Effectiveness of Hyaluronic Acid Gel Injection with and without PRGF for Management of Interdental Papillary Loss: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Sample Size Calculation
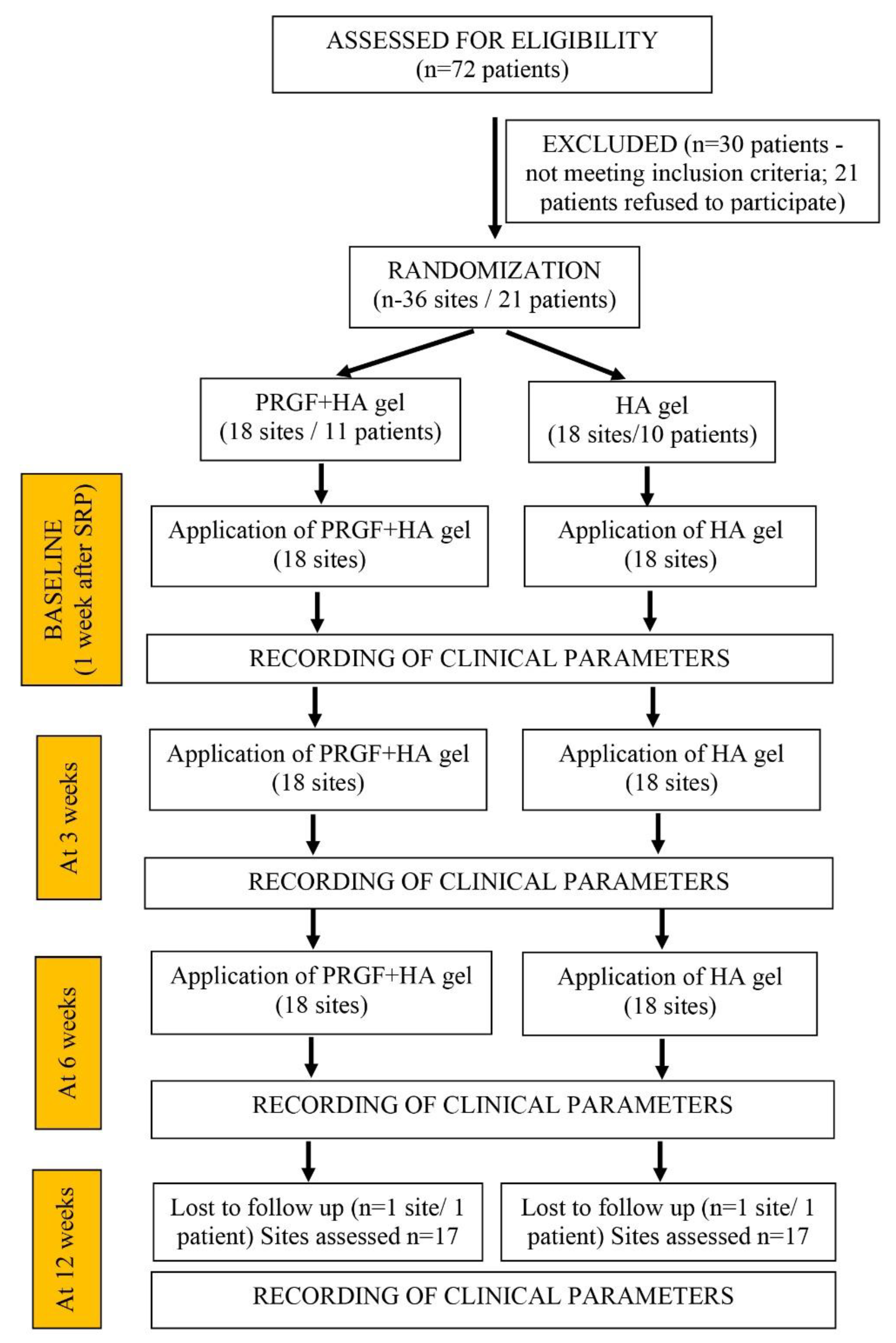
2.3. Randomization and Clinical Procedure
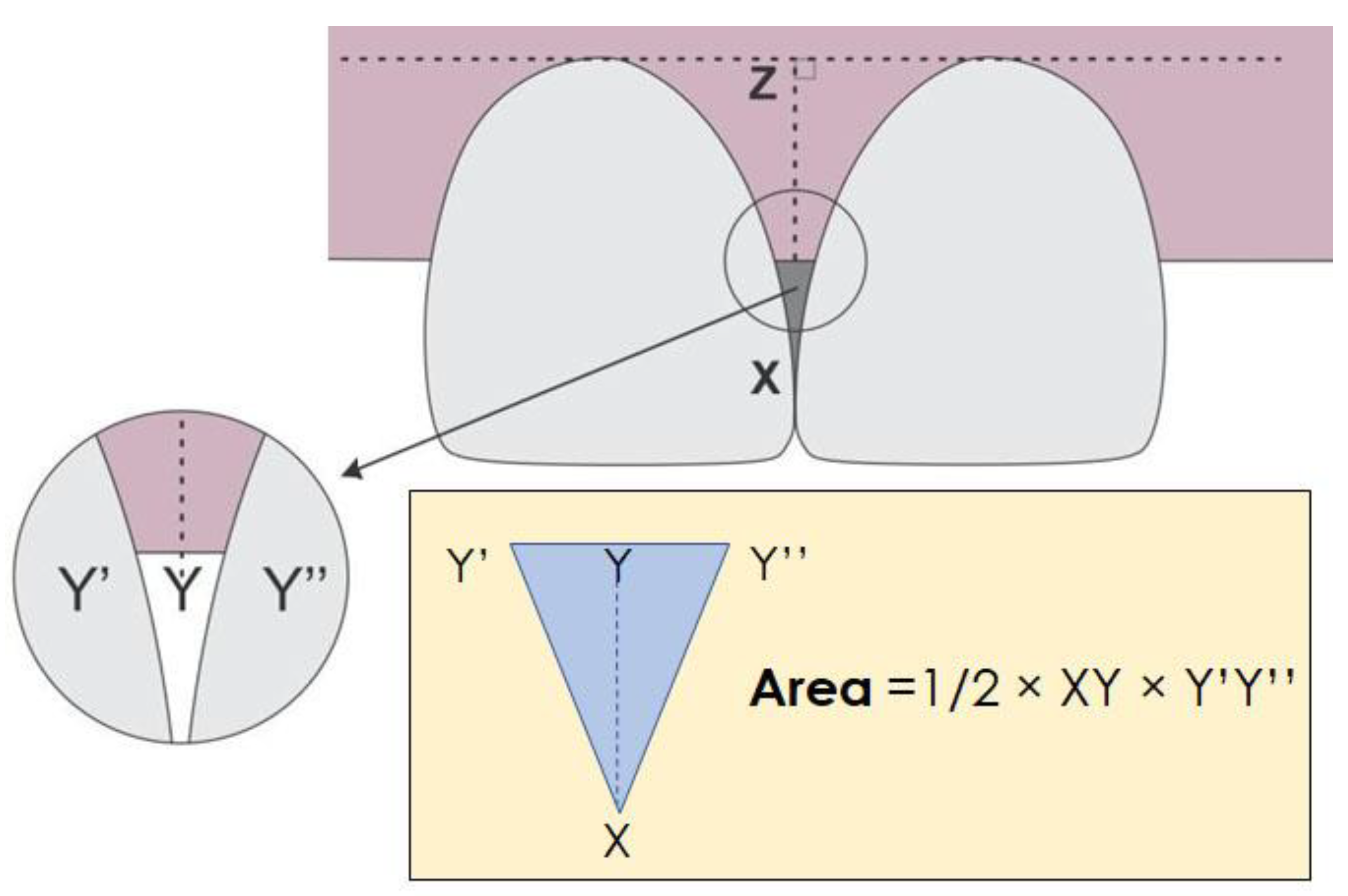
2.4. Outcome Variables
2.5. Statistical Analysis
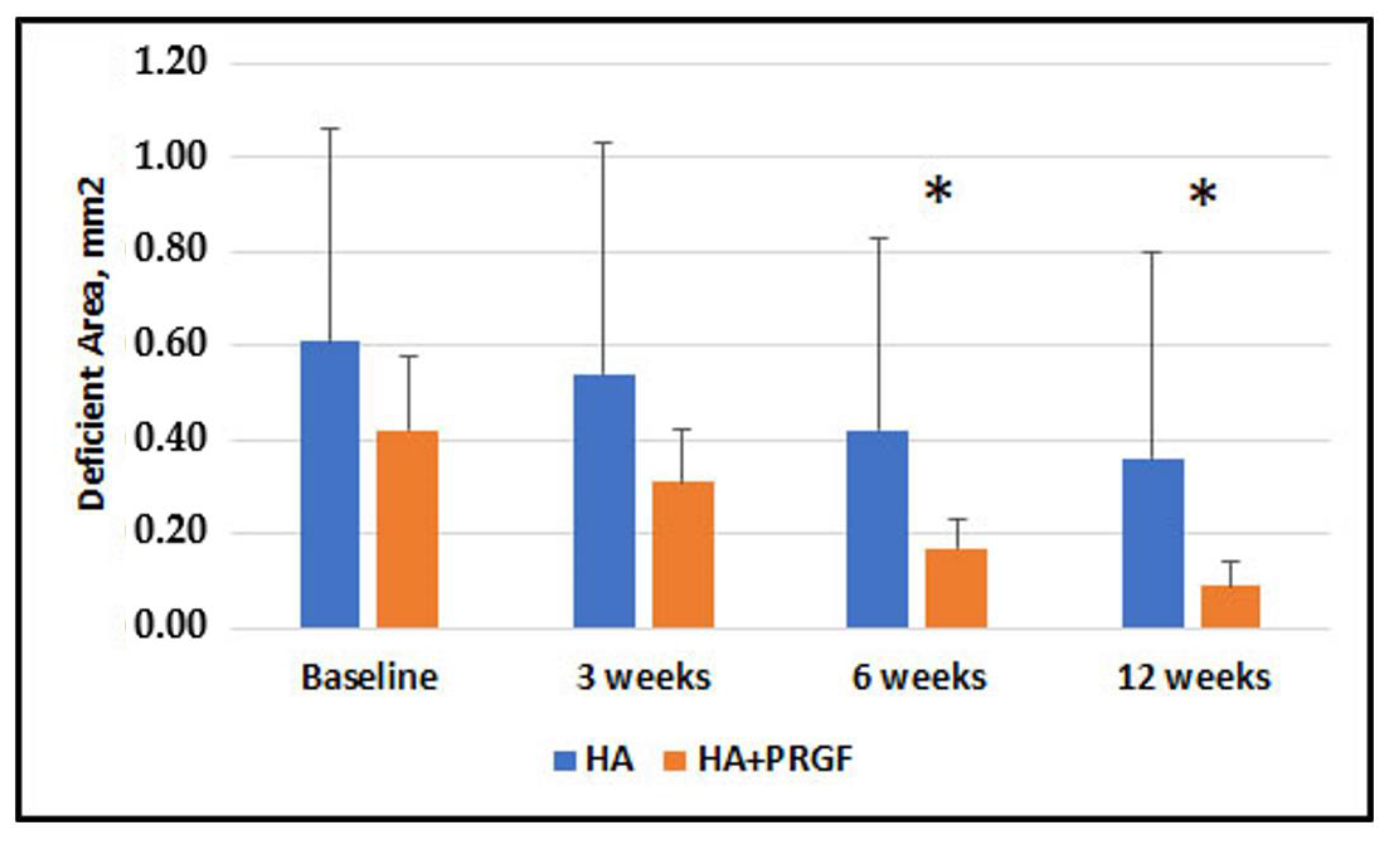
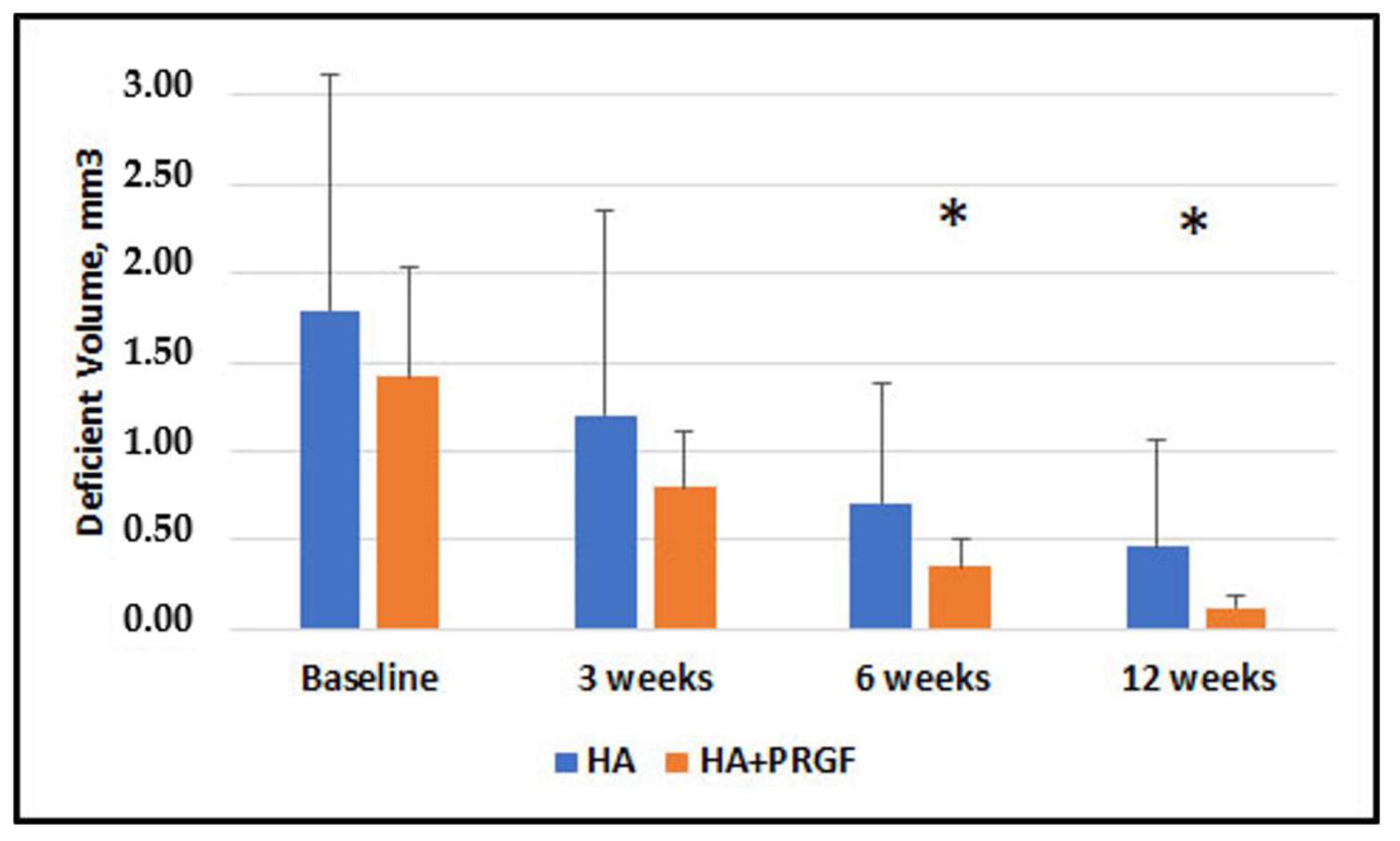
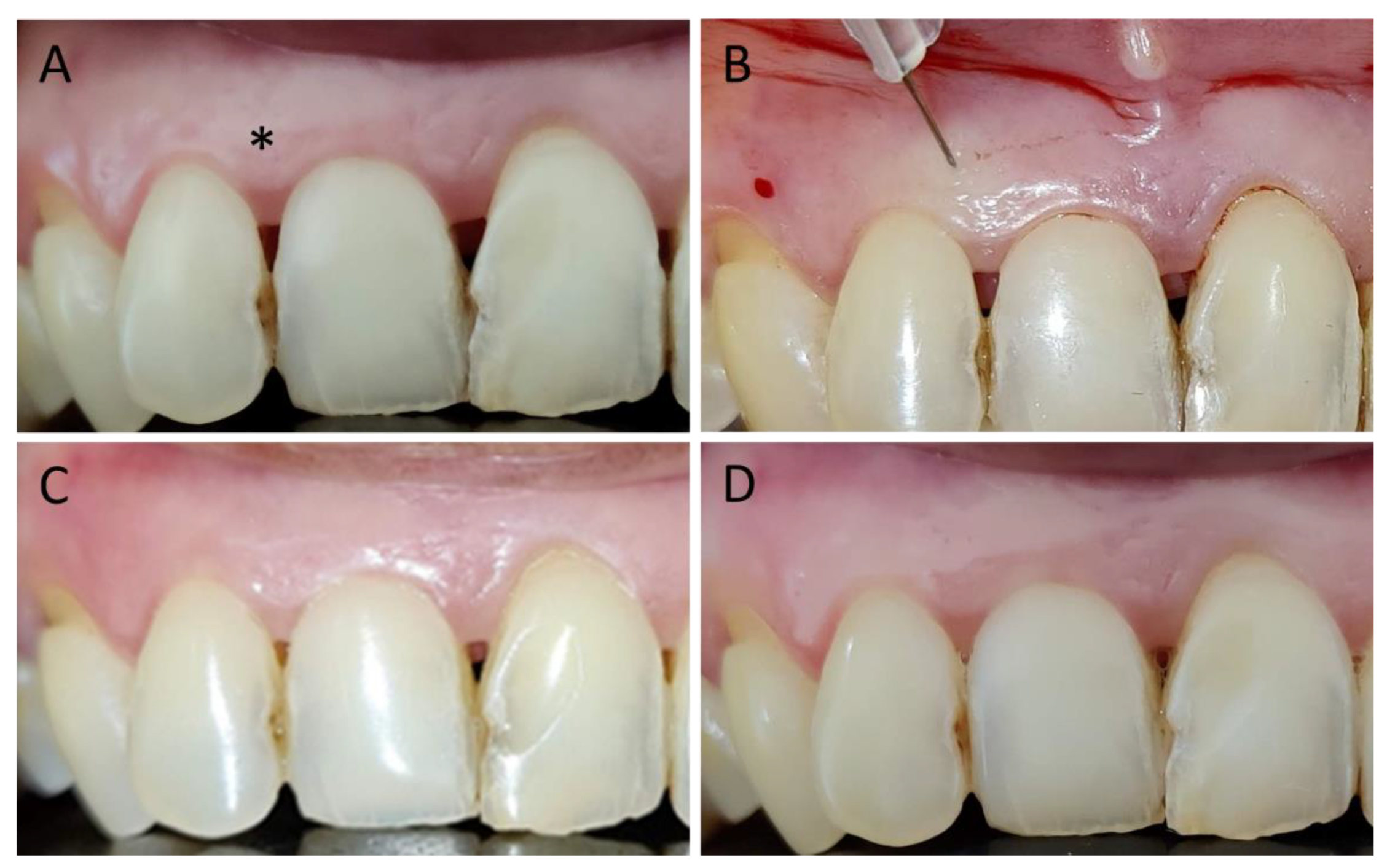
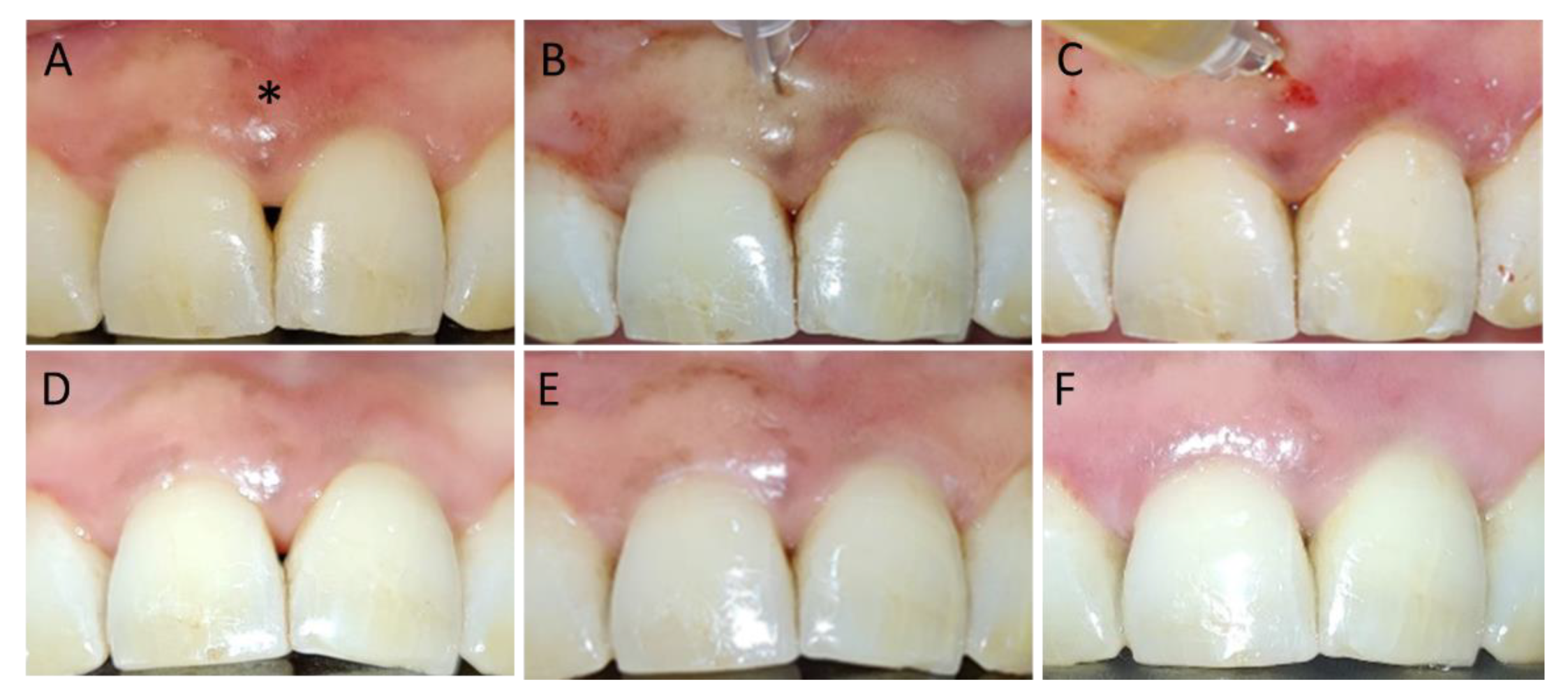
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, V.P.; Uppoor, A.S.; Nayak, D.G.; Shah, D. Black Triangle Dilemma and Its Management in Esthetic Dentistry. Dent. Res. J. 2013, 10, 296–301. [Google Scholar]
- Kokich, V.G. Excellence in Finishing: Modifications for the Perio-Restorative Patient. Semin. Orthod. 2003, 9, 184–203. [Google Scholar] [CrossRef]
- Ficho, A.C.; Souza Faloni, A.P.; Pennisi, P.R.C.; Borges, L.G.F.; Macedo Bernadino, Í.; Paranhos, L.R.; Queiroz, T.P.; Santos, P.L. Is Interdental Papilla Filling Using Hyaluronic Acid a Stable Approach to Treat Black Triangles? A Systematic Review. J. Esthet. Restor. Dent. 2021, 33, 458–465. [Google Scholar] [CrossRef]
- Sharma, A.A.; Park, J.H. Esthetic Considerations in Interdental Papilla: Remediation and Regeneration. J. Esthet. Restor. Dent. 2010, 22, 18–28. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Re, S. Interdental Papilla Augmentation Procedure Following Orthodontic Treatment in a Periodontal Patient. J. Periodontol. 2005, 76, 655–661. [Google Scholar] [CrossRef]
- Prato, G.P.P.; Rotundo, R.; Cortellini, P.; Tinti, C.; Azzi, R. Interdental Papilla Management: A Review and Classification of the Therapeutic Approaches. J. Prosthet. Dent. 2004, 92, 476. [Google Scholar] [CrossRef]
