Establishing the Callus-Based Isolation of Extracellular Vesicles from Cissus quadrangularis and Elucidating Their Role in Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Surface Sterilization
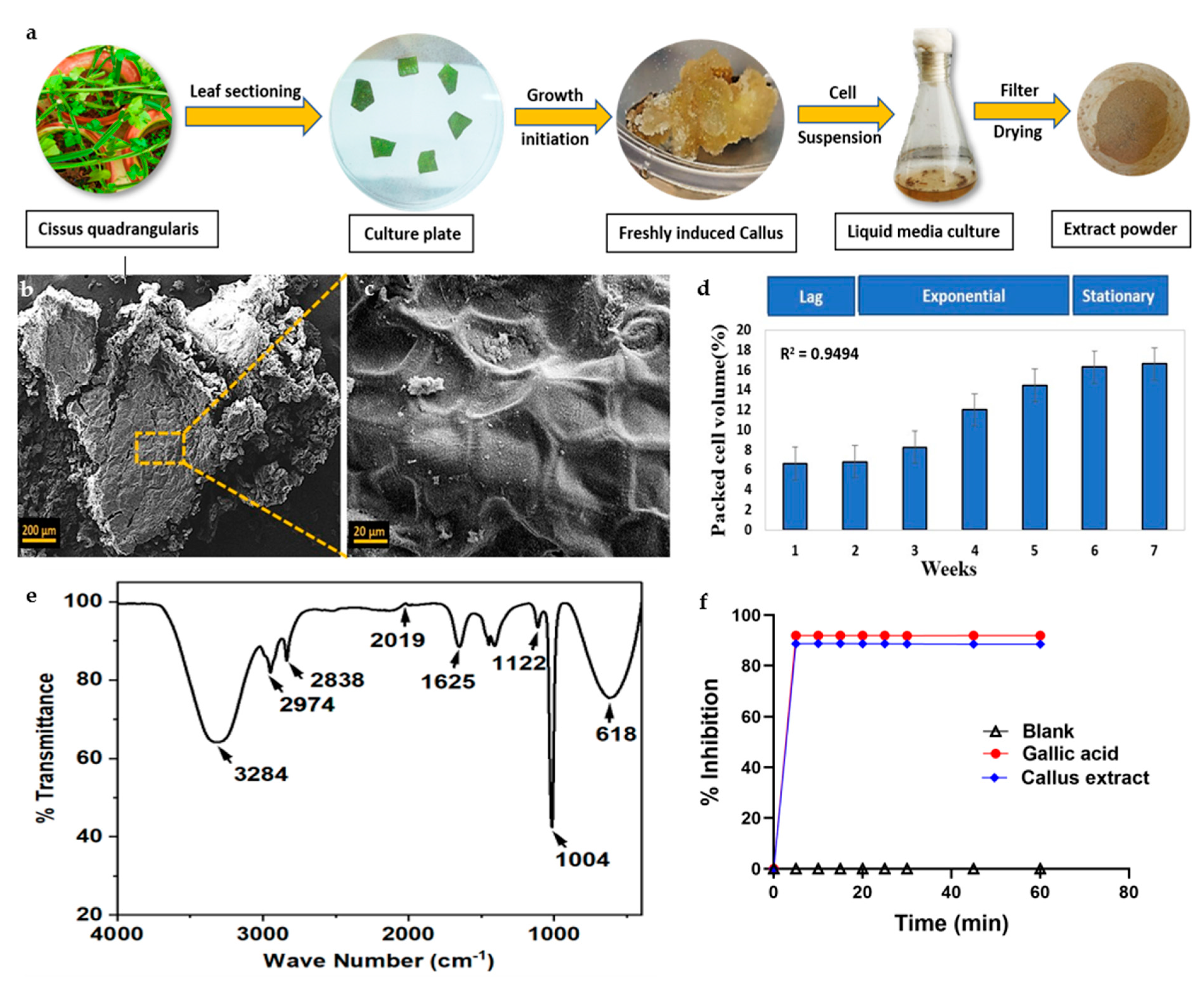
2.2.1. Cissus Cell Induction
2.2.2. Callus Suspension Culture and Growth Kinetics
2.2.3. Cellular Extraction from the Callus
2.3. Characterization of Callus and Biochemical Analysis of Cell Extract
2.3.1. Scanning Electron Microscopy
2.3.2. Fourier-Transform Infrared Spectroscopy
2.3.3. Quantification of Total Phenolics
2.3.4. Measurement of Antioxidant Activity by DPPH Assay
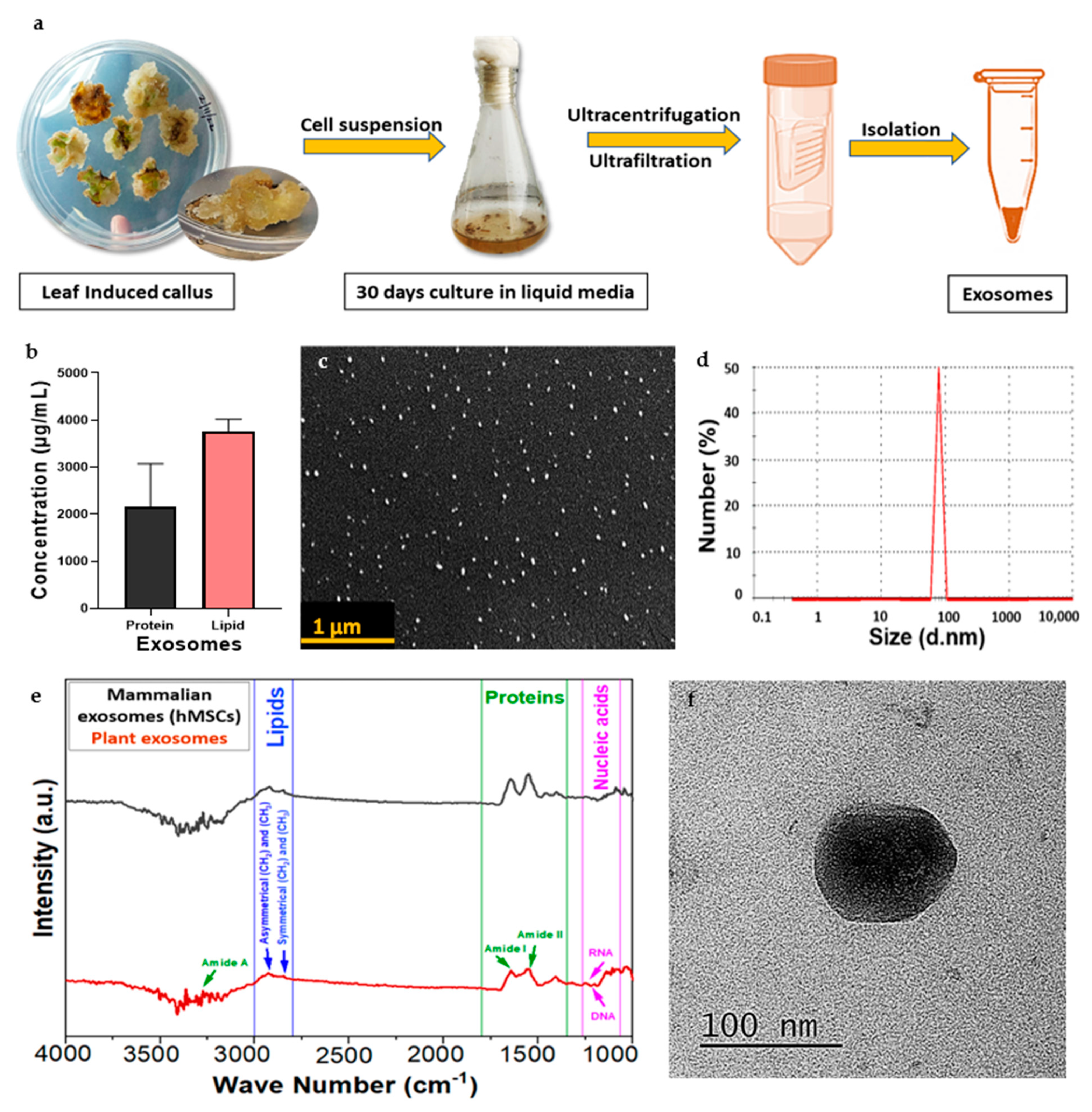
2.4. Exosome Isolation from CQ Cells
2.5. Characterization of CQ Exosomes
2.5.1. Protein Content
2.5.2. Lipid Content
2.5.3. Dynamic Light Scattering
2.5.4. Scanning Electron Microscopy
2.5.5. Transmission Electron Microscopy
2.5.6. Fourier-Transform Infrared Spectrum Analysis
2.6. In Vitro Cellular Studies of CQ Exosomes
2.6.1. Exosome Internalization by hMSCs
2.6.2. Cellular Viability under Oxidative Stress
2.6.3. Wound Scratch Assay
2.6.4. Cellular Proliferation in Response to Exosomes
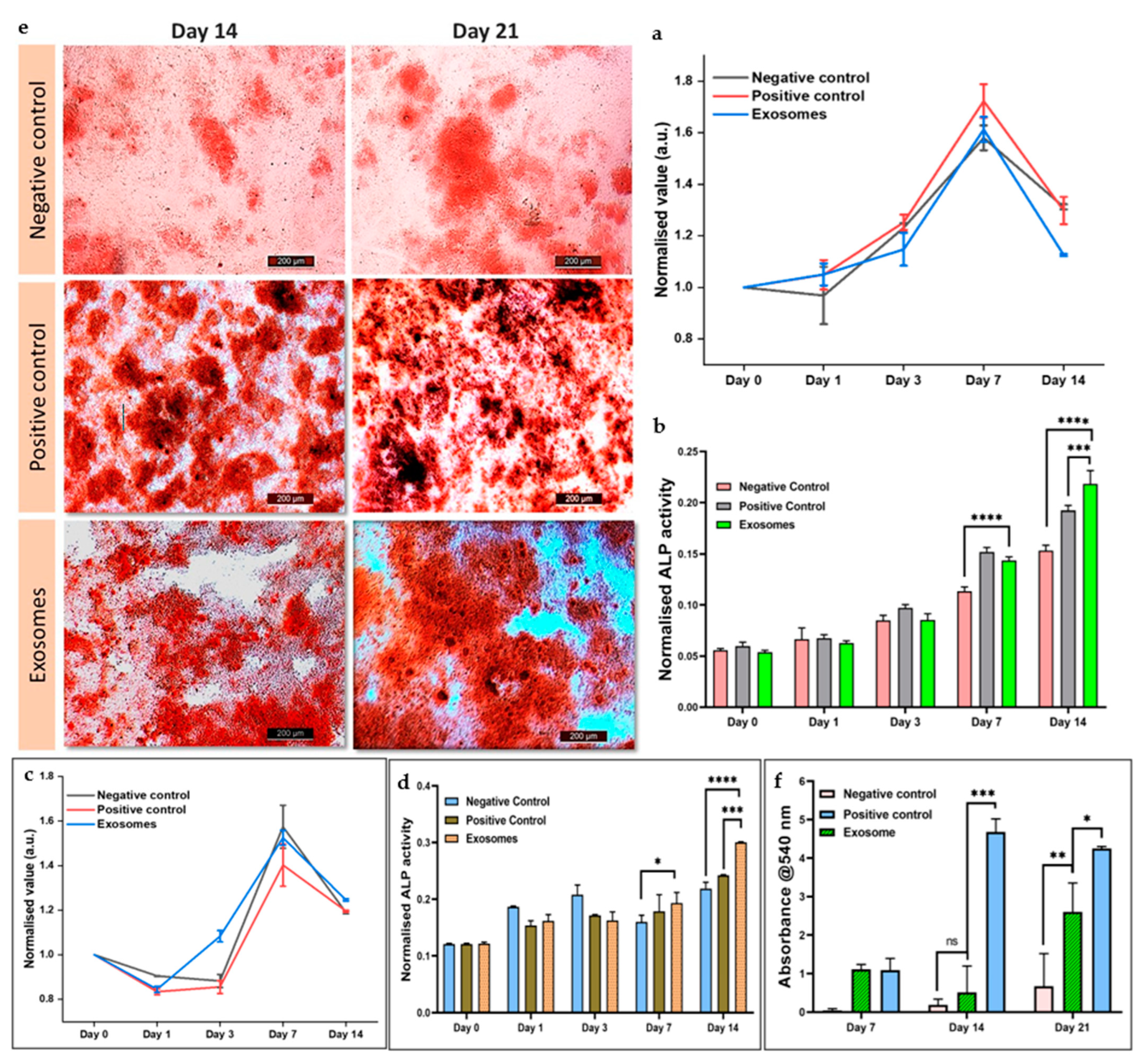
2.6.5. Effect of CQ Exosomes in Cellular Differentiation
2.6.6. Alizarin Red Staining
2.6.7. Statistical Methods
3. Results and Discussion
3.1. Callus Formation and Growth Curve
3.2. Callus Characterization and Biochemical Evaluation of Cell Extract
3.2.1. Scanning Electron Microscopy
3.2.2. Fourier-Transform Infrared Spectroscopy
3.2.3. Antioxidant Activity
3.3. Isolation of Exosomes
3.4. Characterization of CQ Exosomes
3.4.1. Estimation of Protein and Lipid Content
3.4.2. Dynamic Light Scattering
3.4.3. Scanning Electron Microscopy
3.4.4. Transmission Electron Microscopy
3.4.5. Fourier-Transform Infrared Spectrum Analysis
3.5. In Vitro Cellular Studies of CQ Exosomes
3.5.1. Exosome Internalization
3.5.2. Cellular Viability under Oxidative Stress
3.5.3. Wound Scratch Assay
