Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of Experimental Scaffolds
2.2. Scaffold Characterization
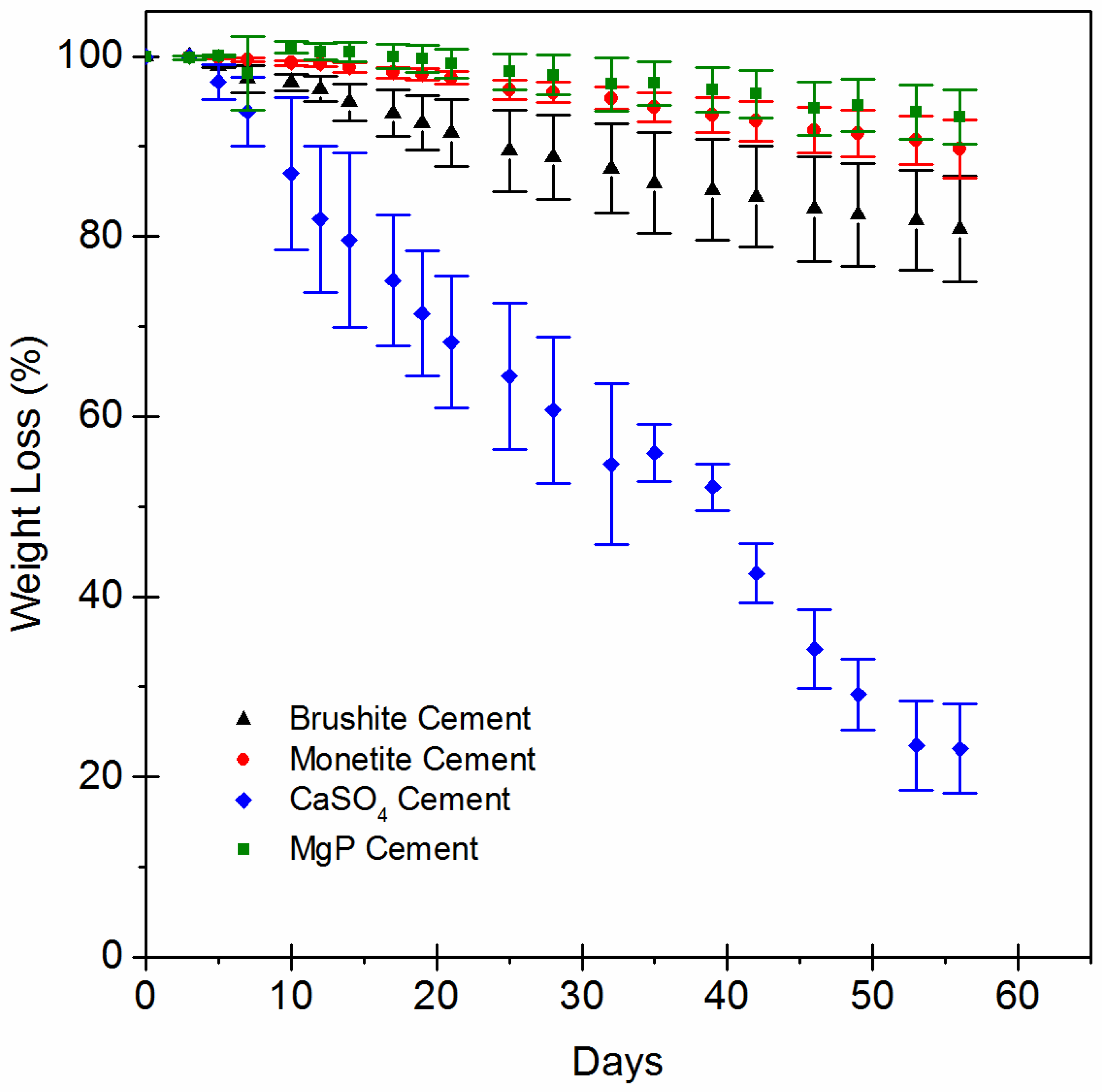
2.3. In Vitro Evaluation of Scaffold Dissolution
2.4. Animal Model and Surgical Procedure
2.5. Evaluation of Scaffold Dissolution and Blood Vessel Density in the Animal Explants
2.6. Immunohistochemical (IHC) Staining
2.7. Statistical Analysis
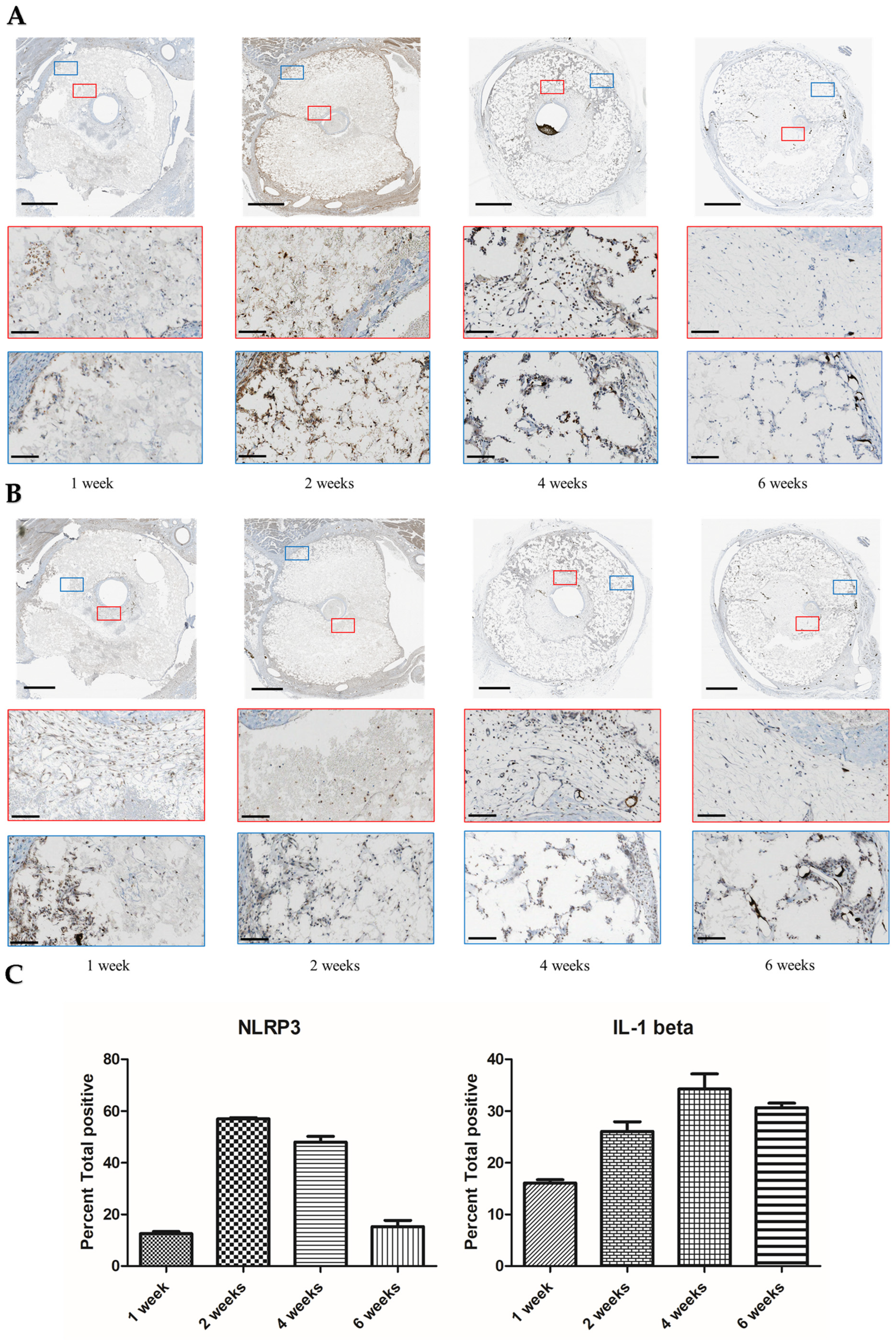
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Soucacos, P.N.; Kokkalis, Z.T.; Piagkou, M.; Johnson, E.O. Vascularized bone grafts for the management of skeletal defects in orthopaedic trauma and reconstructive surgery. Injury 2013, 44 (Suppl. 1), S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Bumbasirevic, M.; Stevanovic, M.; Bumbasirevic, V.; Lesic, A.; Atkinson, H.D. Free vascularised fibular grafts in orthopaedics. Int. Orthop. 2014, 38, 1277–1282. [Google Scholar] [CrossRef]
- Muramatsu, K.; Hashimoto, T.; Tominaga, Y.; Taguchi, T. Vascularized bone graft for oncological reconstruction of the extremities: Review of the biological advantages. Anticancer. Res. 2014, 34, 2701–2707. [Google Scholar] [PubMed]
- Allsopp, B.J.; Hunter-Smith, D.J.; Rozen, W.M. Vascularized versus Nonvascularized Bone Grafts: What Is the Evidence? Clin. Orthop. Relat. Res. 2016, 474, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.S.; Stinner, D.J.; Obremskey, W.T. Treatment of Traumatic Segmental Long-Bone Defects: A Critical Analysis Review. JBJS Rev. 2014, 2, e1. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Podlesh, S.; Anthony, J.P.; Alexander, J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J. Oral. Maxillofac. Surg. 1997, 55, 1200–1206. [Google Scholar] [CrossRef]