- Zetu, L.; Wang, H.-L. Management of Inter-Dental/Inter-Implant Papilla. J. Clin. Periodontol. 2005, 32, 831–839. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, J.D.; Storrer, C.M.; Sousa, A.M.; Lopes, T.R.; de Sousa Vieira, J.; Deliberador, T.M. Papillary Regeneration: Anatomical Aspects and Treatment Approaches. RSBO 2013, 9, 448–456. [Google Scholar] [CrossRef]
- Bacos, J.T.; Dayan, S.H. Superficial Dermal Fillers with Hyaluronic Acid. Facial Plast. Surg. FPS 2019, 35, 219–223. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic Acid: Perspectives in Dentistry. A Systematic Review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Ni, J.; Zhong, Z.; Wu, Y.; Shu, R.; Wu, Y.; Li, C. Hyaluronic Acid vs. Physiological Saline for Enlarging Deficient Gingival Papillae: A Randomized Controlled Clinical Trial and an in Vitro Study. Ann. Transl. Med. 2021, 9, 759. [Google Scholar] [CrossRef]
- Pitale, U.; Pal, P.; Thakare, G.; Verma, M.; Dhakad, S.; Pandey, R. Minimally Invasive Therapy for Reconstruction of Lost Interdental Papilla by Using Injectable Hyaluronic Acid Filler. J. Indian Soc. Periodontol. 2021, 25, 22. [Google Scholar] [CrossRef]
- Alhabashneh, R.; Alomari, S.; Khaleel, B.; Qinawi, H.; Alzaubi, M. Interdental Papilla Reconstruction Using Injectable Hyaluronic Acid: A 6 Month Prospective Longitudinal Clinical Study. J. Esthet. Restor. Dent. 2021, 33, 531–537. [Google Scholar] [CrossRef]
- Pi, S.; Choi, Y.J.; Hwang, S.; Lee, D.-W.; Yook, J.I.; Kim, K.-H.; Chung, C.J. Local Injection of Hyaluronic Acid Filler Improves Open Gingival Embrasure: Validation Through a Rat Model. J. Periodontol. 2017, 88, 1221–1230. [Google Scholar] [CrossRef]
