Surface-Modified Silver Nanoparticles and Their Encapsulation in Liposomes Can Treat MCF-7 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Silver Nanoparticles Synthesis
2.3. PVP-AgNPs Synthesis
2.4. BSA-AgNPs Synthesis
2.5. Liposomal Preparation and Encapsulation of AgNPs
2.6. Characterisation of AgNPs
2.6.1. UV-Visible Spectrophotometry
2.6.2. Particle Size Distribution and Zeta Potential Analysis
2.6.3. Fluorescence Analysis
2.6.4. Morphological Analysis Using Scanning Electron Microscopy (SEM)
2.7. Cell Culture and Exposure
2.8. Cell Viability
2.9. Statistical Analysis
3. Results and Discussion
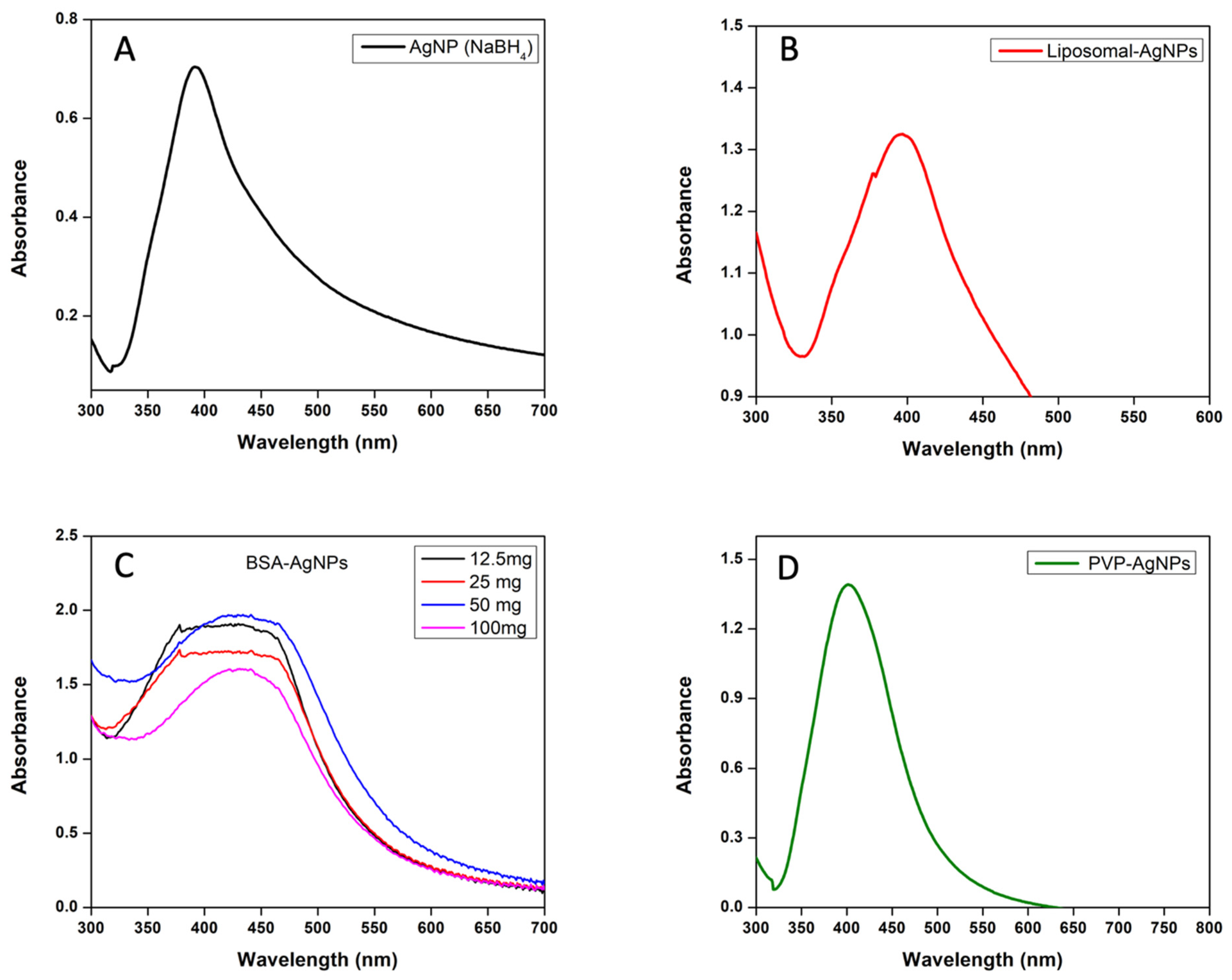
3.1. UV–Visible Spectrophotometer Analysis of AgNPs and Liposomes
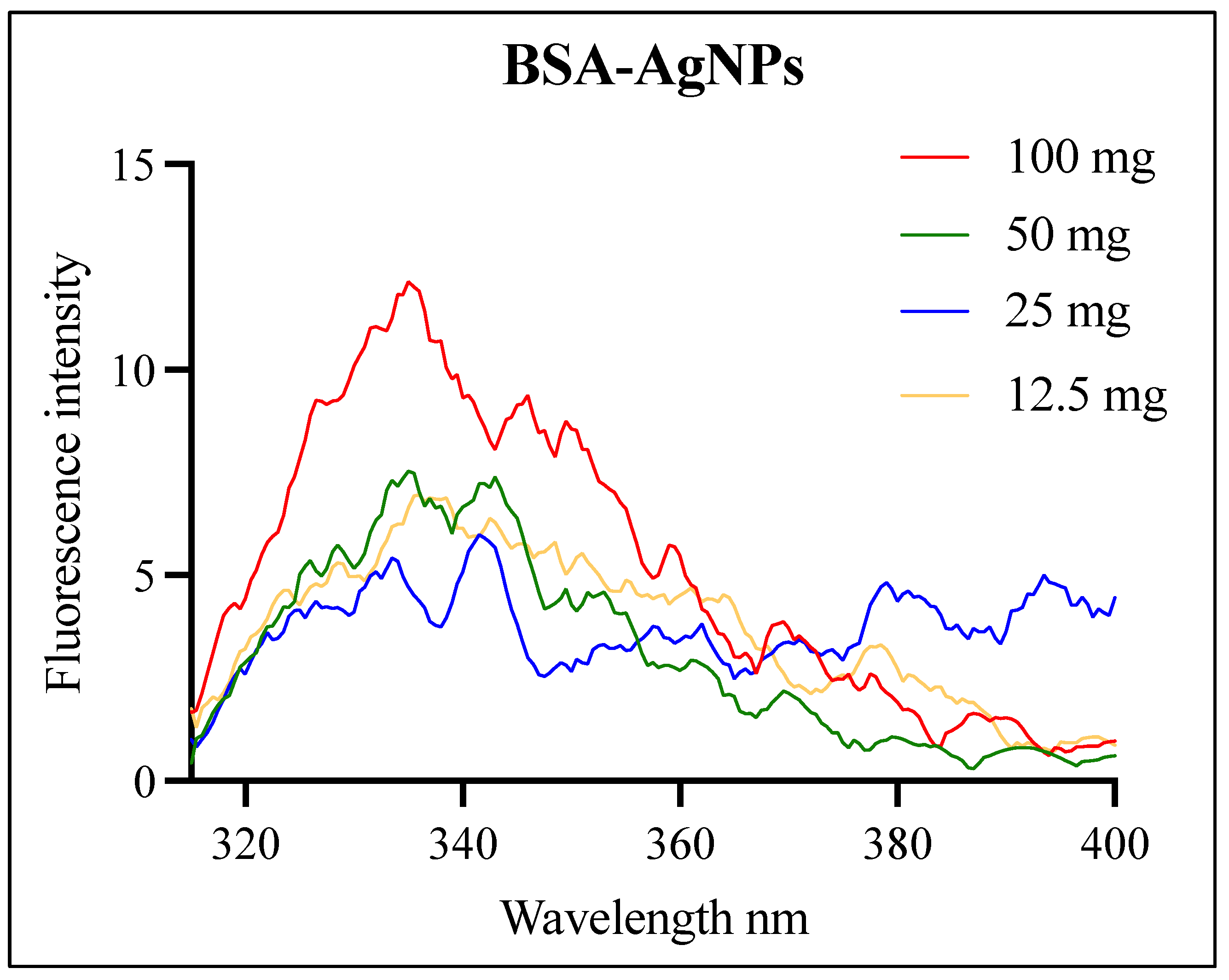
3.2. Fluorescence Analysis of the BSA-AgNPs
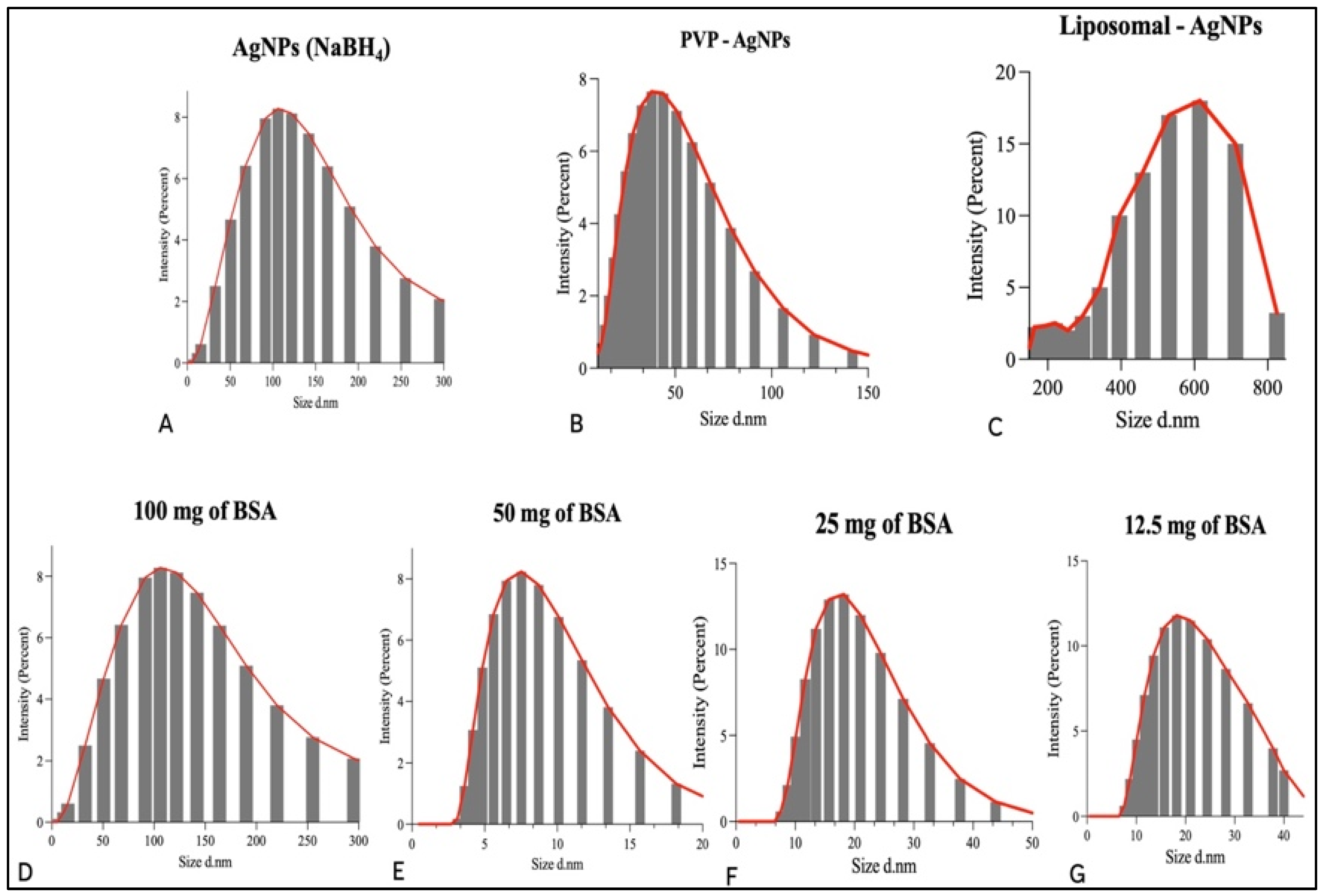
3.3. Particle Size Distribution Analysis and Zeta Potential of AgNPs and Liposomes
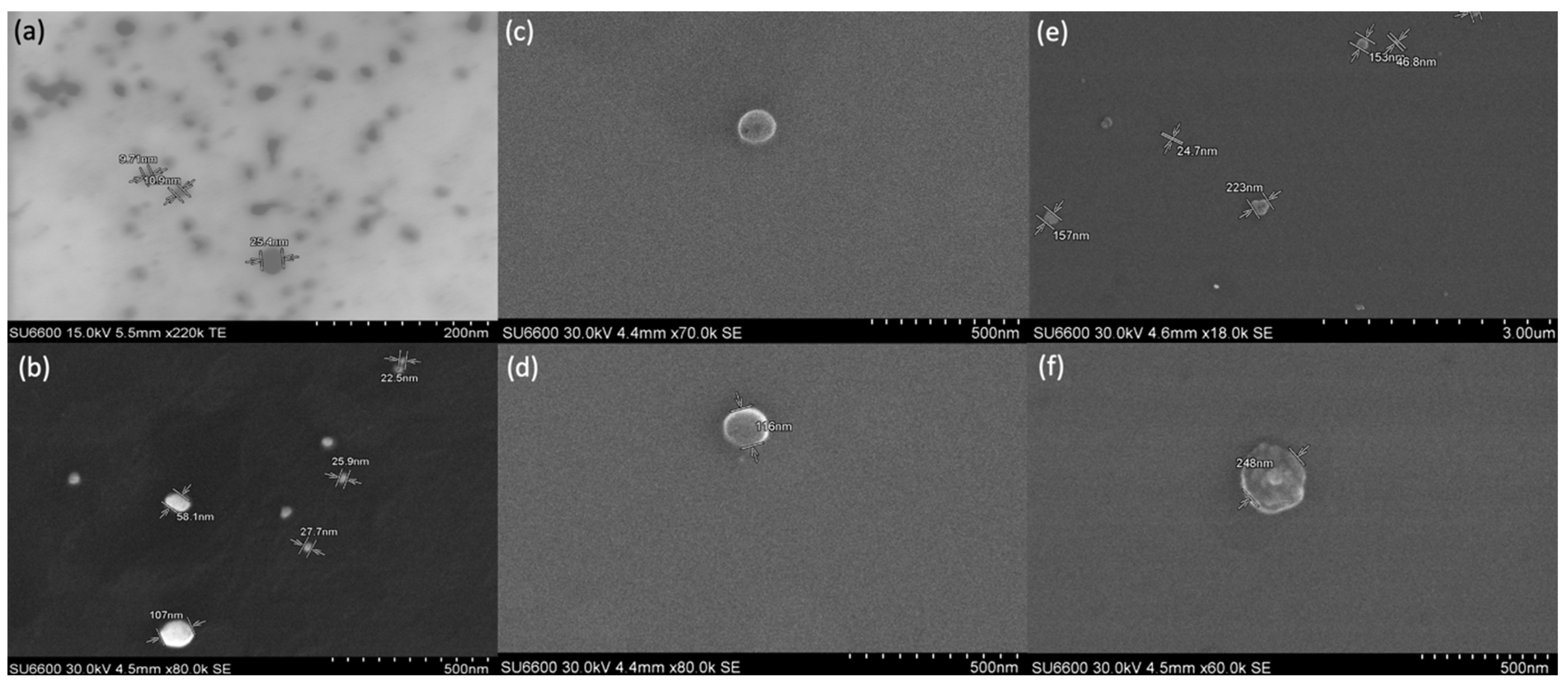
3.4. Scanning Electron Microscopic Analysis of AgNPs and Liposomes
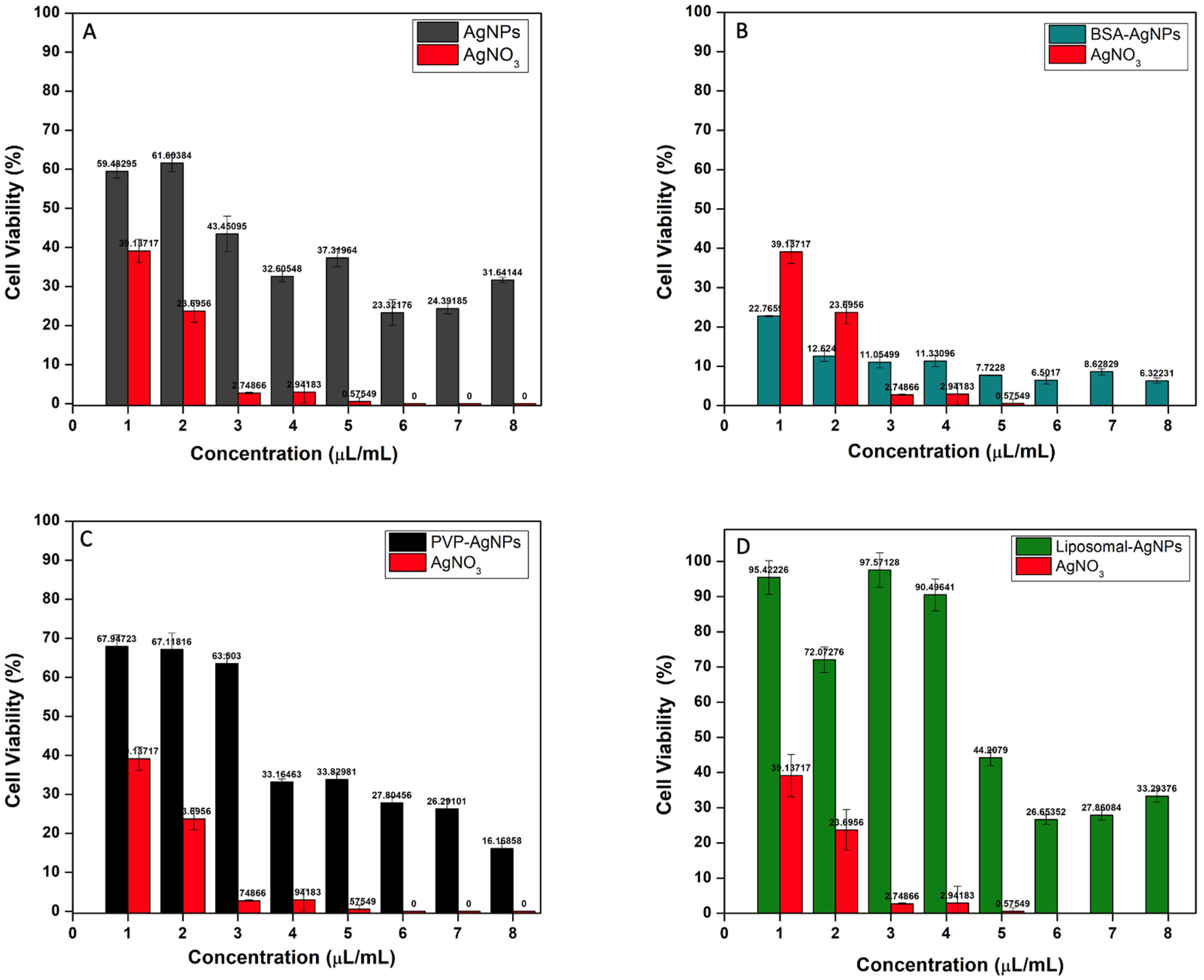
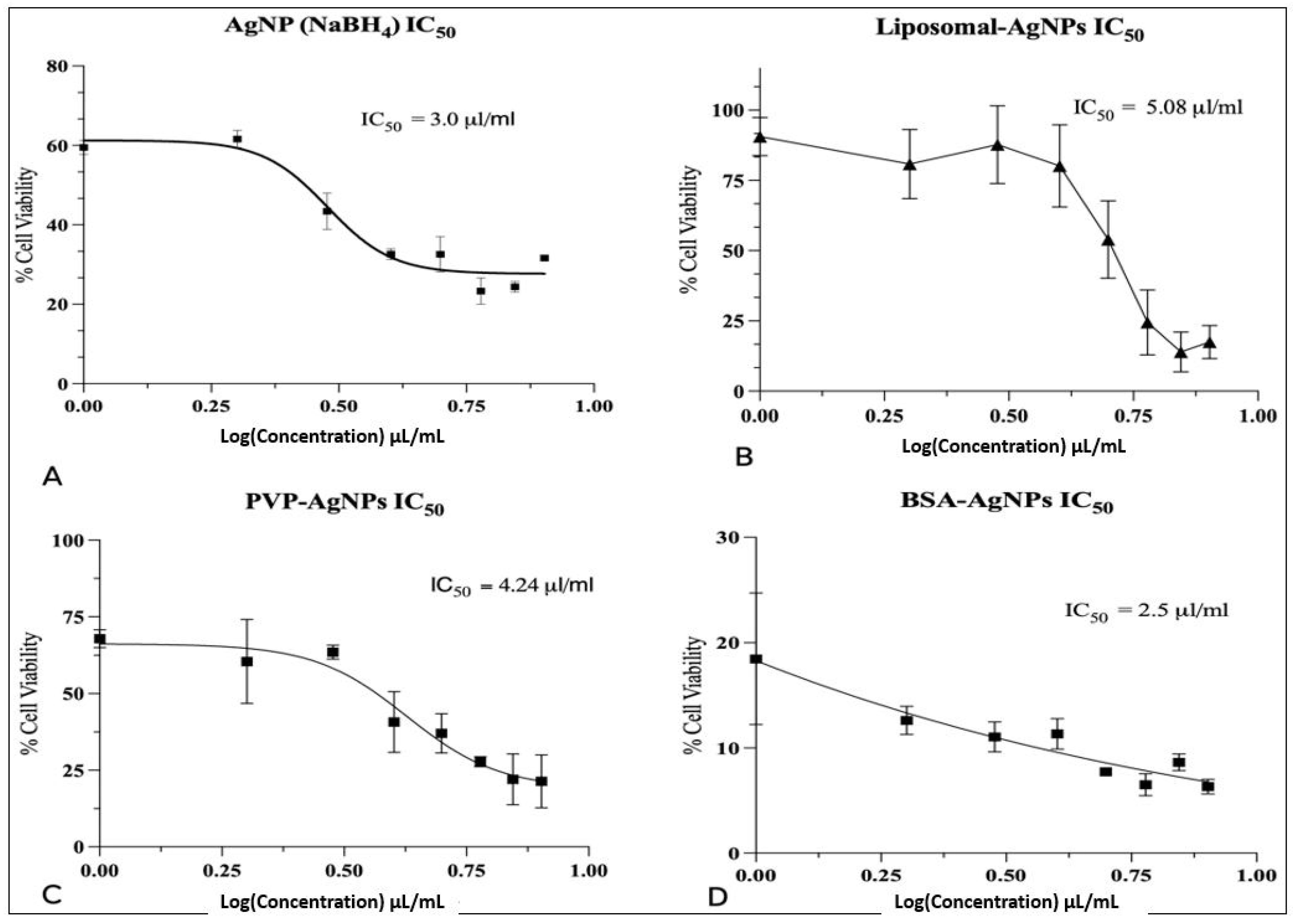
3.5. Cytotoxicity Analysis of AgNPs and Liposomes
3.6. Unmodified AgNPs
3.7. BSA-AgNPs
3.8. PVP-AgNPs