- Olfert, I.M. Physiological Capillary Regression is not Dependent on Reducing VEGF Expression. Microcirculation 2016, 23, 146–156. [Google Scholar] [CrossRef]
- Kress, S.; Baur, J.; Otto, C.; Burkard, N.; Braspenning, J.; Walles, H.; Nickel, J.; Metzger, M. Evaluation of a Miniaturized Biologically Vascularized Scaffold in vitro and in vivo. Sci. Rep. 2018, 8, 4719. [Google Scholar] [CrossRef] [PubMed]
- Dao, K., 3rd; Malgor, R.D.; Montecalvo, J.; Hines, G. Current status of endoscopic vein harvest in cardiac and peripheral vascular surgery. Cardiol Rev 2012, 20, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Beier, J.P.; Kneser, U.; Arkudas, A. Successful human long-term application of in situ bone tissue engineering. J. Cell Mol. Med. 2014, 18, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Charbonnier, B.; Maillard, S.; Sayed, O.; Baradaran, A.; Mangat, H.; Dalisson, B.; Zhang, Z.; Zhang, Y.-L.; Hussain, S.N.A.; Mayaki, D.; et al. Biomaterial-Induction of a Transplantable Angiosome. Adv. Funct. Mater. 2020, 30, 1905115. [Google Scholar] [CrossRef]
- Charbonnier, B.; Baradaran, A.; Sato, D.; Alghamdi, O.; Zhang, Z.; Zhang, Y.L.; Gbureck, U.; Gilardino, M.; Harvey, E.; Makhoul, N.; et al. Material-Induced Venosome-Supported Bone Tubes. Adv. Sci. 2019, 6, 1900844. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Q.; Fu, L.; Zheng, S.; Wang, C.; Han, L.; Gong, Z.; Wang, Z.; Tang, H.; Zhang, Y. CD301b+ macrophages mediate angiogenesis of calcium phosphate bioceramics by CaN/NFATc1/VEGF axis. Bioact. Mater. 