- Bertl, K.; Gotfredsen, K.; Jensen, S.S.; Bruckmann, C.; Stavropoulos, A. Can Hyaluronan Injections Augment Deficient Papillae at Implant-Supported Crowns in the Anterior Maxilla? A Randomized Controlled Clinical Trial with 6 Months Follow-Up. Clin. Oral Implants Res. 2017, 28, 1054–1061. [Google Scholar] [CrossRef]
- Awartani, F.A.; Tatakis, D.N. Interdental Papilla Loss: Treatment by Hyaluronic Acid Gel Injection: A Case Series. Clin. Oral Investig. 2016, 20, 1775–1780. [Google Scholar] [CrossRef]
- Lee, W.-P.; Kim, H.-J.; Yu, S.-J.; Kim, B.-O. Six Month Clinical Evaluation of Interdental Papilla Reconstruction with Injectable Hyaluronic Acid Gel Using an Image Analysis System: Six Month Clinical Evaluation of Interdental Papilla Reconstruction. J. Esthet. Restor. Dent. 2016, 28, 221–230. [Google Scholar] [CrossRef]
- Becker, W.; Gabitov, I.; Stepanov, M.; Kois, J.; Smidt, A.; Becker, B.E. Minimally Invasive Treatment for Papillae Deficiencies in the Esthetic Zone: A Pilot Study. Clin. Implant Dent. Relat. Res. 2010, 12, 1–8. [Google Scholar] [CrossRef]
- Sadat Mansouri, S.; Ghasemi, M.; Salmani, Z.; Shams, N. Clinical Application of Hyaluronic Acid Gel for Reconstruction of Interdental Papilla at the Esthetic Zone. J. Iran. Dent. Assoc. 2013, 25, 208–213. [Google Scholar]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of PRP, PRF and CGF in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Ní Ríordáin, R. Autologous Platelet Concentrates in Oral Surgery: Protocols, Properties, and Clinical Applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Karanxha, L.; Panda, S.; Bucchi, C.; Nadathur Doraiswamy, J.; Sankari, M.; Ramamoorthi, S.; Varghese, S.; Taschieri, S. Autologous Platelet Concentrates for Treating Periodontal Infrabony Defects. Cochrane Database Syst. Rev. 2018, 11, CD011423. [Google Scholar] [CrossRef]