3.9. Liposomal-AgNPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, H.J.; Ekweremadu, C.; Patel, N. Advanced Drug Delivery System with Nanomaterials for Personalised Medicine to Treat Breast Cancer. J. Drug Deliv. Sci. Technol. 2019, 52, 1051–1060. [Google Scholar] [CrossRef]
- Nounou, M.I.; Elamrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast Cancer: Conventional Diagnosis and Treatment Modalities and Recent Patents and Technologies Supplementary Issue: Targeted Therapies in Breast Cancer Treatment. Breast Cancer 2015, 9, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Nan, A. Combination Drug Delivery Approaches in Metastatic Breast Cancer. J. Drug Deliv. 2012, 2012, 915375. [Google Scholar] [CrossRef]
- Boas, D.; Remennik, S.; Reches, M. Peptide-Capped Au and Ag Nanoparticles: Detection of Heavy Metals and Photochemical Core/Shell Formation. J. Colloid Interface Sci. 2023, 631, 66–76. [Google Scholar] [CrossRef]
- Yusuf, A.; Brophy, A.; Gorey, B.; Casey, A. Liposomal Encapsulation of Silver Nanoparticles Enhances Cytotoxicity and Causes Induction of Reactive Oxygen Species-Independent Apoptosis. J. Appl. Toxicol. 2018, 38, 616–627. [Google Scholar] [CrossRef]
