Effect of Highly Hydrophilic Superparamagnetic Iron Oxide Nanoparticles on Macrophage Function and Survival
Abstract
:1. Introduction
2. Materials and Methods
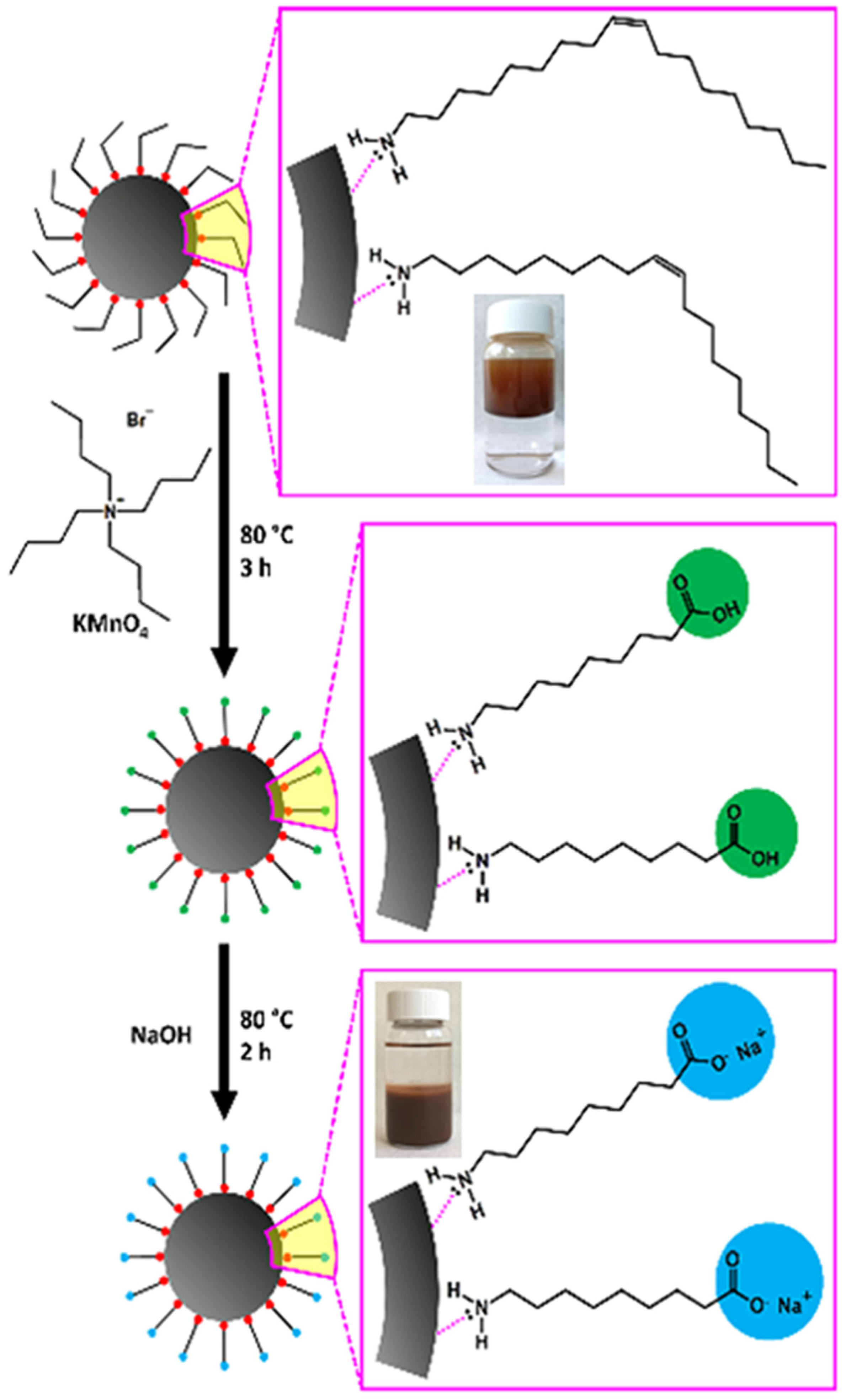
2.1. Synthesis and Characterisation of SPIONs
2.2. Cell Line and Antibodies
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Detection of Apoptosis (Annexin-PI Assay)
2.6. Cell Cycle Assay
2.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results and Discussion
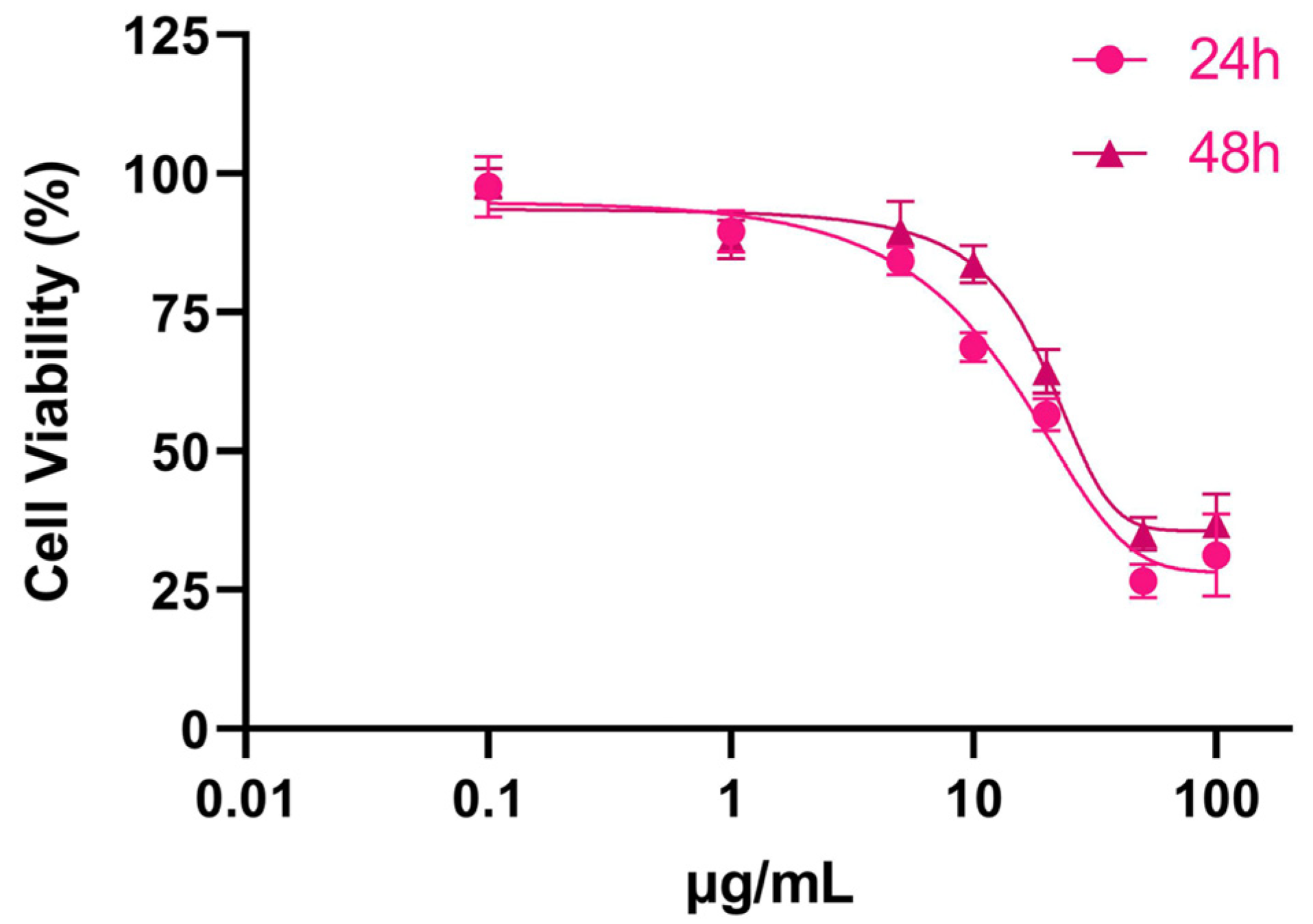
3.1. Cytotoxicity of SPIONs
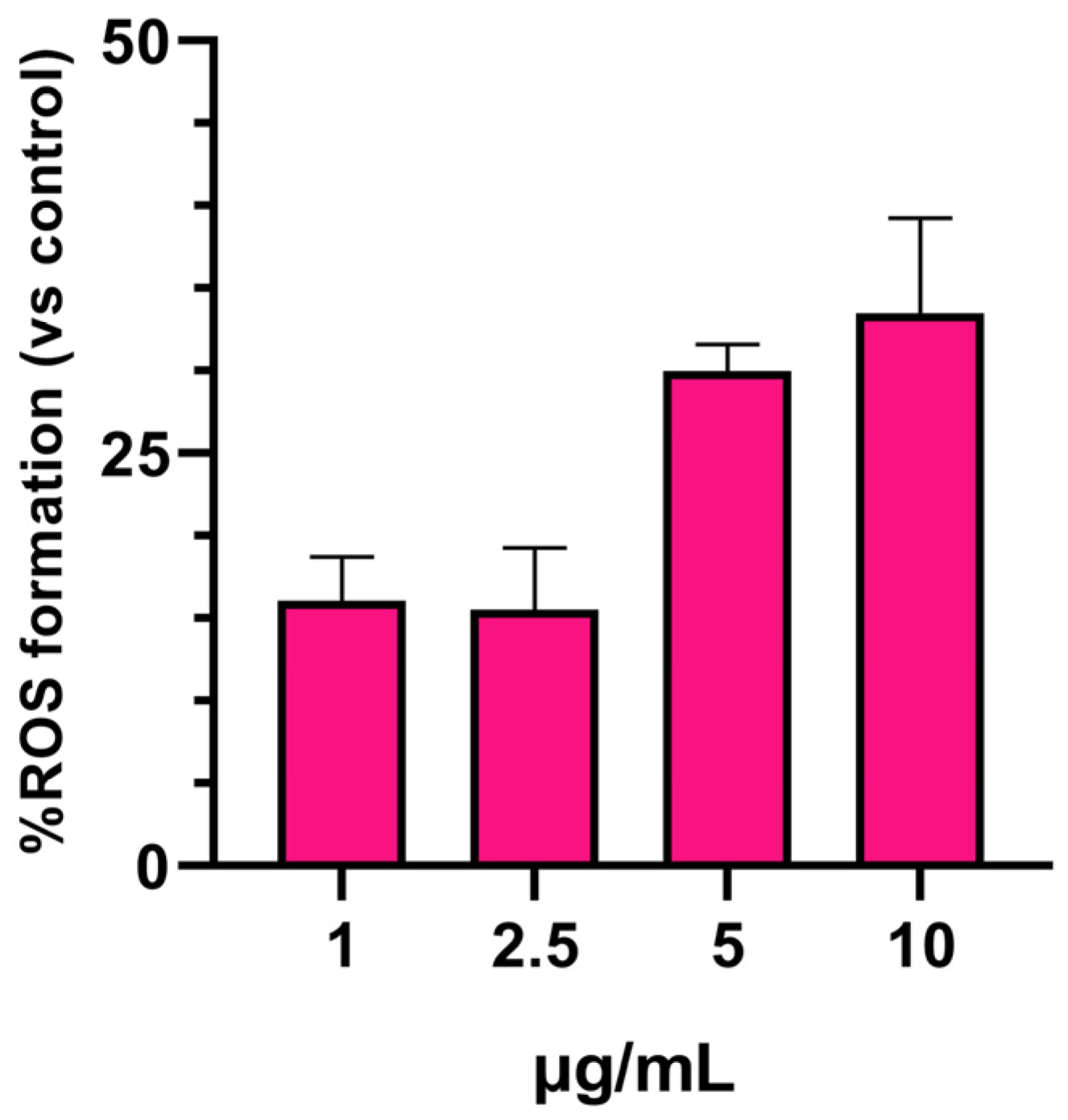
3.2. SPIONs-Induced ROS Generation
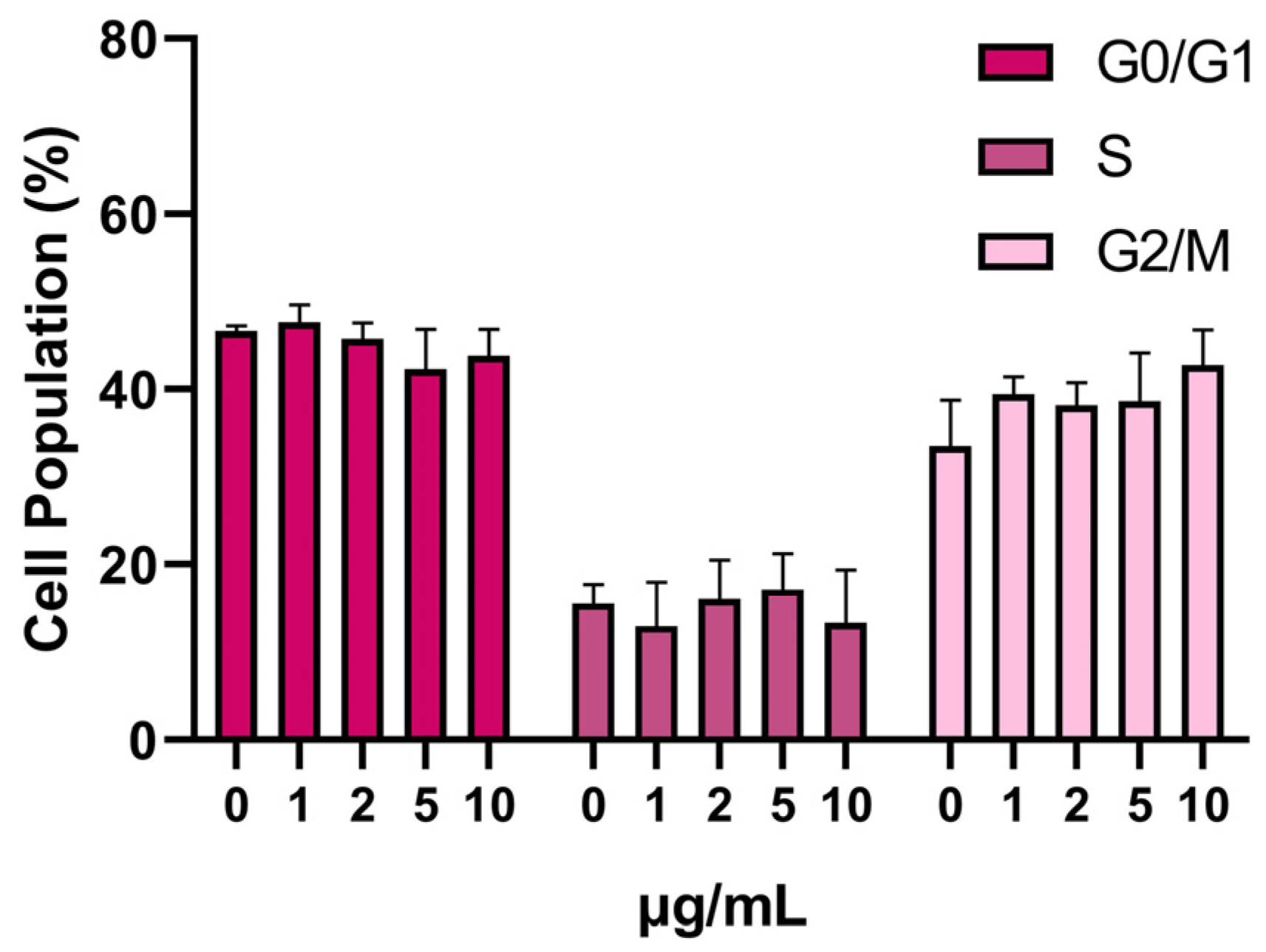
3.3. SPION Influence on the Cell Cycle
3.4. Apoptosis
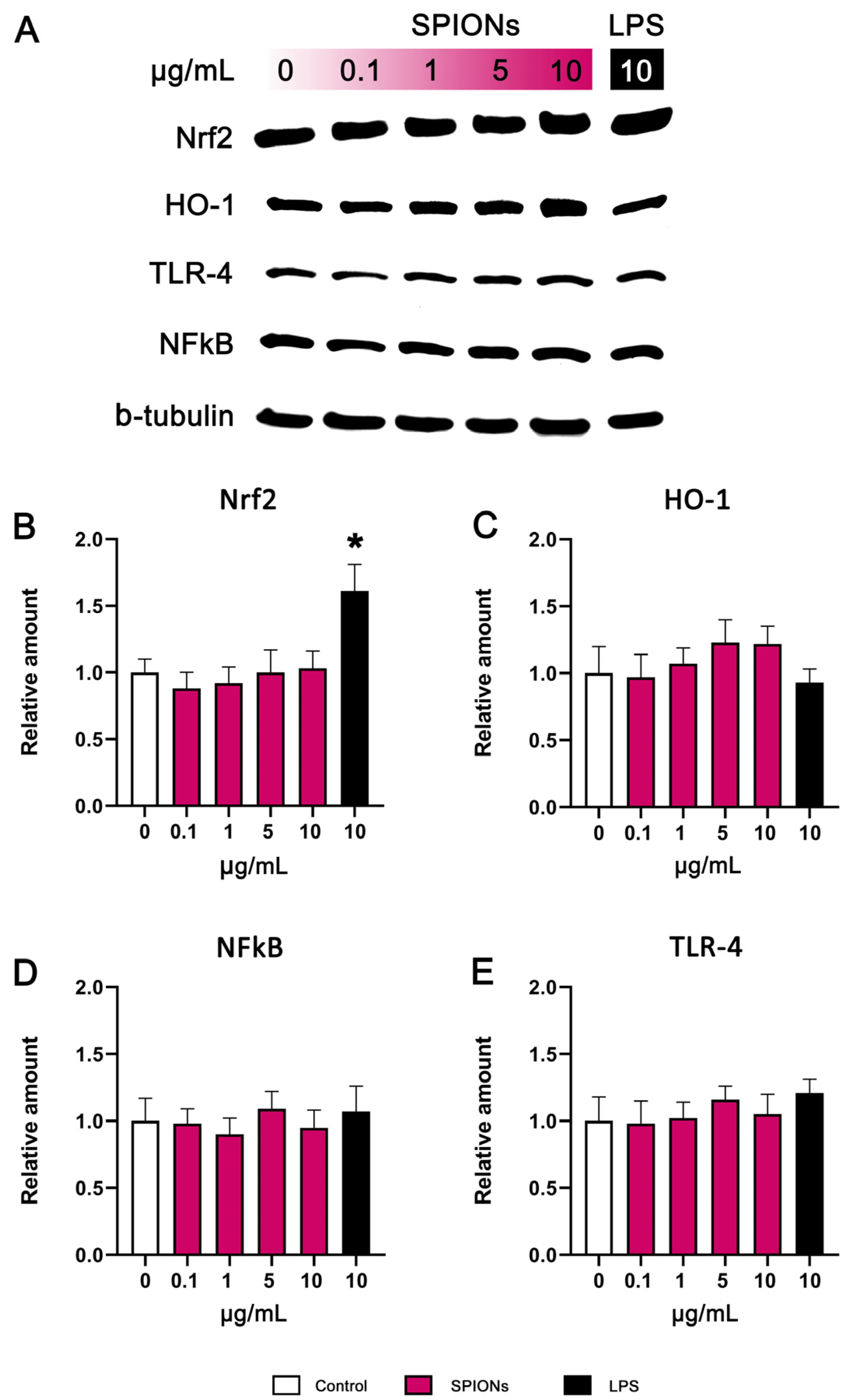
3.5. Western Blotting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Borges, R.; Ferreira, L.M.; Rettori, C.; Lourenço, I.M.; Seabra, A.B.; Müller, F.A.; Ferraz, E.P.; Marques, M.M.; Miola, M.; Baino, F.; et al. Superparamagnetic and Highly Bioactive SPIONS/Bioactive Glass Nanocomposite and Its Potential Application in Magnetic Hyperthermia. Biomater. Adv. 2022, 135, 112655. [Google Scholar] [CrossRef] [PubMed]
- Schemberg, J.; El Abbassi, A.; Lindenbauer, A.; Chen, L.-Y.; Grodrian, A.; Nakos, X.; Apte, G.; Khan, N.; Kraupner, A.; Nguyen, T.-H.; et al. Synthesis of Biocompatible Superparamagnetic Iron Oxide Nanoparticles (SPION) under Different Microfluidic Regimes. ACS Appl. Mater. Interfaces 2022, 14, 48011–48028. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, C.; Bandyopadhyaya, R. Mechanistic Study on Magnetite Nanoparticle Formation by Thermal Decomposition and Coprecipitation Routes. J. Phys. Chem. C 2011, 115, 1380–1387. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.; Sanchez-Dominguez, M.; Boutonnet, M.; Järås, S.; Civera, C.; Solans, C.; Kuttuva, G.R. Comparison and Functionalization Study of Microemulsion-Prepared Magnetic Iron Oxide Nanoparticles. Langmuir 2012, 28, 8479–8485. [Google Scholar] [CrossRef] [PubMed]
