Enhancing Tissue Equivalence in 7Li Heavy Ion Therapy with MC Algorithm Optimized Polymer-Based Bioinks
Abstract
:1. Introduction
2. Material and Method
3. Result
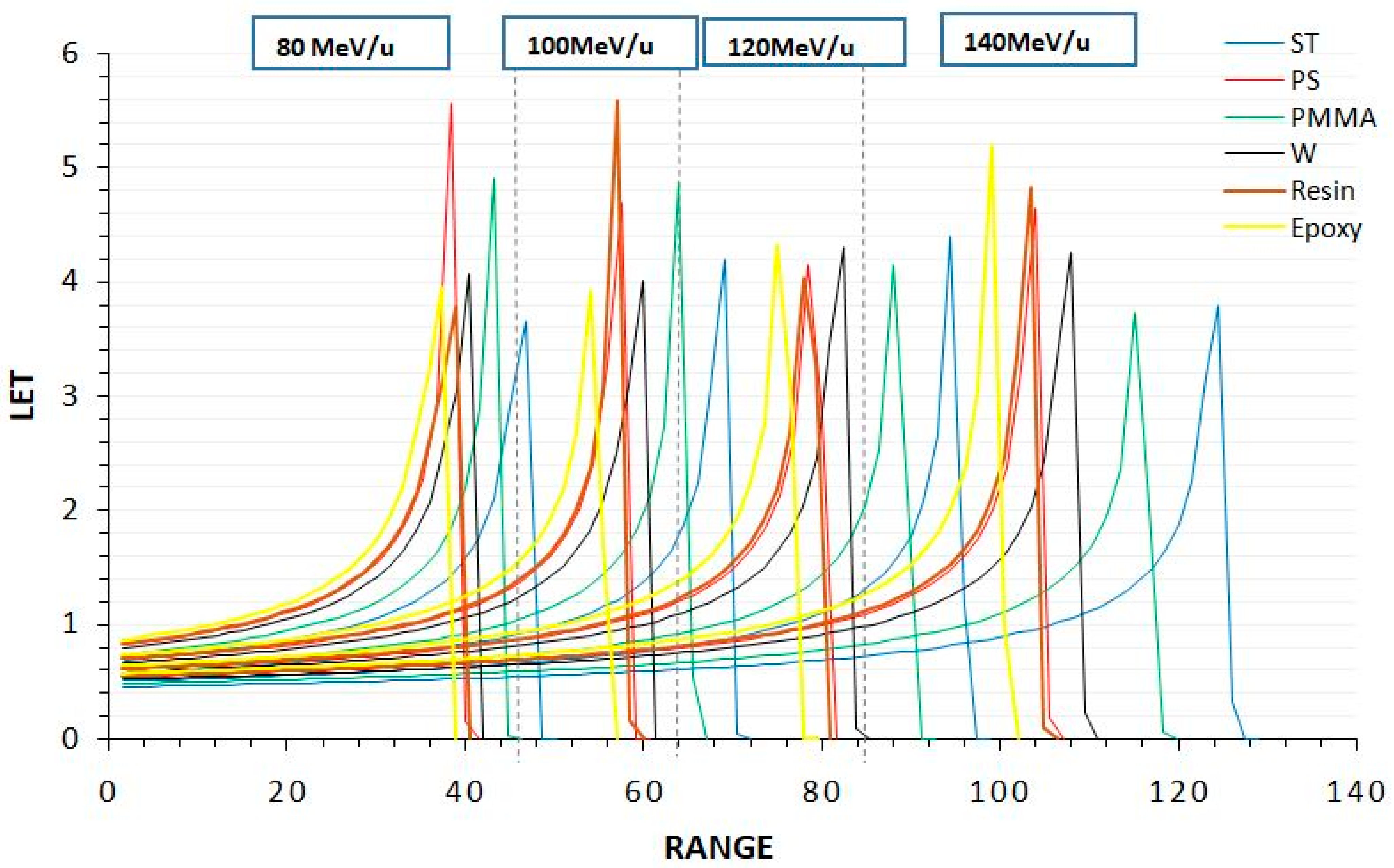
3.1. Bragg Cure
3.2. Recoils
3.3. Collision Events
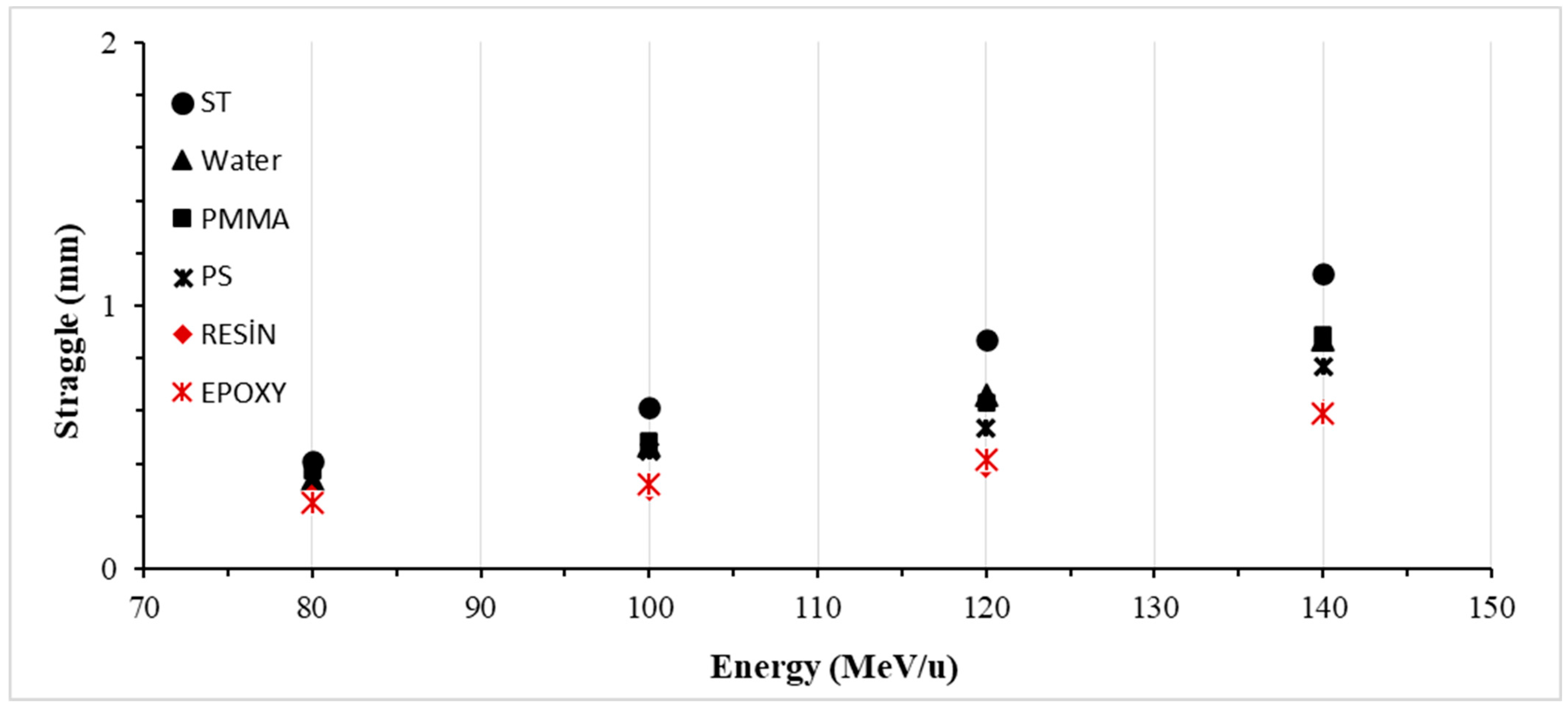
3.4. Lateral Straggle
4. Discussion
5. Conclusions
- Utilizing Li ions as intermediate ions in heavy ion therapy and examining them in terms of ionization, recoils, and lateral straggle.
- Conducting a radiological investigation of polymer biomaterials used in phantom production or as body soft tissue replacements with heavy ions.
- Analyzing the structure of crystalline injection zones in polymer materials and investigating changes in polymer bonds caused by heavy ions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, O. Simulation based analysis of 4He, 7Li, 8Be and 10B ions for heavy ion therapy. Int. J. Radiat. Res. 2023, 21, 131–137. [Google Scholar]
- Mattei, I.; Bini, F.; Collamati, F.; De Lucia, E.; Frallicciardi, P.M.; Iarocci, E.; Mancini-Terracciano, C.; Marafini, M.; Muraro, S.; Paramatti, R.; et al. Secondary radiation measurements for particle therapy applications: Prompt photons produced by 4He.12C and 16O ion beams in a PMMA target. Phys. Med. 2017, 62, 1438–1455. [Google Scholar] [CrossRef]
- Musha, A.; Kubo, N.; Kawamura, H.; Okano, N.; Sato, H.; Okada, K.; Osu, N.; Yumisaki, H.; Adachi, A.; Takayasu, Y.; et al. Carbon-ion radiotherapy for inoperable head and neck bone and soft-tissue sarcoma: Prospective observational study. Antıcancer Res. 2022, 42, 1439–1446. [Google Scholar] [CrossRef]
- Brownstein, J.M.; Wisdom, A.J.; Castle, K.D.; Mowery, Y.M.; Guida, P.; Lee, C.-L.; Tommasino, F.; La Tessa, C.; Scifoni, E.; Gao, J.; et al. Characterizing the Potency and Impact of Carbon Ion Therapy in a Primary Mouse Model of Soft Tissue Sarcoma Relative Potency of Carbon Ions in Primary Mouse Sarcomas. Mol. Cancer Ther. 2018, 17, 858–868. [Google Scholar] [CrossRef]
- Horst, F.; Schardt, D.; Iwase, H.; Schuy, C.; Durante, M.; Weber, U. Physical characterization of 3He ion beams for radiotherapy and comparison with 4He. Phys. Med. Biol. 2021, 66, 095009. [Google Scholar] [CrossRef]
