "In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers" Journal of Functional Biomaterials 14, no. 10: 486. https://doi.org/10.3390/jfb14100486
APA StyleBihn, S. K., Son, K., Son, Y.-T., Dahal, R. H., Kim, S., Kim, J., Hwang, J. H., Kwon, S.-M., Lee, J. H., Kim, H. D., Lee, J.-M., Jin, M.-U., & Lee, K.-B. (2023). In Vitro Biofilm Formation on Zirconia Implant Surfaces Treated with Femtosecond and Nanosecond Lasers. Journal of Functional Biomaterials, 14(10), 486. https://doi.org/10.3390/jfb14100486