- Ozel, F.; Kockar, H. Growth and Characterizations of Magnetic Nanoparticles under Hydrothermal Conditions: Reaction Time and Temperature. J. Magn. Magn. Mater. 2015, 373, 213–216. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, J.; Luo, Y.; Zeng, R.; Zhang, C.; Zhou, W.; Guo, K.; Wu, H.; Sha, W.; Chen, H. Targeted Extracellular Vesicle Delivery Systems Employing Superparamagnetic Iron Oxide Nanoparticles. Acta Biomater. 2021, 134, 13–31. [Google Scholar] [CrossRef]
- Xiao, Y.; Du, J. Superparamagnetic Nanoparticles for Biomedical Applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Pernia Leal, M.; Luisa García-Martín, M. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2018, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Sabantina, L. Electrospun Magnetic Nanofiber Mats for Magnetic Hyperthermia in Cancer Treatment Applications—Technology, Mechanism, and Materials. Polymers 2023, 15, 1902. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, T.S.; Lu, Y.J.; Chen, J.P. Optimization of the Preparation of Magnetic Liposomes for the Combined Use of Magnetic Hyperthermia and Photothermia in Dual Magneto-Photothermal Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 5187. [Google Scholar] [CrossRef]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1740. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted Superparamagnetic Iron Oxide Nanoparticles for Early Detection of Cancer: Possibilities and Challenges. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting Strategies for Superparamagnetic Iron Oxide Nanoparticles in Cancer Therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Fadeyev, F.A.; Blyakhman, F.A.; Safronov, A.P.; Melnikov, G.Y.; Nikanorova, A.D.; Novoselova, I.P.; Kurlyandskaya, G.V. Biological Impact of γ-Fe2O3 Magnetic