- Ozcan Bulut, S.; Ilhan, D.; Karabulut, E.; Caglayan, F.; Keceli, H.G. Efficacy of Platelet-Rich Fibrin and Connective Tissue Graft in Papilla Reconstruction. J. Esthet. Restor. Dent. 2022, 34, 1096–1104. [Google Scholar] [CrossRef]
- Puri, K.; Khatri, M.; Bansal, M.; Kumar, A.; Rehan, M.; Gupta, A. A Novel Injectable Platelet-Rich Fibrin Reinforced Papilla Reconstruction Technique. J. Indian Soc. Periodontol. 2022, 26, 412. [Google Scholar] [CrossRef]
- Panda, S.; Satpathy, A.; Chandra Das, A.; Kumar, M.; Mishra, L.; Gupta, S.; Srivastava, G.; Lukomska-Szymanska, M.; Taschieri, S.; Del Fabbro, M. Additive Effect of Platelet Rich Fibrin with Coronally Advanced Flap Procedure in Root Coverage of Miller’s Class I and II Recession Defects—A PRISMA Compliant Systematic Review and Meta-Analysis. Materials 2020, 13, 4314. [Google Scholar] [CrossRef]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The Use of Plasma Rich in Growth Factors (PRGF) in Guided Tissue Regeneration and Guided Bone Regeneration. A Review of Histological, Immunohistochemical, Histomorphometrical, Radiological and Clinical Results in Humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Panda, S.; Purkayastha, A.; Mohanty, R.; Nayak, R.; Satpathy, A.; Das, A.C.; Kumar, M.; Mohanty, G.; Panda, S.; Fabbro, M.D. Plasma Rich in Growth Factors (PRGF) in Non-Surgical Periodontal Therapy: A Randomized Clinical Trial. Braz. Oral Res. 2020, 34, e034. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous Platelets as a Source of Proteins for Healing and Tissue Regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Vandana, K.L. Use of Different Concentrations of Hyaluronic Acid in Interdental Papillary Deficiency Treatment: A Clinical Study. J. Indian Soc. Periodontol. 2019, 23, 35–41. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; De la Fuente, M.; Zalduendo, M.M.; Orive, G. Plasma Rich in Growth Factors (PRGF-Endoret) Stimulates Tendon and Synovial Fibroblasts Migration and Improves the Biological Properties of Hyaluronic Acid. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1657–1665. [Google Scholar] [CrossRef]
- Godfrey, L.; Martínez-Escribano, J.; Roo, E.; Pino, A.; Anitua, E. Plasma Rich in Growth Factor Gel as an Autologous Filler for Facial Volume Restoration. J. Cosmet. Dermatol. 2020, 19, 2552–2559. [Google Scholar] [CrossRef]
- Lee, W.-P.; Seo, Y.-S.; Kim, H.-J.; Yu, S.-J.; Kim, B.-O. The Association between Radiographic Embrasure Morphology and Interdental Papilla Reconstruction Using Injectable Hyaluronic Acid Gel. J. Periodontal Implant Sci. 2016, 46, 277. [Google Scholar] [CrossRef] [Green Version]
- Chaulkar, P.; Mali, R.; Mali, A.; Lele, P.; Patil, P. A Comparative Evaluation of Papillary Reconstruction by Modified Beagle’s Technique with the Beagle’s Surgical Technique: A Clinical and Radiographic Study. J. Indian Soc. Periodontol. 2017, 21, 218. [Google Scholar] [CrossRef]
- Nordland, W.P.; Sandhu, H.S. Microsurgical Technique for Augmentation of the Interdental Papilla: Three Case Reports. Int. J. Periodontics Restorative Dent. 2008, 28, 543–549. [Google Scholar]
- Nordland, W.P.; Tarnow, D.P. A Classification System for Loss of Papillary Height. J. Periodontol. 1998, 69, 1124–1126. [Google Scholar] [CrossRef] [Green Version]
- Abdelraouf, S.A.; Dahab, O.A.; Elbarbary, A.; El-Din, A.M.; Zaki, B.M. Assessment of Hyaluronic Acid Gel Injection in the Reconstruction of Interdental Papilla: A Randomized Clinical Trial. Open Access Maced. J. Med. Sci. 2019, 7, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Karanxha, L.; Goker, F.; Satpathy, A.; Taschieri, S.; Francetti, L.; Das, A.C.; Kumar, M.; Panda, S.; Fabbro, M.D. Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 1347. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Sankari, M.; Satpathy, A.; Jayakumar, D.; Mozzati, M.; Mortellaro, C.; Gallesio, G.; Taschieri, S.; Del Fabbro, M. Adjunctive Effect of Autologus Platelet-Rich Fibrin to Barrier Membrane in the Treatment of Periodontal Intrabony Defects. J. Craniofac. Surg. 2016, 27, 691–696. [Google Scholar] [CrossRef]
- Testori, T.; Panda, S.; Clauser, T.; Scaini, R.; Zuffetti, F.; Capelli, M.; Taschieri, S.; Mortellaro, C.; Del Fabbro, M. Short Implants and Platelet-Rich Fibrin for Transcrestal Sinus Floor Elevation: A Prospective Multicenter Clinical Study. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. S2), 121–135. [Google Scholar]
- Del Fabbro, M.; Panda, S.; Taschieri, S. Adjunctive Use of Plasma Rich in Growth Factors for Improving Alveolar Socket Healing: A Systematic Review. J. Evid. Based Dent. Pract. 2019, 19, 166–176. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Lolato, A.; Panda, S.; Corbella, S.; Satpathy, A.; Das, A.C.; Kumar, M.; Taschieri, S. Methodological Quality Assessment of Systematic Reviews on Autologous Platelet Concentrates for the Treatment of Periodontal Defects. J. Evid. Based Dent. Pract. 2017, 17, 239–255. [Google Scholar] [CrossRef]
| Patient Characteristics | HA Group | HA + PRGF Group |
|---|---|---|
| No. of sites/patients | 18/10 | 18/11 |
| Age, years | 34.63 ± 5.22 | 38.71 ± 8.4 |
| Probing Depth, mm | 2.82 ± 1.1 | 2.54 + 0.71 |
| Clinical Attachment Level, mm | 3.24 ± 0.91 | 2.92 ± 0.97 |
| Gingival Recession, mm | 0.7 ± 0.2 | 0.8 ± 0.3 |
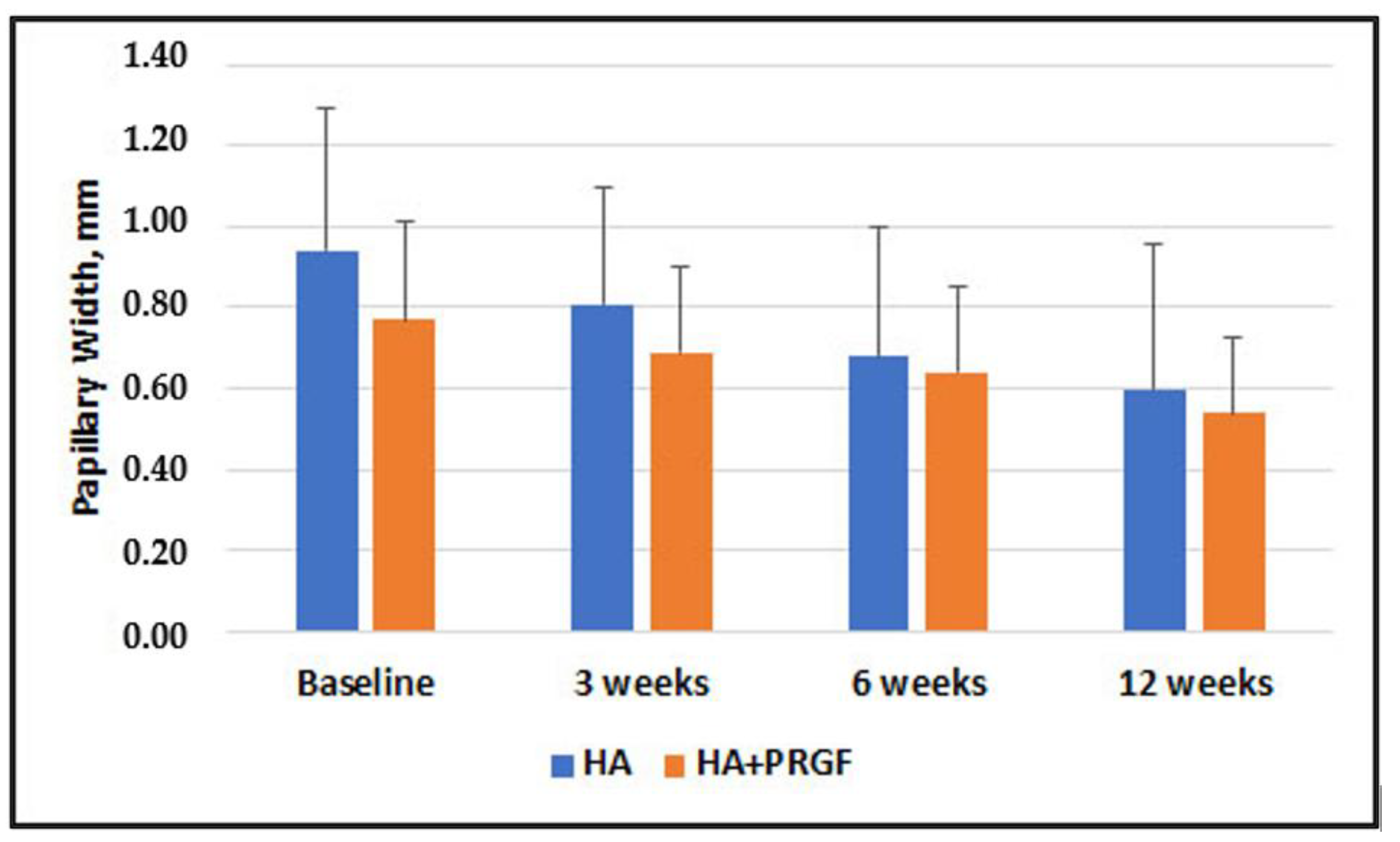