- Sultana, T.; Javed, B.; Raja, N.I.; Mashwani, Z.-R. Silver Nanoparticles Elicited Physiological, Biochemical, and Antioxidant Modifications in Rice Plants to Control Aspergillus Flavus. Green Process. Synth. 2021, 10, 314–324. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-Controlled Silver Nanoparticles Synthesized over the Range 5–100 Nm Using the Same Protocol and Their Antibacterial Efficacy. RSC Adv. 2013, 4, 3974–3983. [Google Scholar] [CrossRef]
- Mariam, J.; Dongre, P.M.; Kothari, D.C. Study of Interaction of Silver Nanoparticles with Bovine Serum Albumin Using Fluorescence Spectroscopy. J. Fluoresc. 2011, 21, 2193–2199. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Udhayakumar, S.; Mani, S.; Dhanapal, M.; Vijaykumar, S.D. Surface Modification of Fe2O3 and MgO Nanoparticles with Agrowastes for the Treatment of Chlorosis in Glycine Max. Nano Converg. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Tsuji, M.; Nishizawa, Y.; Matsumoto, K.; Kubokawa, M.; Miyamae, N.; Tsuji, T. Effects of Chain Length of Polyvinylpyrrolidone for the Synthesis of Silver Nanostructures by a Microwave-Polyol Method. Mater. Lett. 2006, 60, 834–838. [Google Scholar] [CrossRef]
- Kim, D.; Amatya, R.; Hwang, S.; Lee, S.; Min, K.A.; Shin, M.C. BSA-Silver Nanoparticles: A Potential Multimodal Therapeutics for Conventional and Photothermal Treatment of Skin Cancer. Pharmaceutics 2021, 13, 575. [Google Scholar] [CrossRef]