2022, 15, 446–455. [Google Scholar] [CrossRef]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Seime, T.; van Wanrooij, M.; Karlöf, E.; Kronqvist, M.; Johansson, S.; Matic, L.; Gasser, T.C.; Hedin, U. Biomechanical Assessment of Macro-Calcification in Human Carotid Atherosclerosis and Its Impact on Smooth Muscle Cell Phenotype. Cells 2022, 11, 3279. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Zhang, Y.L.; Grover, L.; Merle, G.E.; Tamimi, F.; Barralet, J. In vitro degradation and in vivo resorption of dicalcium phosphate cement based grafts. Acta Biomater. 2015, 26, 338–346. [Google Scholar] [CrossRef]
- Kingery, W.D. Fundamental Study of Phosphate Bonding in Refractories: I., Literature Review. J. Am. Ceram. Soc. 1950, 33, 239–241. [Google Scholar] [CrossRef]
- Cho, C.; Michailidis, V.; Lecker, I.; Collymore, C.; Hanwell, D.; Loka, M.; Danesh, M.; Pham, C.; Urban, P.; Bonin, R.P.; et al. Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci. Rep. 2019, 9, 359. [Google Scholar] [CrossRef]
- Nicholson, A.; Klaunberg, B. Chapter 30—Anesthetic Considerations for In Vivo Imaging Studies. In Anesthesia and Analgesia in Laboratory Animals, 2nd ed.; Fish, R.E., Brown, M.J., Danneman, P.J., Karas, A.Z., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 629–639. [Google Scholar]
- Liu, R.; Jin, J.P. Calponin isoforms CNN1, CNN2 and CNN3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene 2016, 585, 143–153. [Google Scholar] [CrossRef]