Nanoparticles Obtained by Laser Target Evaporation: Focus on Magnetic Biosensor Applications. Biosensors 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.O.; Sanon, N.T.; Carmant, L.; Nguyen, D.K.; Deschênes, S.; Pouliot, P.; Bouthillier, A.; Sawan, M. Superparamagnetic Iron Oxide Nanoparticles-Based Detection of Neuronal Activity. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102478. [Google Scholar] [CrossRef]
- Affatigato, L.; Licciardi, M.; Bonamore, A.; Martorana, A.; Incocciati, A.; Boffi, A.; Militello, V. Ferritin-Coated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier. Molecules 2023, 28, 1163. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Seydi, E.; Rahimi, S.; Tahmasebi, G.; Jahanbani, J.; Pourahmad, J. The Selective Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) on Oral Squamous Cell Carcinoma (OSCC) by Targeting Their Mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J.T.; Vera, A.; Leija, L. Design and Evaluation of Surface Functionalized Superparamagneto-Plasmonic Nanoparticles for Cancer Therapeutics. Int. J. Pharm. 2017, 524, 16–29. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Song, S.C. Thermosensitive/Superparamagnetic Iron Oxide Nanoparticle-Loaded Nanocapsule Hydrogels for Multiple Cancer Hyperthermia. Biomaterials 2016, 106, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Neubert, J.; Glumm, J. Promoting Neuronal Regeneration Using Extracellular Vesicles Loaded with Superparamagnetic Iron Oxide Nanoparticles. Neural Regen. Res. 2016, 11, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Esmaeili, A.; Zarrabi, A.; Zarepour, A. Superparamagnetic Iron Oxide Nanoparticles and Curcumin Equally Promote Neuronal Branching Morphogenesis in the Absence of Nerve Growth Factor in PC12 Cells. Pharmaceutics 2022, 14, 2692. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Montalbano, G.; Gallo-Cordova, A.; Ovejero, J.G.; Izquierdo-Barba, I.; González, B.; Tomasina, C.; Moroni, L.; Vallet-Regí, M.; Vitale-Brovarone, C.; et al. Incorporation of Superparamagnetic Iron Oxide Nanoparticles into Collagen Formulation for 3D Electrospun Scaffolds. Nanomaterials 2022, 12, 181. [Google Scholar] [CrossRef]