3.5.4. Cellular Proliferation in the Presence of Exosomes
3.5.5. Cellular Differentiation in Response to Exosomes
3.5.6. Alizarin Red Staining for Calcium Deposition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent Advancements in the Use of Exosomes as Drug Delivery Systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Kaimuangpak, K.; Tamprasit, K.; Thumanu, K.; Weerapreeyakul, N. Extracellular Vesicles Derived from Microgreens of Raphanus sativus L. Var. Caudatus Alef Contain Bioactive Macromolecules and Inhibit HCT116 Cells Proliferation. Sci. Rep. 2022, 12, 15686. [Google Scholar] [CrossRef] [PubMed]
- Kocholata, M.; Prusova, M.; Auer Malinska, H.; Maly, J.; Janouskova, O. Comparison of Two Isolation Methods of Tobacco-Derived Extracellular Vesicles, Their Characterization and Uptake by Plant and Rat Cells. Sci. Rep. 2022, 12, 19896. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and Opportunities in Exosome Research—Perspectives from Biology, Engineering, and Cancer Therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of Cabbage Exosome-like Nanovesicles and Investigation of Their Biological Activities in Human Cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, K. Plant-Derived Extracellular Vesicles as Oral Drug Delivery Carriers. J. Control. Release 2022, 350, 389–400. [Google Scholar] [CrossRef]
- Alfieri, M.; Leone, A.; Ambrosone, A. Plant-Derived Nano and Microvesicles for Human Health and Therapeutic Potential in Nanomedicine. Pharmaceutics 2021, 13, 498. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-G.; Choi, S.-Y.; Kim, H.; Choi, E.-J.; Lee, E.-J.; Park, P.-J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax Ginseng-Derived Extracellular Vesicles Facilitate Anti-Senescence Effects in Human Skin Cells: An Eco-Friendly and Sustainable Way to Use Ginseng Substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A Call for Rigor and Standardization in Plant Extracellular Vesicle Research. J. Extracell. Vesicles 2021, 10, 12048. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Fuhrmann, G.; Melzig, M.F. Extracellular Vesicles—Connecting Kingdoms. Int. J. Mol. Sci. 2019, 20, 5695. [Google Scholar] [CrossRef]