- Kantemiris, I.; Karaiskos, P.; Papagiannis, P.; Angelopoulos, A. Dose and dose averaged LET comparison of 1H, 4He, 6Li, 8Be, 10B, 12C, 14N and 16O ion beams forming a spread-out Bragg peak. Med. Phys. 2011, 38, 6585–6591. [Google Scholar] [CrossRef]
- Pshenichnov, I.; Mishustin, I.; Greiner, W. Comparative study of depth-dose distributions for beams of light and heavy nuclei in tissue-like media. Nucl. Instrum. Methods B 2008, 266, 1094–1098. [Google Scholar] [CrossRef]
- Ekinci, F. Investigation of tissue equivalence of phantom biomaterials in 4He heavy ion therapy. Radiat. Eff. Defects Solids 2023, 178, 500–509. [Google Scholar] [CrossRef]
- Elsässer, T.; Weyrather, W.K.; Friedrich, T.; Durante, M.; Iancu, G.; Krämer, M.; Kragl, G.; Brons, S.; Winter, M.; Weber, K.J.; et al. Quantification of the relative biological effectiveness for ion beam radiotherapy: Direct experimental comparison of proton and carbon ion beams and a novel approach for treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, O. Recoil Analysis for Heavy Ion Beams. Aksaray J. Sci. Eng. 2022, 6, 123–134. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, T.T.; Zhang, L.F.; Ma, S.; Shen, Y.J.; Feng, C.C.; Nie, P.P.; Yang, Z.R.; Zhu, C. The selective colorimetric probe based on a macrocyclic Sm(III) complex for detecting lithium ion and its performance in the psychiatric drug. Dye. Pigment. 2020, 174, 108027. [Google Scholar] [CrossRef]
- Kamenica, M.; Kothur, R.R.; Willows, A.; Patel, B.A.; Cragg, P.J. Lithium ion sensors. Sensors 2017, 17, 2430. [Google Scholar] [CrossRef]
- Debrot, E.; Newall, M.; Guatelli, S.; Petasecca, M.; Matsufuji, N.; Rosenfeld, A.B. A silicon strip detector array for energy verification and quality assurance in heavy ion therapy. Med. Phys. 2018, 45, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Inaniwa, T.; Furukawa, T.; Sato, S.; Tomitani, T.; Kobayashi, M.; Minohara, S.; Noda, K.; Kanai, T. Development of treatment planning for scanning irradiation at HIMAC. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 2194–2198. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Battistoni, G.; Boehlen, T.; Cerutti, F.; Chin, P.W.; Esposito, L.S.; Fassò, A.; Ferrari, A.; Lechner, A.; Empl, A.; Mairani, A.; et al. Overview of the FLUKA code. Ann. Nucl. Energy 2015, 82, 10–18. [Google Scholar] [CrossRef]