- Miguel, M.G.; Lourenço, J.P.; Faleiro, M.L. Superparamagnetic Iron Oxide Nanoparticles and Essential Oils: A New Tool for Biological Applications. Int. J. Mol. Sci. 2020, 21, 6633. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Liu, X.; Pan, Y.; Sun, Z.; Xue, Y.; Wang, T.; Dou, H.; Hou, Y. SPIONs Enhances IL-10-Producing Macrophages to Relieve Sepsis via Cav1-Notch1/HES1-Mediated Autophagy. Int. J. Nanomed. 2019, 14, 6779–6797. [Google Scholar] [CrossRef]
- Talluri, S.; Malla, R.R. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Diagnosis and Treatment of Breast, Ovarian and Cervical Cancers. Curr. Drug Metab. 2019, 20, 942–945. [Google Scholar] [CrossRef]
- Pongrac, I.M.; Pavičić, I.; Milić, M.; Ahmed, L.B.; Babič, M.; Horák, D.; Vrček, I.V.; Gajović, S. Oxidative Stress Response in Neural Stem Cells Exposed to Different Superparamagnetic Iron Oxide Nanoparticles. Int. J. Nanomed. 2016, 11, 1701–1715. [Google Scholar] [CrossRef]
- Laurent, S.; Burtea, C.; Thirifays, C.; Häfeli, U.O.; Mahmoudi, M. Crucial Ignored Parameters on Nanotoxicology: The Importance of Toxicity Assay Modifications and “Cell Vision”. PLoS ONE 2012, 7, e29997. [Google Scholar] [CrossRef]
- Straczek, T.; Fiejdasz, S.; Rybicki, D.; Goc, K.; Przewoźnik, J.; Mazur, W.; Nowakowska, M.; Zapotoczny, S.; Rumian, S.; Kapusta, C. Dynamics of Superparamagnetic Iron Oxide Nanoparticles with Various Polymeric Coatings. Materials 2019, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive Cytotoxicity Studies of Superparamagnetic Iron Oxide Nanoparticles. Biochem. Biophys. Reports 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall Iron Oxide Nanoparticles Cause Significant Toxicity by Specifically Inducing Acute Oxidative Stress to Multiple Organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Park, E.