Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dental Implants
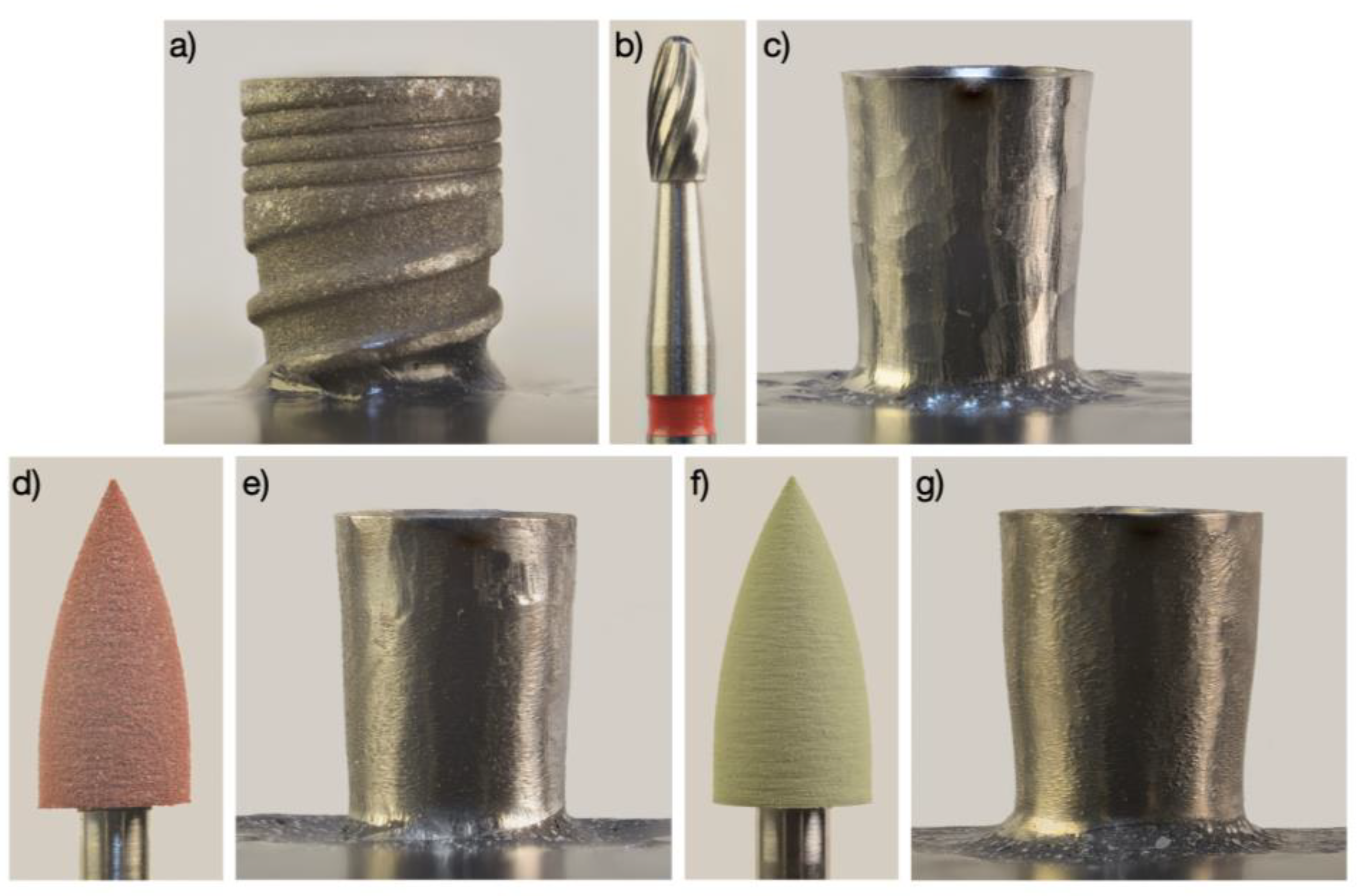