- Sato, N.; Sawasaki, Y.; Senoo, A.; Fuse, Y.; Hirano, Y.; Goto, T. Development of capillary networks from rat microvascular fragments in vitro: The role of myofibroblastic cells. Microvasc. Res. 1987, 33, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.A.; Bean, P.A.; Whitelock, J.M. Inhibition of endothelial cell adhesion and proliferation by extracellular matrix from vascular smooth muscle cells: Role of type V collagen. Atherosclerosis 1998, 141, 141–152. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Lilly, B.; Kennard, S. Differential gene expression in a coculture model of angiogenesis reveals modulation of select pathways and a role for Notch signaling. Physiol Genom. 2009, 36, 69–78. [Google Scholar] [CrossRef]
- Hellström, M.; Kalén, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Delbosc, S.; Glorian, M.; Le Port, A.S.; Béréziat, G.; Andréani, M.; Limon, I. The benefit of docosahexanoic acid on the migration of vascular smooth muscle cells is partially dependent on Notch regulation of MMP-2/-9. Am. J. Pathol. 2008, 172, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Shimokado, K.; Raines, E.W.; Madtes, D.K.; Barrett, T.B.; Benditt, E.P.; Ross, R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell 1985, 43, 277–286. [Google Scholar] [CrossRef]
- Okada, M.; Hojo, Y.; Ikeda, U.; Takahashi, M.; Takizawa, T.; Morishita, R.; Shimada, K. Interaction between monocytes and vascular smooth muscle cells induces expression of hepatocyte growth factor. J. Hypertens 2000, 18, 1825–1831. [Google Scholar] [CrossRef]