-J.; Oh, S.Y.; Lee, S.J.; Lee, K.; Kim, Y.; Lee, B.-S.; Kim, J.S. Chronic Pulmonary Accumulation of Iron Oxide Nanoparticles Induced Th1-Type Immune Response Stimulating the Function of Antigen-Presenting Cells. Environ. Res. 2015, 143, 138–147. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Z.; Ying, H.; Wu, C.; Chen, S. Superparamagnetic Iron Oxide Nanoparticles Attenuate Lipopolysaccharide-Induced Inflammatory Responses through Modulation of Toll-like Receptor 4 Expression. J. Appl. Toxicol. 2020, 40, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, S.; Maurizi, L.; Nury, T.; Sallem, F.; Boudon, J.; Riedinger, J.M.; Millot, N.; Bouyer, F.; Lizard, G. Cellular Interactions of Functionalized Superparamagnetic Iron Oxide Nanoparticles on Oligodendrocytes without Detrimental Side Effects: Cell Death Induction, Oxidative Stress and Inflammation. Colloids Surf. B Biointerfaces 2018, 170, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Ranjbary, A.G.; Saleh, G.K.; Azimi, M.; Karimian, F.; Mehrzad, J.; Zohdi, J. Superparamagnetic Iron Oxide Nanoparticles Induce Apoptosis in HT-29 Cells by Stimulating Oxidative Stress and Damaging DNA. Biol. Trace Elem. Res. 2023, 201, 1163–1173. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Q.; Wang, J.; Xue, R.; He, M. Acute Iron Oxide Nanoparticles Exposure Induced Murine Eosinophilic Airway Inflammation via TLR2 and TLR4 Signaling. Environ. Toxicol. 2022, 37, 925–935. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, I.; Chakrabarti, M.; Mukherjee, A. Genotoxicity and Biocompatibility of Superparamagnetic Iron Oxide Nanoparticles: Influence of Surface Modification on Biodistribution, Retention, DNA Damage and Oxidative Stress. Food Chem. Toxicol. 2020, 136, 110989. [Google Scholar] [CrossRef]