- Boehmler, D.J.; O’Dell, Z.J.; Chung, C.; Riley, K.R. Bovine Serum Albumin Enhances Silver Nanoparticle Dissolution Kinetics in a Size-and Concentration-Dependent Manner. Langmuir 2020, 36, 1053–1061. [Google Scholar] [CrossRef]
- Yusuf, A.; Casey, A. Evaluation of Silver Nanoparticle Encapsulation in DPPC-Based Liposome by Different Methods for Enhanced Cytotoxicity. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 860–871. [Google Scholar] [CrossRef]
- Olusanya, T.; Ahmad, R.H.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, X.; Xu, W.; Zhang, R.; Wu, G. Biosynthesis of Bimetallic Au-Ag Nanoparticles Using Escherichia Coli and Its Biomedical Applications. ACS Biomater. Sci. Eng. 2020, 6, 680–689. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis Muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Mathew, G.M.; Pancrecious, J.K.; Nair, J.B.; Rajan, T.P.D.; Maiti, K.K.; Pai, B.C. Biogenic Ag Nanoparticles from Neem Extract: Their Structural Evaluation and Antimicrobial Effects against Pseudomonas Nitroreducens and Aspergillus Unguis (NII 08123). ACS Biomater. Sci. Eng. 2019, 6, 235–245. [Google Scholar] [CrossRef]