- Dai, S.; Wei, D.; Wu, Z.; Zhou, X.; Wei, X.; Huang, H.; Li, G. Phase I Clinical Trial of Autologous Ascites-Derived Exosomes Combined With GM-CSF for Colorectal Cancer. Mol. Ther. 2008, 16, 782–790. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A Bacterial Extracellular Vesicle-based Intranasal Vaccine against SARS-CoV-2 Protects against Disease and Elicits Neutralizing Antibodies to Wild-type and Delta Variants. J. Extracell. Vesicles 2022, 11, 12192. [Google Scholar] [CrossRef]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front. Immunol. 2018, 9, 2538. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from Human Adipose Stem Cells Promote Wound Healing by Optimizing Cellular Functions via AKT and ERK Signaling Pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Rutter, B.; Rutter, K.; Innes, R. Isolation and Quantification of Plant Extracellular Vesicles. Bio-Protocol 2017, 7, 2533. [Google Scholar] [CrossRef]
- Hamid, H.S.; Patil, S. A Phytochemical and Pharmacological Review of an Indian Plant: Cissus quadrangularis. In Proceedings of the 2nd International Electronic Conference on Biomedicines, Online, 1–31 March 2023; MDPI: Basel, Switzerland, 2023; p. 20. [Google Scholar]
- Jaiganesh, K.P.; Prathap, B.; Baskaran, D.; Mageswaran, M.; Pravin, G. Review on Ethnobotony, Phytochemistry and Pharmacology of Cissus quadrangularis, Linn. World J. Pharm. Res. 2021, 10, 408–428. Available online: https://wjpr.s3.ap-south-1.amazonaws.com/article_issue/89e06d3e7f936da352479060e2339950.pdf (accessed on 27 October 2023).