- Karouta, N.; Simos, Y.V.; Basina, G.; Spyrou, K.; Subrati, M.; Chatzikonstantinou, A.V.; Hammami, M.A.; Tzitzios, V.; Alhassan, S.M.; Al Wahedi, Y.; et al. Highly Hydrophilic Oleylamine-Modified Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications. ACS Appl. Nano Mater. 2023, 6, 2770–2783. [Google Scholar] [CrossRef]
- Tzitzios, V.; Georgakilas, V.; Zafiropoulou, I.; Boukos, N.; Basina, G.; Niarchos, D.; Petridis, D. A General Chemical Route for the Synthesis of Capped Nanocrystalline Materials. J. Nanosci. Nanotechnol. 2008, 8, 3117–3122. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Grosse, S.; Mollnes, T.E.; Ali, S.; Stenvik, J.; Nilsen, A.M. Iron Oxide Nanoparticles Enhance Toll-like Receptor-Induced Cytokines in a Particle Size- and Actin-Dependent Manner in Human Blood. Nanomedicine 2018, 13, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Rivera, V.; Uribe-Ramírez, M.; González-Pozos, S.; Lozano, O.; Lucas, S.; De Vizcaya-Ruiz, A. Protein Corona Acts as a Protective Shield against Fe 3 O 4 -PEG Inflammation and ROS-Induced Toxicity in Human Macrophages. Toxicol. Lett. 2016, 240, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Nwasike, C.; Purr, E.; Nagi, J.S.; Mahler, G.J.; Doiron, A.L. Incorporation of Targeting Biomolecule Improves Interpolymer Complex-Superparamagnetic Iron Oxide Nanoparticles Attachment to and Activation of T2 MR Signals in M2 Macrophages. Int. J. Nanomedicine 2023, 18, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron Homeostasis and Oxidative Stress: An Intimate Relationship. Biochim. Biophys. Acta—Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Nwasike, C.; Yoo, E.; Purr, E.; Doiron, A.L. Activatable Superparamagnetic Iron Oxide Nanoparticles Scavenge Reactive Oxygen Species in Macrophages and Endothelial Cells. RSC Adv. 2020, 10, 41305–41314. [Google Scholar] [CrossRef]