- Niita, K.; Sato, T.; Iwase, H.; Nose, H.; Nakashima, H.; Sihver, L. PHITS—A particle and heavy ion transport code system. Radiat. Meas. 2006, 41, 1080–1090. [Google Scholar] [CrossRef]
- Zuber, S.H.; Yusof, M.F.M.; Hashikin, N.A.A.; Samson, D.O.; Aziz, M.Z.A.; Hashim, R. Rhizophora spp. as potential phantom material in medical physics applications—A review. Radiat. Phys. Chem. 2021, 189, 109731. [Google Scholar] [CrossRef]
- Ramos, S.M.O.; Thomas, S.; Berdeguez, M.B.T.; Sa, L.V.; Souza, S.A.L. Anthropomorphic phantoms-potential for more studies and training in radiology. Int. J. Radiol. Radiat. Ther. 2017, 2, 00033. [Google Scholar]
- Zhang, J.; Lu, Y.; Hsi, W.; Zhang, J.; Sheng, Y.; Shi, L.; Wang, W.; Lu, J.; Zhou, R.; Cheng, J. Evaluation of Proton Therapy Accuracy Using a PMMA Phantom and PET Prediction Module. Front. Oncol. 2018, 13, 523. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Biersack, J.P.; Ziegler, M.D. SRIM—The Stopping and Range of Ions in Matter; SRIM Co.: Boston, MA, USA, 2008; ISBN 0-9654207-1-X. [Google Scholar]
- Stoller, R.; Toloczko, M.; Was, G.; Certain, A.; Dwaraknath, S.; Garner, F. On the use of SRIM for Computing Radiation Damage Exposure. Nucl. Instrum. Methods Phys. Res. B 2013, 310, 75–80. [Google Scholar] [CrossRef]
- Torrisi, L.; Cutroneo, M.; Torrisi, A.; Silipigni, L.; Havranek, V. Small-field Dosimetry Based on Reduced Graphene Oxide under MeV Helium Beam Irradiation. Radiat. Eff. Defects Solids 2020, 175, 120–135. [Google Scholar] [CrossRef]
- Kanematsu, N.; Koba, Y.; Ogata, R. Evaluation of plastic materials for range shifting range compensation and solid phantom dosimetry in carbon-ion radiotherapy. Med. Phys. 2013, 40, 041724. [Google Scholar] [CrossRef]
- Kum, O. Estimation of cooling water activation in high-energy heavy ion medical accelerator. Radiat. Phys. Chem. 2021, 181, 109258. [Google Scholar] [CrossRef]