| Papillary Width, mm | 0.94 ± 0.35 | 0.77 ± 0.24 |
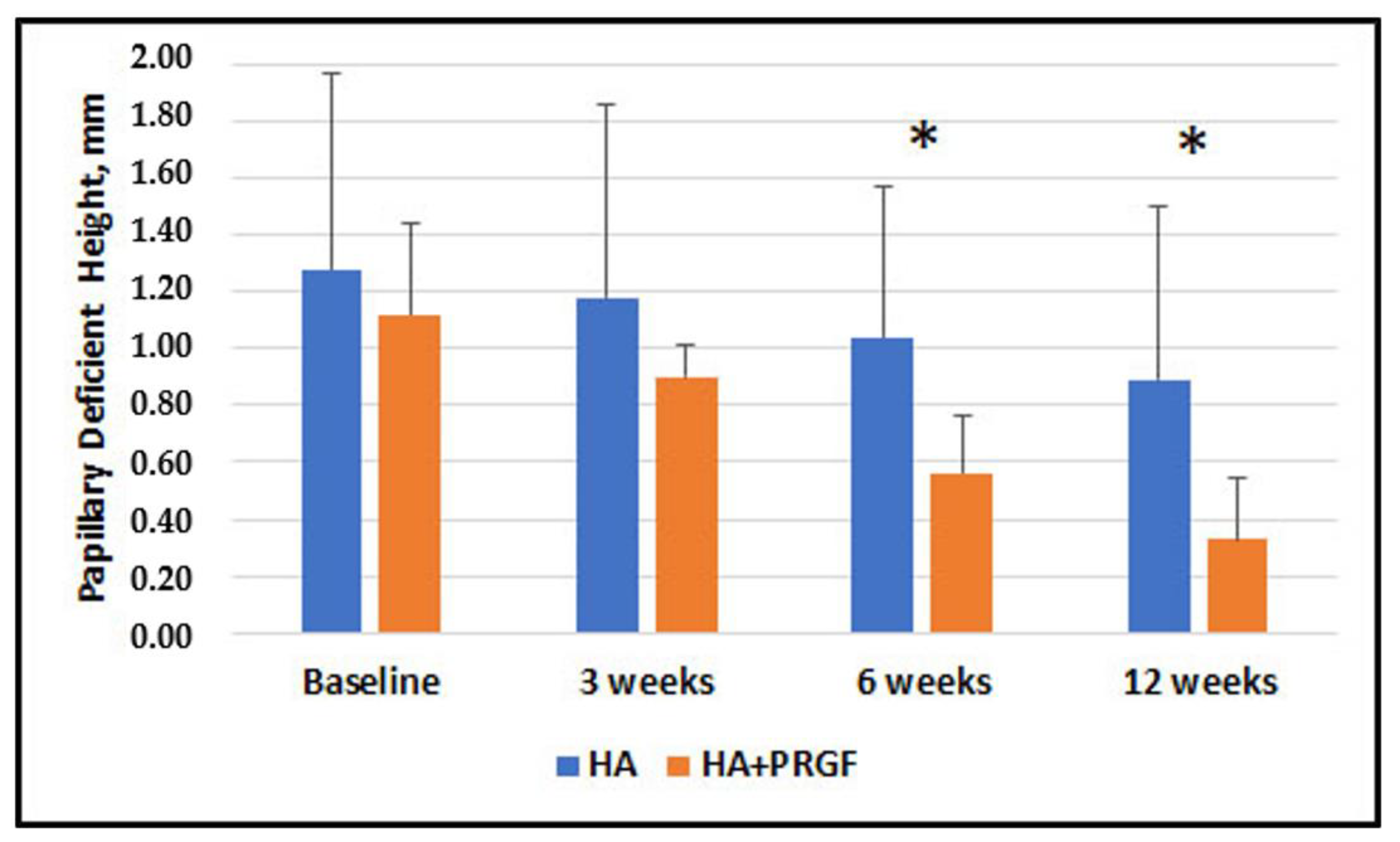
| Papillary Deficient Height, mm | 1.28 ± 0.69 | 1.12 ± 0.32 |
| Deficient Area, mm2 | 0.61 + 0.45 | 0.42 + 0.16 |
| Deficient Volume, mm3 | 1.79 ± 1.32 | 1.42 ± 0.62 |
| Contact point to Bone Crest, mm | 5.26 ± 1.2 | 5.92 ± 0.72 |
| Baseline | 3 Weeks | 6 Weeks | 12 Weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference (SE) | 95% CI of the Difference | p-Value | Mean Difference (SE) | 95% CI of the Difference | p-Value | Mean Difference (SE) | 95% CI of the Difference | p-Value | Mean Difference (SE) | 95% CI of the Difference | p-Value | |
| Papillary Width, mm | 0.18 (0.10) | −0.02, 0.38 | 0.08 | 0.12 (0.08) | −0.05, 0.29 | 0.16 | 0.04 (0.09) | −0.14, 0.23 | 0.63 | 0.05 (0.10) | −0.15, 0.25 | 0.59 |
| Papillary Deficient Height, mm | 0.16 (0.18) | −0.20, 0.53 | 0.37 | 0.28 (0.16) | −0.05, 0.61 | 0.09 | 0.49 (0.13) | 0.22, 0.76 | 0.001 * | 0.56 (0.16) | 0.24, 0.88 | 0.001 * |
| Deficient Area, mm2 | 0.19 (0.11) | −0.04, 0.42 | 0.11 | 0.22 (0.12) | −0.02, 0.47 | 0.07 | 0.24 (0.10) | 0.04, 0.44 | 0.019 * | 0.27 (0.11) | 0.05, 0.49 | 0.017 * |
| Deficient Volume, mm3 | 0.37 (0.34) | −0.33, 1.07 | 0.29 | 0.41 (0.28) | −0.16, 0.98 | 0.16 | 0.36 (0.16) | 0.03, 0.69 | 0.032 * | 0.33 (0.15) | 0.03, 0.64 | 0.032 * |
| Time and Group | N | Mean, % | Std. Deviation, % | p-Value | |
|---|---|---|---|---|---|
| 3 Weeks | HA | 18 | 12.07 | 8.96 | 0.340 |
| HA + PRGF | 18 | 15.94 | 14.41 | ||
| 6 Weeks | HA | 18 | 20.24 | 10.80 | 0.000 |
| HA + PRGF | 18 | 49.30 | 18.01 | ||
| 12 Weeks | HA | 17 | 57.62 | 21.78 | 0.006 |
| HA + PRGF | 17 | 77.42 | 16.70 | ||
| Time and Group | N | Mean, % | Std. Deviation, % | p-Value | |
|---|---|---|---|---|---|
| 3 Weeks | HA | 18 | 23.60 | 15.87 | 0.000 |