- Lafuente-Gómez, N.; Wang, S.; Fontana, F.; Dhanjani, M.; García-Soriano, D.; Correia, A.; Castellanos, M.; Rodriguez Diaz, C.; Salas, G.; Santos, H.A.; et al. Synergistic Immunomodulatory Effect in Macrophages Mediated by Magnetic Nanoparticles Modified with MiRNAs. Nanoscale 2022, 14, 11129–11138. [Google Scholar] [CrossRef] [PubMed]
- Dolci, S.; Domenici, V.; Vidili, G.; Orecchioni, M.; Bandiera, P.; Madeddu, R.; Farace, C.; Peana, M.; Tiné, M.R.; Manetti, R.; et al. Immune Compatible Cystine-Functionalized Superparamagnetic Iron Oxide Nanoparticles as Vascular Contrast Agents in Ultrasonography. RSC Adv. 2016, 6, 2712–2723. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gamba, P.; Testa, G.; Rossin, D.; Biasi, F.; Poli, G.; Leonarduzzi, G. Relation between TLR4/NF-ΚB Signaling Pathway Activation by 27-Hydroxycholesterol and 4-Hydroxynonenal, and Atherosclerotic Plaque Instability. Aging Cell 2015, 14, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential Therapeutic Targets for Inflammation in Toll-like Receptor 4 (TLR4)-Mediated Signaling Pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta—Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, C.; Kim, M.B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.K.; Lee, J.Y. Astaxanthin Exerts Anti-Inflammatory and Antioxidant Effects in Macrophages in NRF2-Dependent and Independent Manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Charrier, J.G.; Wu, D.; McFall, A.S.; Li, W.; Abid, A.; Kennedy, I.M.; Anastasio, C. Physicochemical Properties of Iron Oxide Nanoparticles That Contribute to Cellular ROS-Dependent Signaling and Acellular Production of Hydroxyl Radical. Free Radic. Res. 2016, 50, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Pérez-Yagüe, S.; Morales, M.P.; Barber, D.F. Polyethylenimine-Coated SPIONs Trigger Macrophage Activation through TLR-4 Signaling and ROS