- Foster, D.G.; Artur, E.D. Avarege Neutronic Properties of “Prompt” Fission Poducts; LA-9168-MS, DE32:009080; Los Alamos National Laboratory Report; Los Alamos National Lab.: Los Alamos, NM, USA, 1982. [Google Scholar]
- Kantz, M.R.; Newman, H.D., Jr.; Stigale, F.H. The skin-core morphology and structure–property relationships in injection-molded polypropylene. J. Appl. Polym. Sci. 1972, 16, 1249–1260. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Song, B.; Li, Y.; Liu, L. Detecting crystallization structure evolution of polypropylene injection-molded bar induced by nucleating agent. Polym. Eng. Sci. 2008, 48, 1532–1541. [Google Scholar] [CrossRef]
- Piorkowska, E.; Rutledge, G.C. (Eds.) Handbook of Polymer Crystallization; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Groom, D.E.; Klein, S.R. Passage of particles through matter. Eur. Phys. J. C-Part. Fields 2000, 15, 163–173. [Google Scholar] [CrossRef]
- Was, S.G. Fundamentals of Radiation Materials Science. In Fundamentals of Radiation Materials Science Metals and Alloys; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Stoneham, A.M.; Matthews, J.R.; Ford, I.J. Innovative materials for fusion power plant structures: Separating functions. J. Phys. Condens. Matter 2004, 16, S2597. [Google Scholar] [CrossRef]
- Lee, E.H. Ion-beam modification of polymeric materials fundamental principles and applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1999, 151, 29–41. [Google Scholar] [CrossRef]
- Ziegler, J.F. SRIM: The Stopping and Range of Ion in Matter. Publisher of Website. Available online: https://www.srim.org (accessed on 13 March 2020).
- Ekinci, F.; Bostanci, E.; Güzel, M.S.; Dagli, O. Effect of different embolization materials on proton beam stereotactic radiosurgery Arteriovenous Malformation dose distributions using the Monte Carlo simulation code. J. Radiat. Res. Appl. Sci. 2022, 15, 191–197. [Google Scholar] [CrossRef]
- Ekinci, F. Ionization and phonon production by 10B ions in radiotherapy applications. Commun. Fac. Sci. Univ. Ank. Ser. A2–A3 Phys. Sci. Eng. 2023, 65, 30–37. [Google Scholar]