2.2. Implantoplasty Procedure
2.3. Surface Topography Analyses
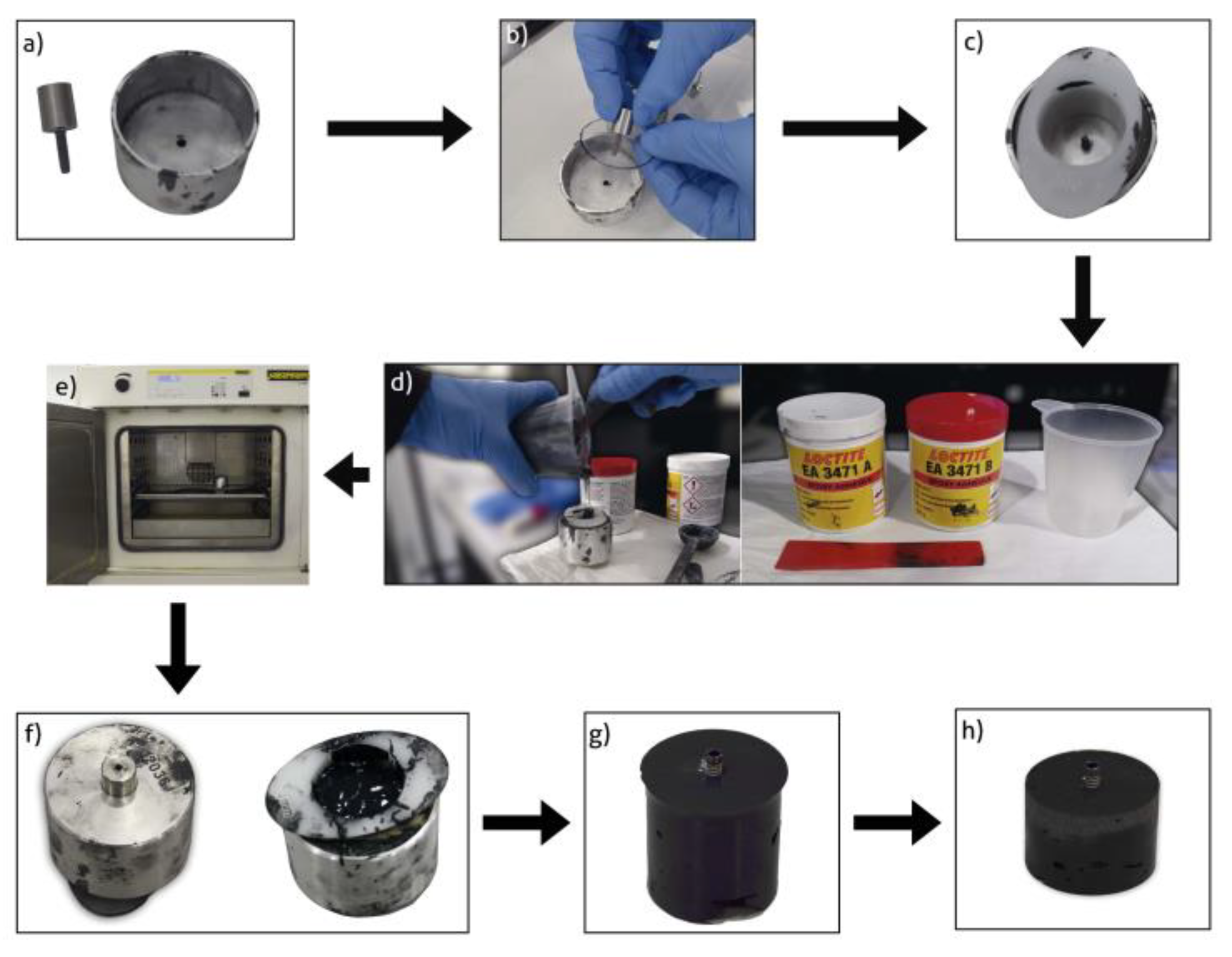