- Butoi, E.; Gan, A.M.; Tucureanu, M.M.; Stan, D.; Macarie, R.D.; Constantinescu, C.; Calin, M.; Simionescu, M.; Manduteanu, I. Cross-talk between macrophages and smooth muscle cells impairs collagen and metalloprotease synthesis and promotes angiogenesis. Biochim. Biophys Acta 2016, 1863, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Burger, F.; Baptista, D.; Roth, A.; da Silva, R.F.; Montecucco, F.; Mach, F.; Brandt, K.J.; Miteva, K. NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Dautova, Y.; Kapustin, A.N.; Pappert, K.; Epple, M.; Okkenhaug, H.; Cook, S.J.; Shanahan, C.M.; Bootman, M.D.; Proudfoot, D. Calcium phosphate particles stimulate interleukin-1β release from human vascular smooth muscle cells: A role for spleen tyrosine kinase and exosome release. J. Mol. Cell Cardiol. 2018, 115, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskard, D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Dautova, Y.; Kozlova, D.; Skepper, J.N.; Epple, M.; Bootman, M.D.; Proudfoot, D. Fetuin-A and albumin alter cytotoxic effects of calcium phosphate nanoparticles on human vascular smooth muscle cells. PLoS ONE 2014, 9, e97565. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kohno, H.; Matsuoka, Y.; Murakami, M.; Nakatsuka, R.; Hase, M.; Yasuda, K.; Uemura, Y.; Sasaki, Y.; Fukuhara, S.; et al. CXCL8 enhances the angiogenic activity of umbilical cord blood-derived outgrowth endothelial cells in vitro. Cell Biol. Int. 2011, 35, 201–208. [Google Scholar] [CrossRef]
- Jung, Y.D.; Fan, F.; McConkey, D.J.; Jean, M.E.; Liu, W.; Reinmuth, N.; Stoeltzing, O.; Ahmad, S.A.; Parikh, A.A.; Mukaida, N.; et al. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine 2002, 18, 206–213. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Hari, A.; Zhang, Y.; Tu, Z.; Detampel, P.; Stenner, M.; Ganguly, A.; Shi, Y. Activation of NLRP3 inflammasome by crystalline structures via cell surface contact. Sci. Rep. 2014, 4, 7281. [Google Scholar] [CrossRef]