- Javed, B.; Mashwani, Z.; Sarwer, A.; Raja, N.I.; Nadhman, A. Synergistic Response of Physicochemical Reaction Parameters on Biogenesis of Silver Nanoparticles and Their Action against Colon Cancer and Leishmanial Cells. Artif. Cells Nanomed Biotechnol. 2020, 48, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Javed, B.; Mashwani, Z.-R. Synergistic Effects of Physicochemical Parameters on Bio-Fabrication of Mint Silver Nanoparticles: Structural Evaluation and Action Against HCT116 Colon Cancer Cells. Int. J. Nanomed. 2020, 15, 3621–3637. [Google Scholar] [CrossRef] [PubMed]
- Al-Saidi, W.A.; Feng, H.; Fichthorn, K.A. Adsorption of Polyvinylpyrrolidone on Ag Surfaces: Insight into a Structure-Directing Agent. Nano Lett. 2012, 12, 997–1001. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Tufoni, M.; Bonavita, M.B. Clinical Use of Albumin. Blood Transfus. 2013, 11, s18–s25. [Google Scholar]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Köller, M.; Epple, M. The Dissolution and Biological Effects of Silver Nanoparticles in Biological Media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- M, J.F.; P, L. Apoptotic Efficacy of Biogenic Silver Nanoparticles on Human Breast Cancer MCF-7 Cell Lines. Prog. Biomater. 2015, 4, 113. [Google Scholar] [CrossRef]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A. Epidermal Growth Factor-Labeled Liposomes as a Way to Target the Toxicity of Silver Nanoparticles into EGFR-Overexpressing Cancer Cells in Vitro. Toxicol. Appl. Pharmacol. 