- Gupta, S.; Qayoom, I.; Gupta, P.; Gupta, A.; Singh, P.; Singh, S.; Kumar, A. Exosome-Functionalized, Drug-Laden Bone Substitute along with an Antioxidant Herbal Membrane for Bone and Periosteum Regeneration in Bone Sarcoma. ACS Appl. Mater. Interfaces 2023, 15, 8824–8839. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar Mehta, S.; Qayoom, I.; Gupta, S.; Singh, S.; Kumar, A. Biofunctionalization with Cissus quadrangularis Phytobioactives Accentuates Nano-Hydroxyapatite Based Ceramic Nano-Cement for Neo-Bone Formation in Critical Sized Bone Defect. Int. J. Pharm. 2023, 642, 123110. [Google Scholar] [CrossRef]
- Sharma, N.; Nathawat, R.S.; Gour, K.; Patni, V. Establishment of Callus Tissue and Effect of Growth Regulators on Enhanced Sterol Production in Cissus quadrangularis L. Int. J. Pharmacol. 2011, 7, 653–658. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Ihsan-ul-haq Production of Commercially Important Secondary Metabolites and Antioxidant Activity in Cell Suspension Cultures of Artemisia absinthium L. Ind. Crops Prod. 2013, 49, 400–406. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Somatic Embryogenesis of Date Palm (Phoenix dactylifera L.) Through Cell Suspension Culture. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, 2nd ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; pp. 357–366. [Google Scholar]
- Giri, L.; Dhyani, P.; Rawat, S.; Bhatt, I.D.; Nandi, S.K.; Rawal, R.S.; Pande, V. In Vitro Production of Phenolic Compounds and Antioxidant Activity in Callus Suspension Cultures of Habenaria Edgeworthii: A Rare Himalayan Medicinal Orchid. Ind. Crops Prod. 2012, 39, 1–6. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.L.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Abdi, A.A.; Jouyandeh, M.; Vahabi, H.; Shabanian, M.; Lafon-Pham, D.; Gabrion, X.; Laheurte, P.; Nahavandi, A.M.; Saeb, M.R. Correlating the Photophysical Properties with the Cure Index of Epoxy Nanocomposite Coatings. J. Inorg. Organomet. Polym. Mater. 2021, 31, 923–933. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Damania, A.; Jaiman, D.; Teotia, A.K.; Kumar, A. Mesenchymal Stromal Cell-Derived Exosome-Rich Fractionated Secretome Confers a Hepatoprotective Effect in Liver Injury. Stem Cell Res. Ther. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nikhil, A.; Kumar, A. Preparation of Thermo-Responsive Polymer Encapsulated Exosomes and Its Role as a Therapeutic Agent for Blood Clot Lysis. Colloids Surf. B Biointerfaces 2022, 216, 112580. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; Mitchell, A.J.; Searles, C.D. An Accurate, Precise Method for General Labeling of Extracellular Vesicles. MethodsX 2015, 2, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Teotia, A.K.; Qayoom, I.; Shiekh, P.A.; Andrabi, S.M.; Kumar, A. Periosteum-Mimicking Tissue-Engineered Composite for Treating Periosteum Damage in Critical-Sized Bone Defects. Biomacromolecules 2021, 22, 3237–3250. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Toussaint, J. Citrate-Coated Platinum Nanoparticles Exhibit a Primary Particle-Size Dependent Effect on Stimulating Melanogenesis in Human Melanocytes. Cosmetics 2020, 7, 88. [Google Scholar] [CrossRef]
- Sabokbar, A.; Millett, P.J.; Myer, B.; Rushton, N. A Rapid, Quantitative Assay for Measuring Alkaline Phosphatase Activity in Osteoblastic Cells in Vitro. Bone Miner. 1994, 27, 57–67. [Google Scholar] [CrossRef]
- Kasi, S.D.; Ramasamy, J.M.; Nagaraj, D.; Santiyagu, V.; Ponraj, J.S. Biogenic Synthesis of Copper Oxide Nanoparticles Using Leaf Extracts of Cissus quadrangularis and Piper betle and Its Antibacterial Effects. Micro Nano Lett. 2021, 16, 419–424. [Google Scholar] [CrossRef]
- Di Gioia, S.; Hossain, M.N.; Conese, M. Biological Properties and Therapeutic Effects of Plant-Derived Nanovesicles. Open Med. 2020, 15, 1096–1122. [Google Scholar] [CrossRef]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Rapid Generation of Mitochondrial Superoxide Induces Mitochondrion-Dependent but Caspase-Independent Cell Death in Hippocampal Neuronal Cells That Morphologically Resembles Necroptosis. Toxicol. Appl. Pharmacol. 2012, 262, 156–166. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohammed-Geba, K.; Tawfic, A.A.; El-Magd, M.A. Camel Milk Exosomes Modulate Cyclophosphamide-Induced Oxidative Stress and Immuno-Toxicity in Rats. Food Funct. 2019, 10, 7523–7532. [Google Scholar] [CrossRef]