- Abe, M. Charged particle radiotherapy at the Hyogo Ion Beam Medical Center: Characteristics, technology and clinical results. Proc. Jpn. Acad. Ser. B 2007, 83, 151–163. [Google Scholar] [CrossRef]
- Nishio, T.; Miyatake, A.; Ogino, T.; Nakagawa, K.; Saijo, N.; Esumi, H. The development and clinical use of a beam on-line PET system mounted on a rotating gantry port in proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Parodi, K.; Paganetti, H.; Shih, H.A.; Michaud, S.; Loeffler, J.S.; DeLaney, T.F.; Liebsch, N.J.; Munzenrider, J.E.; Fischman, A.J.; Knopf, A.; et al. Patient study of in vivo verification of beam delivery and range, using positron emission tomography and computed tomography imaging after proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 920–934. [Google Scholar] [CrossRef]
- Priegnitz, M.; Fiedler, F.; Kunath, D.; Laube, K.; Enghardt, W. An experiment-based approach for predicting positron emitter distributions produced during therapeutic ion irradiation. IEEE Trans. Nucl. Sci. 2011, 59, 77–87. [Google Scholar] [CrossRef]
- Kempe, J.; Gudowska, I.; Brahme, A. Depth absorbed dose and LET distributions of therapeutic H, He, Li and C beams. Med. Phys. 2007, 34, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Verona, C.; Magrin, G.; Solevi, P.; Bandorf, M.; Marinelli, M.; Stock, M.; Rinati, G.V. Toward the use of single crystal diamond based detector for ion-beam therapy microdosimetry. Radiat. Meas. 2018, 110, 25–31. [Google Scholar] [CrossRef]
- Vernimmen, F. Intracranial stereotactic radiation therapy with charged particle beams: An opportunity to regain the momentum. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 52–55. [Google Scholar] [CrossRef]
- Park, S.H.; Kano, H.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for cerebellopontine angle meningiomas. J. Neurosurg. 2014, 120, 708–715. [Google Scholar] [CrossRef]
- Dokic, I.; Mairani, A.; Niklas, M.; Zimmermann, F.; Chaudhri, N.; Krunic, D.; Tessonnier, T.; Ferrari, A.; Parodi, K.; Jäkel, O.; et al. Next generation multi-scale biophysical characterization of high precision cancer particle radiotherapy using clinical proton, helium-, carbon-and oxygen ion beams. Oncotarget 2016, 7, 56676. [Google Scholar] [CrossRef] [PubMed]