2022, 443, 116009. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Mousavi, M.P.S.; Chen, L.D.; Bühlmann, P.; Haynes, C.L. Characterization of Silver Ion Dissolution from Silver Nanoparticles Using Fluorous-Phase Ion-Selective Electrodes and Assessment of Resultant Toxicity to Shewanella Oneidensis. Chem. Sci. 2013, 4, 2564–2572. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible Particle-Specific Toxicity Mechanism of Silver Nanoparticles: The Role of Ag+ Ion Release in the Cytosol. Nanomedicine 2015, 11, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Leng, W.; Vikesland, P.J. Controlled Evaluation of the Impacts of Surface Coatings on Silver Nanoparticle Dissolution Rates. Environ. Sci. Technol. 2018, 52, 2726–2734. [Google Scholar] [CrossRef]
- Nogueira, D.R.; Mitjans, M.; Rolim, C.M.B.; Vinardell, M.P. Mechanisms Underlying Cytotoxicity Induced by Engineered Nanomaterials: A Review of In Vitro Studies. Nanomaterials 2014, 4, 454. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, G.; Lee, S. A Gold Nanoparticle and Aflatoxin B1-BSA Conjugates Based Lateral Flow Assay Method for the Analysis of Aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef]
- Javed, B.; Raja, N.I.; Nadhman, A.; Mashwani, Z.-R. Understanding the Potential of Bio-Fabricated Non-Oxidative Silver Nanoparticles to Eradicate Leishmania and Plant Bacterial Pathogens. Appl. Nanosci. 2020, 10, 2057–2067. [Google Scholar] [CrossRef]
- Javed, B.; Nadhman, A.; Razzaq, A.; Mashwani, Z. One-Pot Phytosynthesis of Nano-Silver from Mentha longifolia L.: Their Characterization and Evaluation of Photodynamic Potential. Mater. Res. Express 2020, 7, 055401. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In Vitro Toxicity of Nanoparticles in BRL 3A Rat Liver Cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Lodeiro, C.; Capelo, J.L.; Lorenzo, J.; Oliveira, E. Magnetic, Fluorescent and Hybrid Nanoparticles: From Synthesis to Application in Biosystems. Mater. Sci. Eng. C 2020, 106, 110104. [Google Scholar] [CrossRef]
| Colloidal Sample | Zeta Potential (mV) |
|---|---|
| AgNPs (NaBH4) | −41.33 |
| AgNPs (NaBH4 and Na3C6H5O7) | −0.73 |
| PVP-AgNPs | −33.0 |
| Liposomal-AgNPs | −30.66 |
| BSA 100 mg | −19.66 |
| BSA 50 mg | 18.46 |
| BSA 25 mg | −0.07 |
| BSA 12.5 mg | 1.75 |
| Sample | Concentration (μL/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| AgNPs (NaBH4) | 59.5% | 63% | 43% | 33.6% | 32.6% | 23.3% | 24.4% | 31.5% |
| PVP-AgNPs | 67.9% | 60.4% | 63.4% | 40.7% | 37% | 27.3% | 22.1% | 21.4% |