Production and Modulate Podosome Dynamics. Biomaterials 2015, 52, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, L.; Zhu, W.; Li, D.; Yang, L.; Duan, J.; Cai, Z.; Nie, Y.; Zhang, Y.; Gong, Q.; et al. Iron Oxide Nanoparticles Promote Macrophage Autophagy and Inflammatory Response through Activation of Toll-like Receptor-4 Signaling. Biomaterials 2019, 203, 23–30. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korakaki, E.; Simos, Y.V.; Karouta, N.; Spyrou, K.; Zygouri, P.; Gournis, D.P.; Tsamis, K.I.; Stamatis, H.; Dounousi, E.; Vezyraki, P.; et al. Effect of Highly Hydrophilic Superparamagnetic Iron Oxide Nanoparticles on Macrophage Function and Survival. J. Funct. Biomater. 2023, 14, 514. https://doi.org/10.3390/jfb14100514
Korakaki E, Simos YV, Karouta N, Spyrou K, Zygouri P, Gournis DP, Tsamis KI, Stamatis H, Dounousi E, Vezyraki P, et al. Effect of Highly Hydrophilic Superparamagnetic Iron Oxide Nanoparticles on Macrophage Function and Survival. Journal of Functional Biomaterials. 2023; 14(10):514. https://doi.org/10.3390/jfb14100514
Chicago/Turabian StyleKorakaki, Efterpi, Yannis Vasileios Simos, Niki Karouta, Konstantinos Spyrou, Panagiota Zygouri, Dimitrios Panagiotis Gournis, Konstantinos Ioannis Tsamis, Haralambos Stamatis, Evangelia Dounousi, Patra Vezyraki, and et al. 2023. "Effect of Highly Hydrophilic Superparamagnetic Iron Oxide Nanoparticles on Macrophage Function and Survival" Journal of Functional Biomaterials 14, no. 10: 514. https://doi.org/10.3390/jfb14100514
APA StyleKorakaki, E., Simos, Y. V., Karouta, N., Spyrou, K., Zygouri, P., Gournis, D. P., Tsamis, K. I., Stamatis, H., Dounousi, E., Vezyraki, P., & Peschos, D. (2023). Effect of Highly Hydrophilic Superparamagnetic Iron Oxide Nanoparticles on Macrophage Function and Survival. Journal of Functional Biomaterials, 14(10), 514. https://doi.org/10.3390/jfb14100514











_Stamatis.png)