| Biomaterials | Atomic Percent | Mass Percent | Atomic Number Density | Mass Density | Displacement | Binding | Surface |
|---|---|---|---|---|---|---|---|
| ST | H 54.6; C 32.9; N 0.862; O 7.89; Mg 3.63; CI 1.72 | H 8.12; C 58.3; N 1.78; O 18.6; Mg 13.0; CI 8.99 | 8.88 | 1.0 | H 10; C 28; N 28; O 28; Mg 25; CI 25 | H 3; C 3; N 3; O 3; Mg 3; CI 3 | H 2; C 7.41; N 2; O 2; Mg 1.24; CI 2 |
| W | O 33.3; H 66.6 | O 88.8; H 11.1 | 10.02 | 1.0 | O 28; H 10 | O 3; H 3 | O 2; H 2 |
| PMMA | H 53.3; C 33.3; O 13.3 | H 8.05; C 59.9; O 31.9 | 8.57 | 0.95 | H 10; C 28; O 28 | H 3; C 3; O 3 | H 2; C 7.41; O 2 |
| PS | H 50; C 50 | H 7.74; C 92.2 | 9.81 | 1.06 | H 10; C 28 | H 3; C 3 | H 2; C 7.41 |
| Resin | C 42; N 1.3; O 7.9; P 1.3; CI 1.3; H 46 | C 64; N 2.3; O 16.2; P 5.4; CI 5.9; H 6.2 | 8.52 | 1.11 | C 28; N 28; O 28; P 25; CI 25; H 10 | C 3; N 3; O 3; P 3; CI 3; H 3 | C 7.41; N 2; O 2; P 3.27; CI 3; H 2 |
| Epoxy | C 42.2; N 2.2; O 8.9; P 2.2; CI 2.3; H 42.2 | C 58.1; N 3.6; O 16.3; P 7.9; CI 9.1; H 4.9 | 8.16 | 1.18 | C 28; N 28; O 28; P 25; CI 25; H 10 | C 3; N 3; O 3; P 3; CI 3; H 3 | C 7.41; N 2; O 2; P 3.27; CI 2; H 2 |
| Energy | Bragg Peak Position (mm) | |||||
|---|---|---|---|---|---|---|
| W | PMMA | PS | ST | Resin | Epoxy | |
| 80 | 40.5 | 43.2 | 38.4 | 46.8 | 39.1 | 36.1 |
| 100 | 60.2 | 64.1 | 57.6 | 69.1 | 57.2 | 54.2 |
| 120 | 82.5 | 88.2 | 78.4 | 94.5 | 78.1 | 75.2 |
| 140 | 108.1 | 115.2 | 104 | 124.5 | 103.5 | 99.2 |
| Average | 72.825 | 77.675 | 69.6 | 83.725 | 69.475 | 66.175 |
| S.D. | 25.21 | 26.89 | 24.38 | 28.97 | 24.01 | 23.56 |
| Materials | Energy | T.R. | Contributions to Recoils of Atoms (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | C | O | Cl | Mg | N | P | Ca | F | Si | Na | Al | |||
| ST | 80 | 3.04 | 2.4 | 57.9 | 20.5 | 0.2 | 18.3 | 1.5 | ||||||
| 100 | 3.31 | 2.3 | 51.8 | 19.5 | 0.2 | 20.2 | 7.1 | |||||||
| 120 | 1.31 | 2.5 | 53.3 | 19.3 | 0.1 | 24.3 | 1.5 | |||||||
| 140 | 2.49 | 2.3 | 54.6 | 21.5 | 0.2 | 20.5 | 1.9 | |||||||