- Li, Z.; Hassan, M.Q.; Volinia, S.; van Wijnen, A.J.; Stein, J.L.; Croce, C.M.; Lian, J.B.; Stein, G.S. A MicroRNA Signature for a BMP2-Induced Osteoblast Lineage Commitment Program. Proc. Natl. Acad. Sci. USA 2008, 105, 13906–13911. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Selvamurugan, N. MicroRNAs Expression and Their Regulatory Networks during Mesenchymal Stem Cells Differentiation toward Osteoblasts. Int. J. Biol. Macromol. 2014, 66, 194–202. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, R.; Gupta, S.; Gupta, P.; Nüssler, A.K.; Kumar, A. Establishing the Callus-Based Isolation of Extracellular Vesicles from Cissus quadrangularis and Elucidating Their Role in Osteogenic Differentiation. J. Funct. Biomater. 2023, 14, 540. https://doi.org/10.3390/jfb14110540
Gupta R, Gupta S, Gupta P, Nüssler AK, Kumar A. Establishing the Callus-Based Isolation of Extracellular Vesicles from Cissus quadrangularis and Elucidating Their Role in Osteogenic Differentiation. Journal of Functional Biomaterials. 2023; 14(11):540. https://doi.org/10.3390/jfb14110540
Chicago/Turabian StyleGupta, Ritu, Sneha Gupta, Purva Gupta, Andreas K. Nüssler, and Ashok Kumar. 2023. "Establishing the Callus-Based Isolation of Extracellular Vesicles from Cissus quadrangularis and Elucidating Their Role in Osteogenic Differentiation" Journal of Functional Biomaterials 14, no. 11: 540. https://doi.org/10.3390/jfb14110540
APA StyleGupta, R., Gupta, S., Gupta, P., Nüssler, A. K., & Kumar, A. (2023). Establishing the Callus-Based Isolation of Extracellular Vesicles from Cissus quadrangularis and Elucidating Their Role in Osteogenic Differentiation. Journal of Functional Biomaterials, 14(11), 540. https://doi.org/10.3390/jfb14110540