| BSA-AgNPs | 18.4% | 12.6% | 11.1% | 11.3% | 7.7% | 6.4% | 8.5% | 5.9% |
| Liposomal-AgNPs | 90.6% | 80.5% | 87% | 79.5% | 53.8% | 24.1% | 13.9% | 17% |
| AgNO3 | 31.8% | 19.8% | 8.6% | 2.95% | 0.6% | 0.38% | 0.25% | 0.23% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moors, E.; Sharma, V.; Tian, F.; Javed, B. Surface-Modified Silver Nanoparticles and Their Encapsulation in Liposomes Can Treat MCF-7 Breast Cancer Cells. J. Funct. Biomater. 2023, 14, 509. https://doi.org/10.3390/jfb14100509
Moors E, Sharma V, Tian F, Javed B. Surface-Modified Silver Nanoparticles and Their Encapsulation in Liposomes Can Treat MCF-7 Breast Cancer Cells. Journal of Functional Biomaterials. 2023; 14(10):509. https://doi.org/10.3390/jfb14100509
Chicago/Turabian StyleMoors, Ellenor, Vinayak Sharma, Furong Tian, and Bilal Javed. 2023. "Surface-Modified Silver Nanoparticles and Their Encapsulation in Liposomes Can Treat MCF-7 Breast Cancer Cells" Journal of Functional Biomaterials 14, no. 10: 509. https://doi.org/10.3390/jfb14100509
APA StyleMoors, E., Sharma, V., Tian, F., & Javed, B. (2023). Surface-Modified Silver Nanoparticles and Their Encapsulation in Liposomes Can Treat MCF-7 Breast Cancer Cells. Journal of Functional Biomaterials, 14(10), 509. https://doi.org/10.3390/jfb14100509