- Chen, H.; Agrawal, D.K.; Thankam, F.G. Biomaterials-Driven Sterile Inflammation. Tissue Eng. Part B Rev. 2020, 28, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef]
- Huang, L.; Xie, Y.H.; Xiang, H.B.; Hou, Y.L.; Yu, B. Physiochemical properties of copper doped calcium sulfate in vitro and angiogenesis in vivo. Biotech Histochem. 2021, 96, 117–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Guo, D.; Geng, H.; Dong, C. Effect of Chloride Salts and Bicarbonate on Solubility of CaSO4 in Aqueous Solutions at 37 °C. Procedia Environ. Sci. 2013, 18, 84–91. [Google Scholar] [CrossRef]
- Zia, S.; Ndoye, A.; Lee, T.X.; Webber, R.J.; Grando, S.A. Receptor-mediated inhibition of keratinocyte migration by nicotine involves modulations of calcium influx and intracellular concentration. J. Pharmacol. Exp. Ther. 2000, 293, 973–981. [Google Scholar] [PubMed]
- Fang, K.S.; Farboud, B.; Nuccitelli, R.; Isseroff, R.R. Migration of human keratinocytes in electric fields requires growth factors and extracellular calcium. J. Investig. Derm. 1998, 111, 751–756. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The Role of Calcium in Wound Healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Parys, J.B.; Bultynck, G. Calcium signaling in health, disease and therapy. Biochim. Biophys Acta Mol. Cell Res. 2018, 1865, 1657–1659. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Kao, M.C.; Lin, J.A.; Chen, T.Y.; Cheng, C.F.; Wong, C.S.; Tzeng, I.S.; Huang, C.J. Effects of MgSO4 on inhibiting Nod-like receptor protein 3 inflammasome involve decreasing intracellular calcium. J. Surg. Res. 2018, 221, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, M.; He, X.; Ouyang, D. A mini-review on ion fluxes that regulate NLRP3 inflammasome activation. Acta Biochim. Biophys. Sin. 2021, 53, 131–139. [Google Scholar] [CrossRef]
- Cao, Y. Therapeutic angiogenesis for ischemic disorders: What is missing for clinical benefits? Discov. Med. 2010, 9, 179–184. [Google Scholar]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Giannotti, K.C.; Weinert, S.; Viana, M.N.; Leiguez, E.; Araujo, T.L.S.; Laurindo, F.R.M.; Lomonte, B.; Braun-Dullaeus, R.; Teixeira, C. A Secreted Phospholipase A2 Induces Formation of Smooth Muscle Foam Cells Which Transdifferentiate to Macrophage-Like State. Molecules 2019, 24, 3244. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Bennett, M.; Yu, H.; Clarke, M. Signalling from dead cells drives inflammation and vessel remodelling. Vasc. Pharm. 2012, 56, 187–192. [Google Scholar] [CrossRef]
- Li, G.; Wang, M.; Caulk, A.W.; Cilfone, N.A.; Gujja, S.; Qin, L.; Chen, P.Y.; Chen, Z.; Yousef, S.; Jiao, Y.; et al. Chronic mTOR activation induces a degradative smooth muscle cell phenotype. J. Clin. Investig. 2020, 130, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; van Eys, G.; Bochaton-Piallat, M.-L.; Proudfoot, D.; Biessen, E.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef] [PubMed]
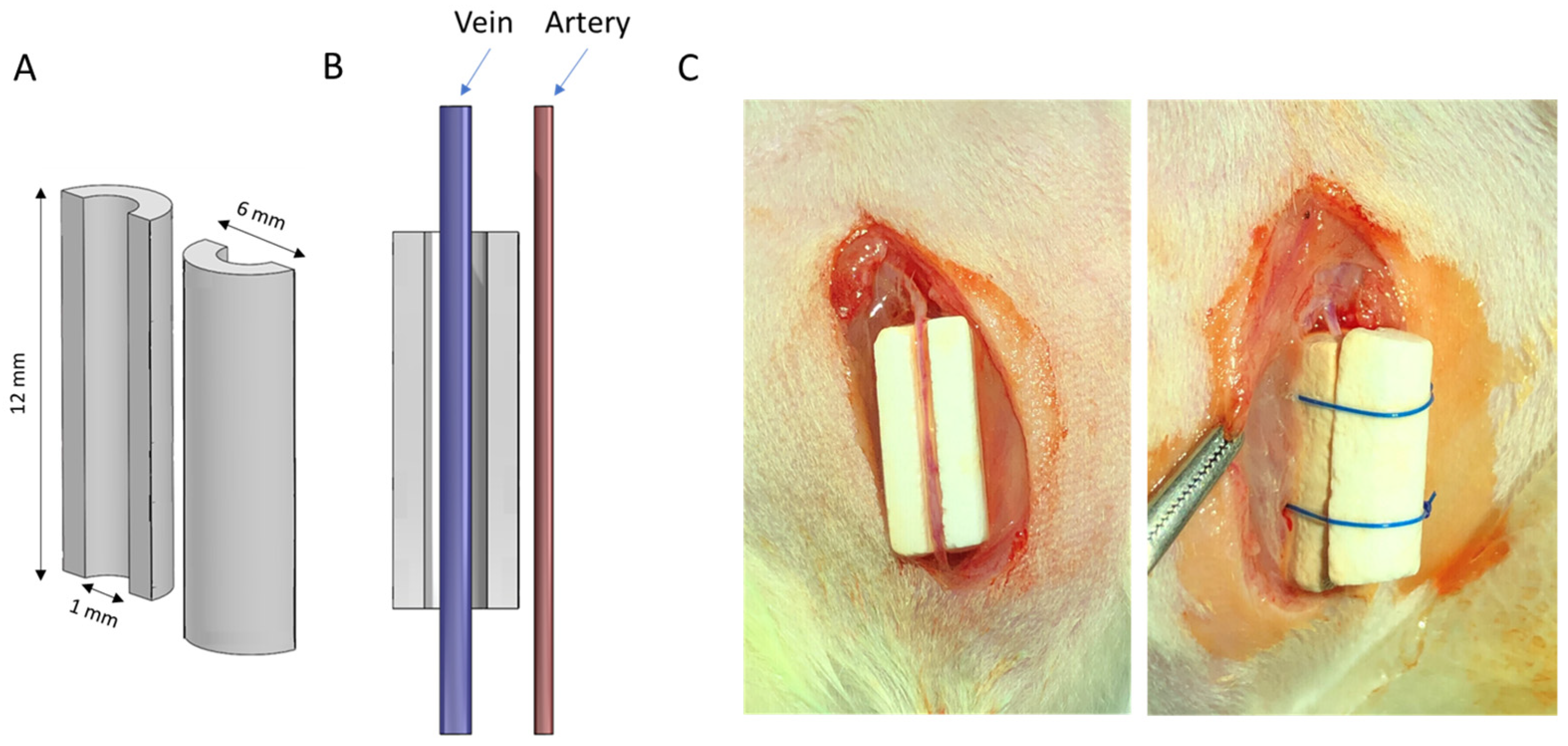
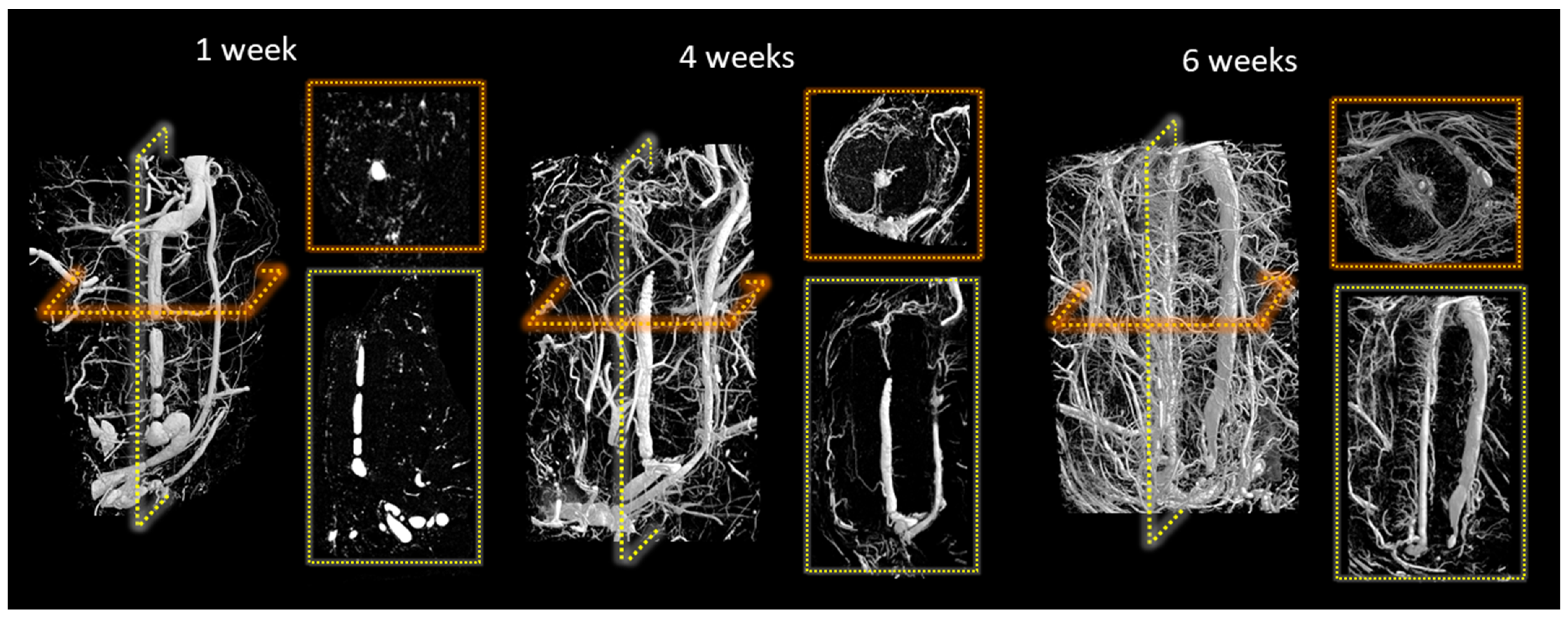