| HA + PRGF | 18 | 42.28 | 11.91 | ||
| 6 Weeks | HA | 18 | 56.03 | 20.55 | 0.006 |
| HA + PRGF | 18 | 73.14 | 13.83 | ||
| 12 Weeks | HA | 17 | 81.42 | 17.20 | 0.033 |
| HA + PRGF | 17 | 91.19 | 5.70 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bal, A.; Panda, S.; Mohanty, R.; Satpathy, A.; Nayak, R.; Tumedei, M.; Argenta, F.; Colapinto, G.; Del Fabbro, M.; Annunziata, M. Effectiveness of Hyaluronic Acid Gel Injection with and without PRGF for Management of Interdental Papillary Loss: A Randomized Clinical Trial. J. Funct. Biomater. 2023, 14, 114. https://doi.org/10.3390/jfb14020114
Bal A, Panda S, Mohanty R, Satpathy A, Nayak R, Tumedei M, Argenta F, Colapinto G, Del Fabbro M, Annunziata M. Effectiveness of Hyaluronic Acid Gel Injection with and without PRGF for Management of Interdental Papillary Loss: A Randomized Clinical Trial. Journal of Functional Biomaterials. 2023; 14(2):114. https://doi.org/10.3390/jfb14020114
Chicago/Turabian StyleBal, Aishwarya, Saurav Panda, Rinkee Mohanty, Anurag Satpathy, Rashmita Nayak, Margherita Tumedei, Francesca Argenta, Gianluca Colapinto, Massimo Del Fabbro, and Marco Annunziata. 2023. "Effectiveness of Hyaluronic Acid Gel Injection with and without PRGF for Management of Interdental Papillary Loss: A Randomized Clinical Trial" Journal of Functional Biomaterials 14, no. 2: 114. https://doi.org/10.3390/jfb14020114
APA StyleBal, A., Panda, S., Mohanty, R., Satpathy, A., Nayak, R., Tumedei, M., Argenta, F., Colapinto, G., Del Fabbro, M., & Annunziata, M. (2023). Effectiveness of Hyaluronic Acid Gel Injection with and without PRGF for Management of Interdental Papillary Loss: A Randomized Clinical Trial. Journal of Functional Biomaterials, 14(2), 114. https://doi.org/10.3390/jfb14020114