| S.D. | 0.89 | 0.1 | 2.6 | 1.1 | 0.1 | 2.5 | 2.7 | |||||||
| Water | 80 | 1.57 | 37.2 | 64.2 | ||||||||||
| 100 | 1.57 | 34.7 | 66.9 | |||||||||||
| 120 | 3.66 | 37.9 | 63.6 | |||||||||||
| 140 | 3.49 | 36.9 | 64.6 | |||||||||||
| S.D. | 1.16 | 1.4 | 1.4 | |||||||||||
| PMMA | 80 | 3.77 | 25.3 | 53.6 | 22.4 | |||||||||
| 100 | 2.86 | 30.7 | 46.1 | 24.7 | ||||||||||
| 120 | 2.13 | 39.6 | 40.8 | 20.7 | ||||||||||
| 140 | 2.59 | 27.7 | 48.5 | 25.3 | ||||||||||
| S.D. | 0.69 | 6.3 | 5.3 | 2.1 | ||||||||||
| PS | 80 | 3.18 | 25.9 | 75.5 | ||||||||||
| 100 | 3.93 | 23.1 | 78.1 | |||||||||||
| 120 | 2.78 | 22.7 | 78.5 | |||||||||||
| 140 | 3.31 | 23.3 | 77.9 | |||||||||||
| S.D. | 0.48 | 1.5 | 1.4 | |||||||||||
| Resin | 80 | 3.28 | 21.2 | 56.3 | 13.3 | 3.8 | 1.6 | 3.8 | ||||||
| 100 | 3.93 | 19.5 | 59.9 | 13.9 | 2.3 | 1.9 | 2.5 | |||||||
| 120 | 2.79 | 22.1 | 54.2 | 14.2 | 4.2 | 2.1 | 3.2 | |||||||
| 140 | 3.28 | 22.6 | 53.3 | 11.6 | 5.3 | 1.6 | 5.6 | |||||||
| S.D. | 0.47 | 1.4 | 2.9 | 1.2 | 1.3 | 0.2 | 1.3 | |||||||
| Epoxy | 80 | 3.416 | 19.2 | 56.5 | 14.8 | 6.6 | ||||||||
| 100 | 3.207 | 20.4 | 54.5 | 15.6 | 5.9 | |||||||||
| 120 | 2.987 | 19.4 | 53.4 | 11.9 | 5.3 | |||||||||
| 140 | 3.553 | 18.1 | 59.1 | 11.4 | 8.5 | |||||||||
| S.D. | 0.247 | 0.9 | 2.5 | 2.1 | 1.4 | |||||||||
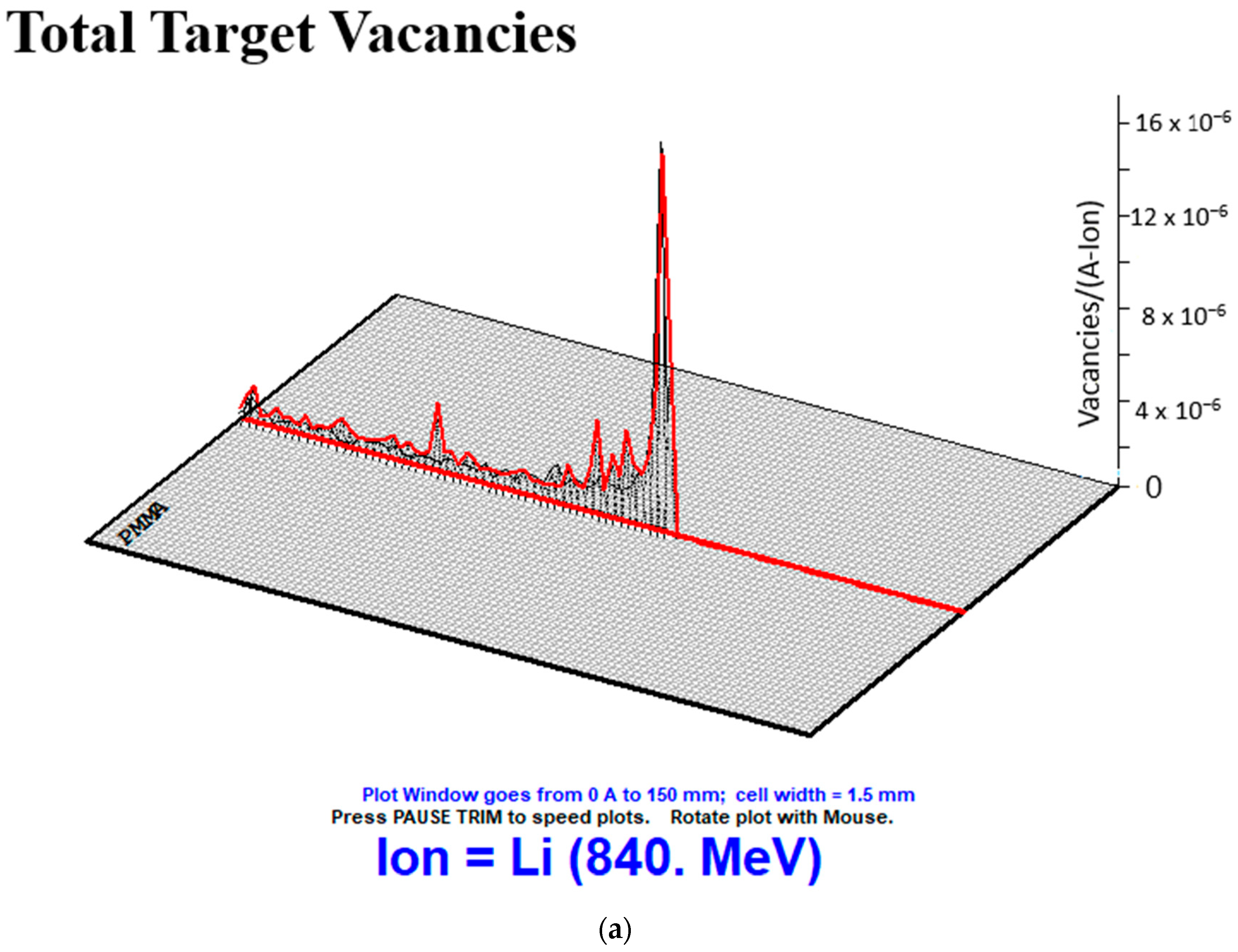
| CE | Biomaterials | Energy (MeV/u) | S.D. | |||
|---|---|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |||
| Total Target Vacanies | Water | 25,840,000 | 29,870,000 | 33,720,000 | 37,290,000 | 4,272,452 |
| PMMA | 20,800,000 | 24,070,000 | 27,280,000 | 30,290,000 | 3,542,563 | |
| PS | 19,060,000 | 22,220,000 | 25,200,000 | 28,040,000 | 3,346,117 | |
| ST | 16,680,000 | 19,310,000 | 21,920,000 | 24,360,000 | 2,868,200 | |
| Resin | 19,780,000 | 22,970,000 | 26,010,000 | 28,960,000 | 3,419,481 | |
| Epoxy | 19,580,000 | 22,730,000 | 25,790,000 | 28,660,000 | 3,388,385 | |
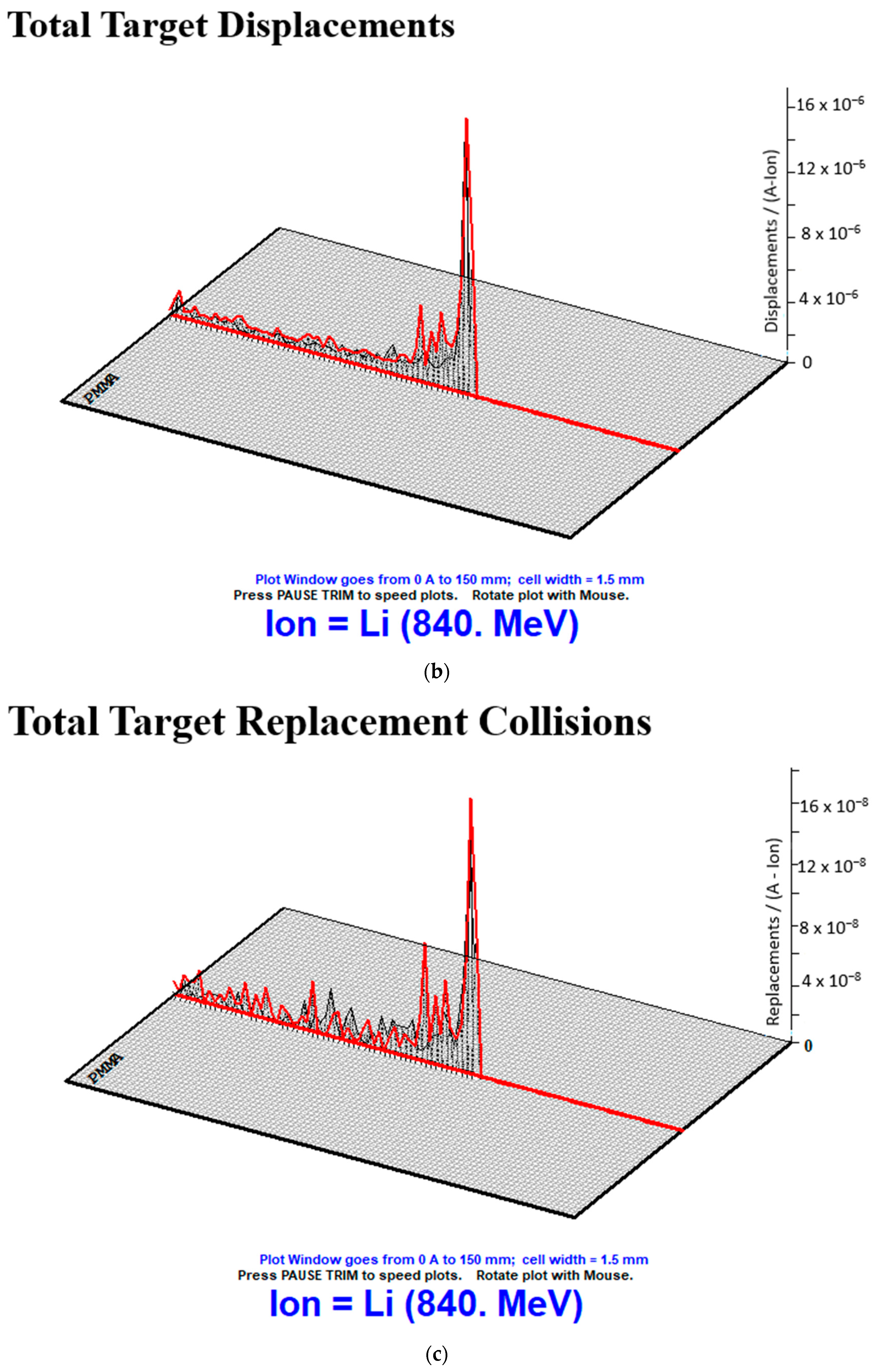
| Total Target Displacements | Water | 26,270,000 | 30,370,000 | 34,290,000 | 37,920,000 | 4,347,404 |
| PMMA | 21,060,000 | 24,370,000 | 27,620,000 | 30,670,000 | 3,587,276 | |