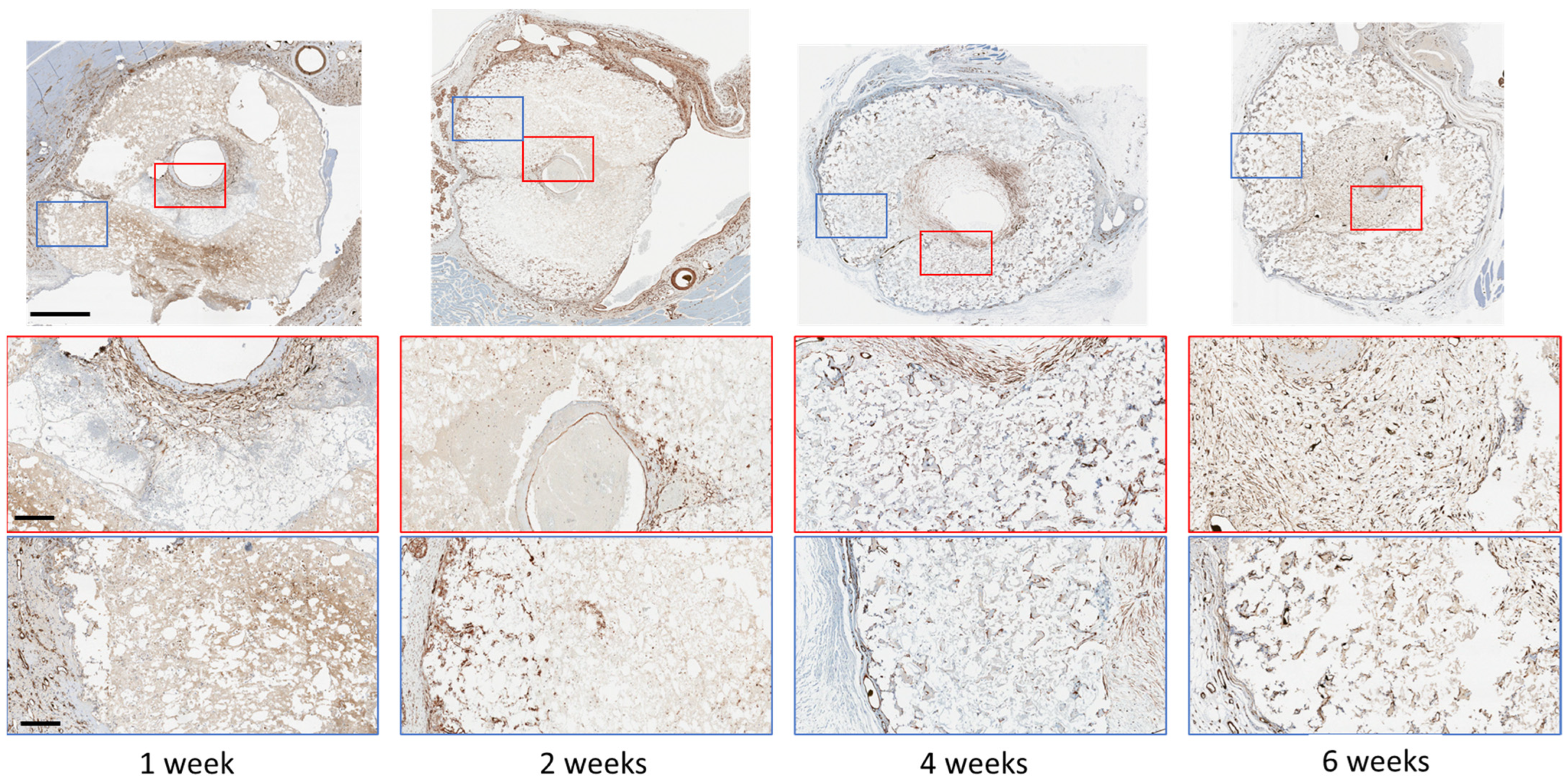

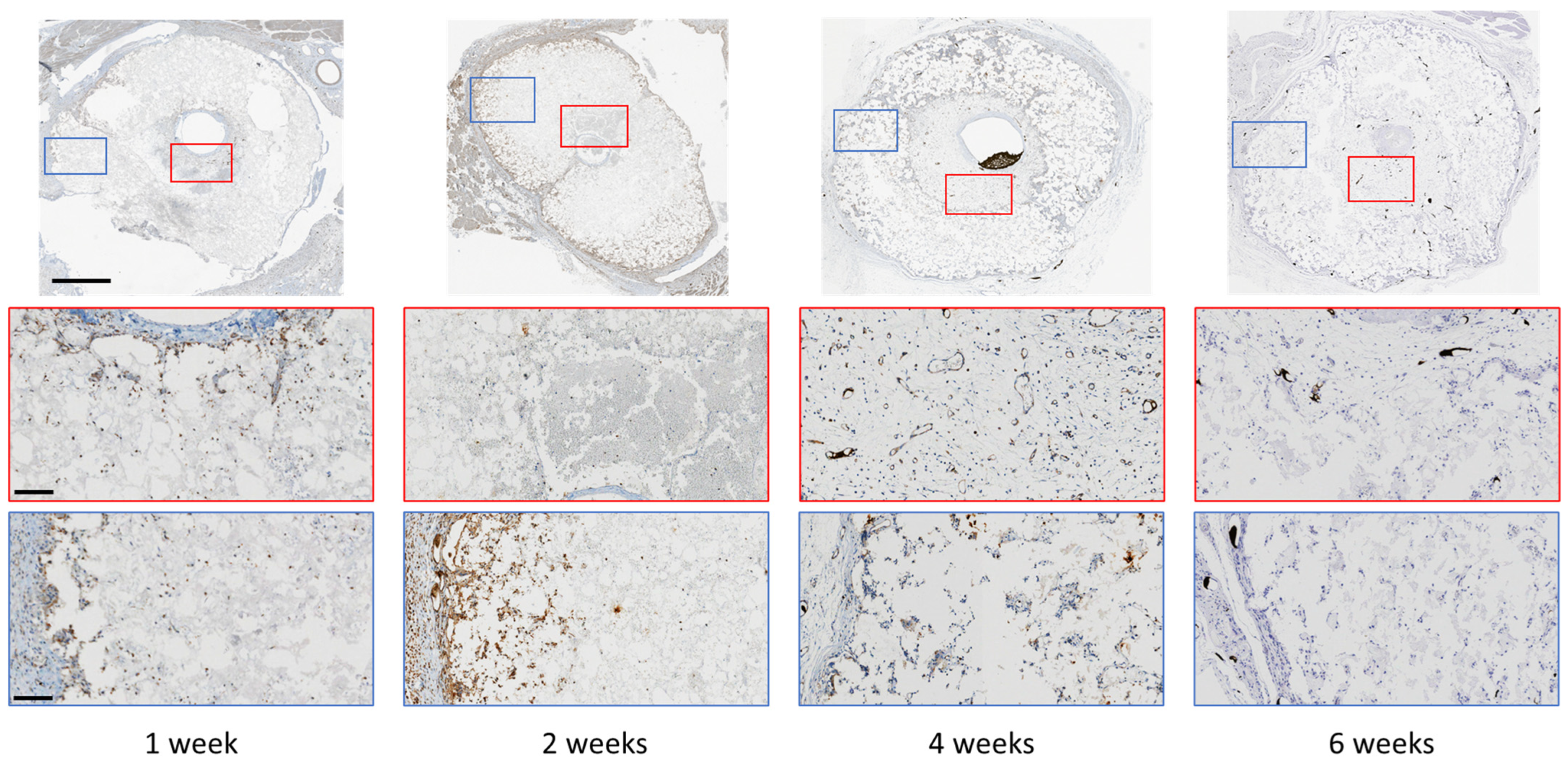
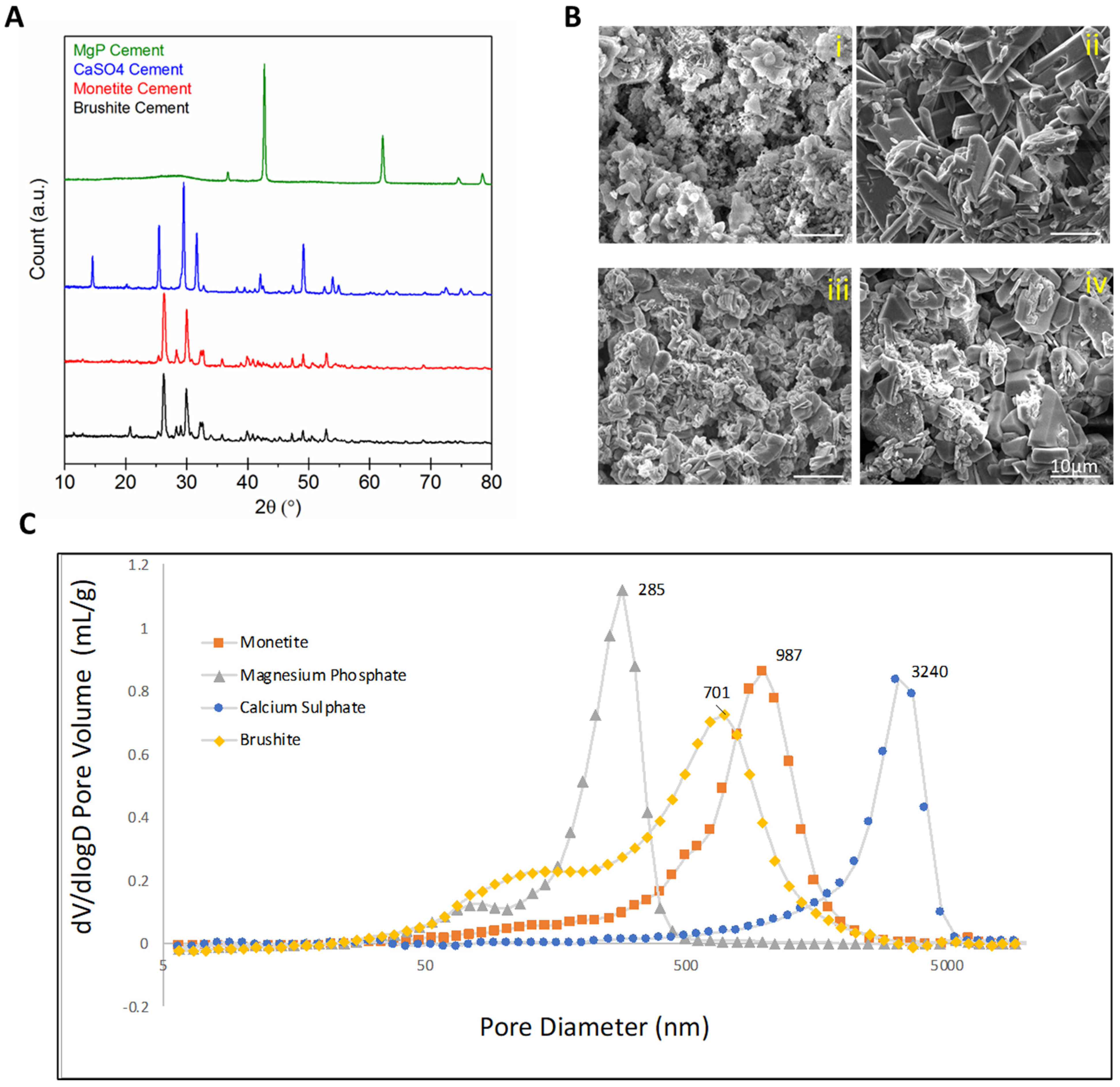

| Scaffold | P:L Ratio (g/mL) | Porosity (%) |
|---|---|---|
| Brushite (CaHPO4·2H2O) | 2:1 | 54.2 (±2.4) |
| Monetite (CaHPO4) | 2:0.7 | 48.0 (±2.5) |
| Calcium sulphate (CaSO4·2H2O) | 2:1 | 45.4 (±1.6) |
| Magnesium phosphate (MgHPO4) | 1:1 | 50.6 (±7.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouhaddi, Y.; Charbonnier, B.; Porge, J.; Zhang, Y.-L.; Garcia, I.; Gbureck, U.; Grover, L.; Gilardino, M.; Harvey, E.; Makhoul, N.; et al. Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds. J. Funct. Biomater. 2023, 14, 105. https://doi.org/10.3390/jfb14020105
Ouhaddi Y, Charbonnier B, Porge J, Zhang Y-L, Garcia I, Gbureck U, Grover L, Gilardino M, Harvey E, Makhoul N, et al. Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds. Journal of Functional Biomaterials. 2023; 14(2):105. https://doi.org/10.3390/jfb14020105
Chicago/Turabian StyleOuhaddi, Yassine, Baptiste Charbonnier, Juliette Porge, Yu-Ling Zhang, Isadora Garcia, Uwe Gbureck, Liam Grover, Mirko Gilardino, Edward Harvey, Nicholas Makhoul, and et al. 2023. "Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds" Journal of Functional Biomaterials 14, no. 2: 105. https://doi.org/10.3390/jfb14020105
APA StyleOuhaddi, Y., Charbonnier, B., Porge, J., Zhang, Y.-L., Garcia, I., Gbureck, U., Grover, L., Gilardino, M., Harvey, E., Makhoul, N., & Barralet, J. (2023). Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds. Journal of Functional Biomaterials, 14(2), 105. https://doi.org/10.3390/jfb14020105