| PS | 19,390,000 | 22,610,000 | 25,630,000 | 28,530,000 | 3,404,247 | |
| ST | 16,860,000 | 19,520,000 | 22,160,000 | 24,630,000 | 2,901,735 | |
| Resin | 19,880,000 | 23,090,000 | 26,140,000 | 29,110,000 | 3,437,372 | |
| Epoxy | 19,790,000 | 22,970,000 | 26,070,000 | 28,960,000 | 3,423,101 | |
| Total Target Replacement Collisions | Water | 440,000 | 510,000 | 570,000 | 630,000 | 70,489 |
| PMMA | 260,000 | 300,000 | 340,000 | 380,000 | 44,721 | |
| PS | 330,000 | 390,000 | 440,000 | 490,000 | 59,319 | |
| ST | 180,000 | 210,000 | 240,000 | 260,000 | 30,311 | |
| Resin | 100,000 | 120,000 | 130,000 | 150,000 | 18,028 | |
| Epoxy | 210,000 | 240,000 | 280,000 | 310,000 | 38,079 | |
| Energy | Lateral Straggle (mm) | |||||
|---|---|---|---|---|---|---|
| Water | PMMA | PS | ST | Resin | Epoxy | |
| 80 | 0.337 | 0.373 | 0.287 | 0.412 | 0.279 | 0.254 |
| 100 | 0.467 | 0.483 | 0.450 | 0.615 | 0.302 | 0.322 |
| 120 | 0.661 | 0.631 | 0.536 | 0.872 | 0.389 | 0.416 |
| 140 | 0.870 | 0.890 | 0.772 | 1.120 | 0.597 | 0.591 |
| Average | 0.584 | 0.594 | 0.511 | 0.755 | 0.392 | 0.396 |
| S.D. | 0.233 | 0.224 | 0.202 | 0.308 | 0.145 | 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekinci, F.; Acici, K.; Asuroglu, T. Enhancing Tissue Equivalence in 7Li Heavy Ion Therapy with MC Algorithm Optimized Polymer-Based Bioinks. J. Funct. Biomater. 2023, 14, 559. https://doi.org/10.3390/jfb14120559
Ekinci F, Acici K, Asuroglu T. Enhancing Tissue Equivalence in 7Li Heavy Ion Therapy with MC Algorithm Optimized Polymer-Based Bioinks. Journal of Functional Biomaterials. 2023; 14(12):559. https://doi.org/10.3390/jfb14120559
Chicago/Turabian StyleEkinci, Fatih, Koray Acici, and Tunc Asuroglu. 2023. "Enhancing Tissue Equivalence in 7Li Heavy Ion Therapy with MC Algorithm Optimized Polymer-Based Bioinks" Journal of Functional Biomaterials 14, no. 12: 559. https://doi.org/10.3390/jfb14120559
APA StyleEkinci, F., Acici, K., & Asuroglu, T. (2023). Enhancing Tissue Equivalence in 7Li Heavy Ion Therapy with MC Algorithm Optimized Polymer-Based Bioinks. Journal of Functional Biomaterials, 14(12), 559. https://doi.org/10.3390/jfb14120559