2.4. Cast Preparation
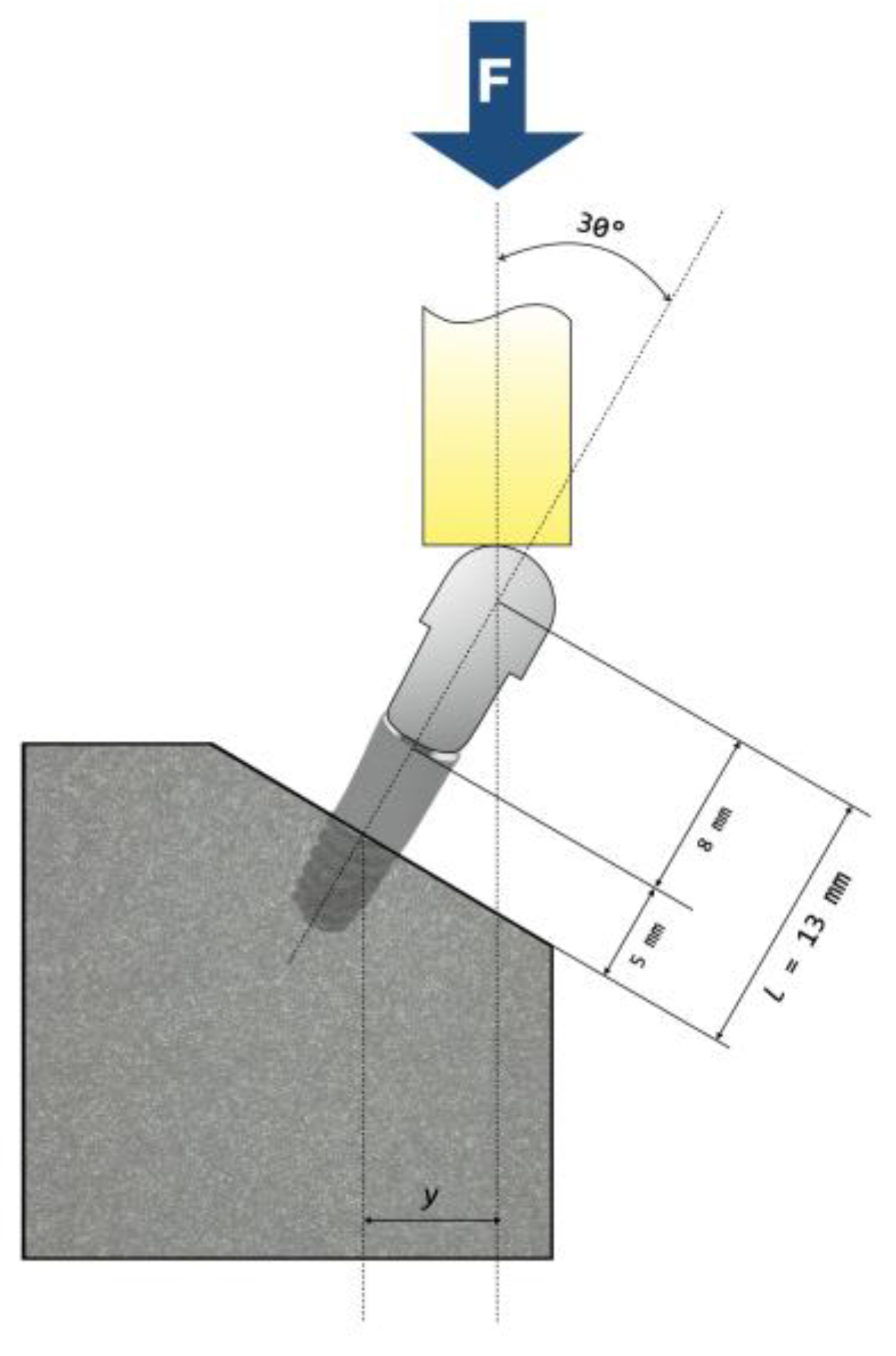
2.5. Fatigue Testing
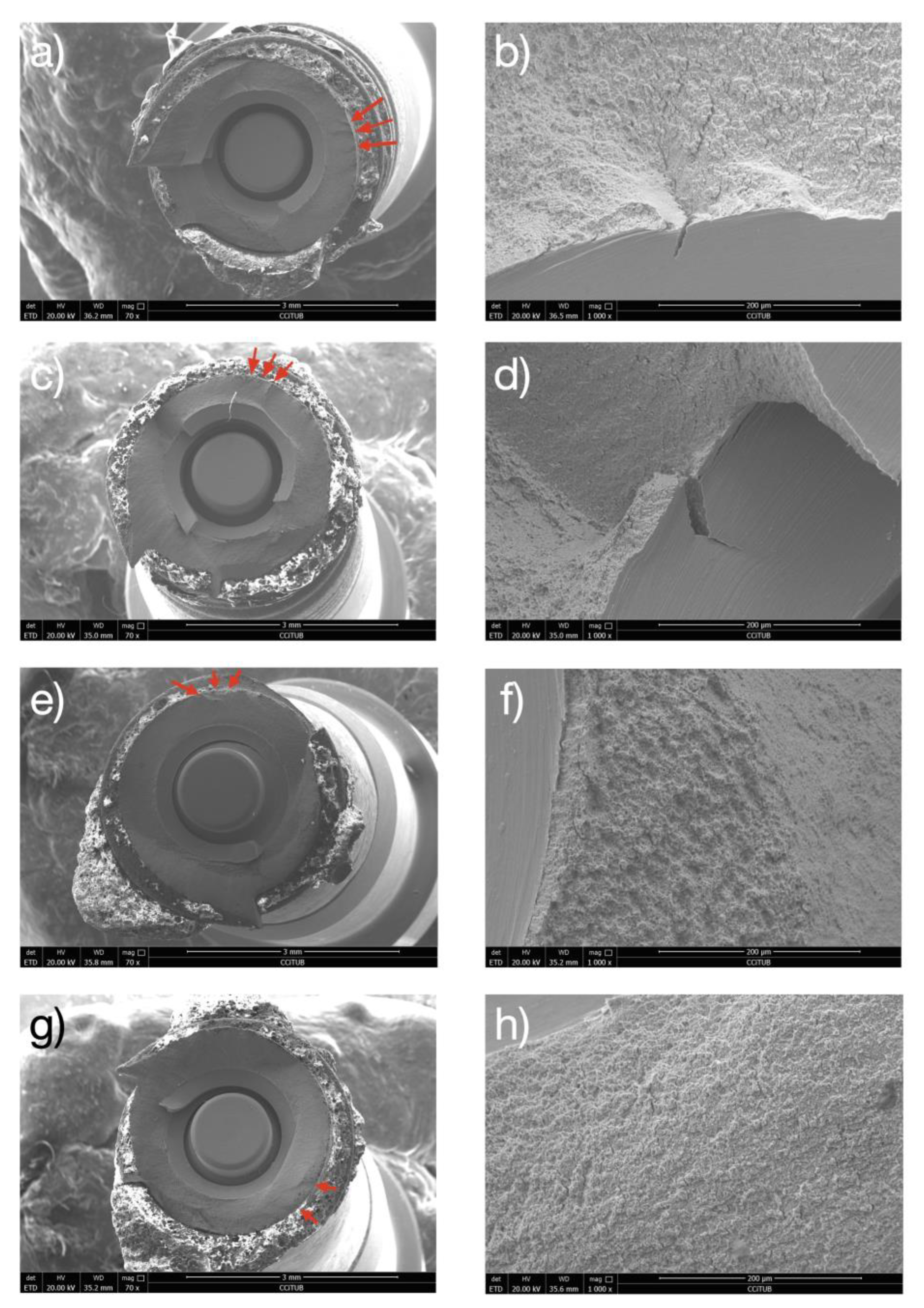
2.6. Fractographic Analysis
2.7. Corrosion Tests
2.8. Statistical Analysis
3. Results
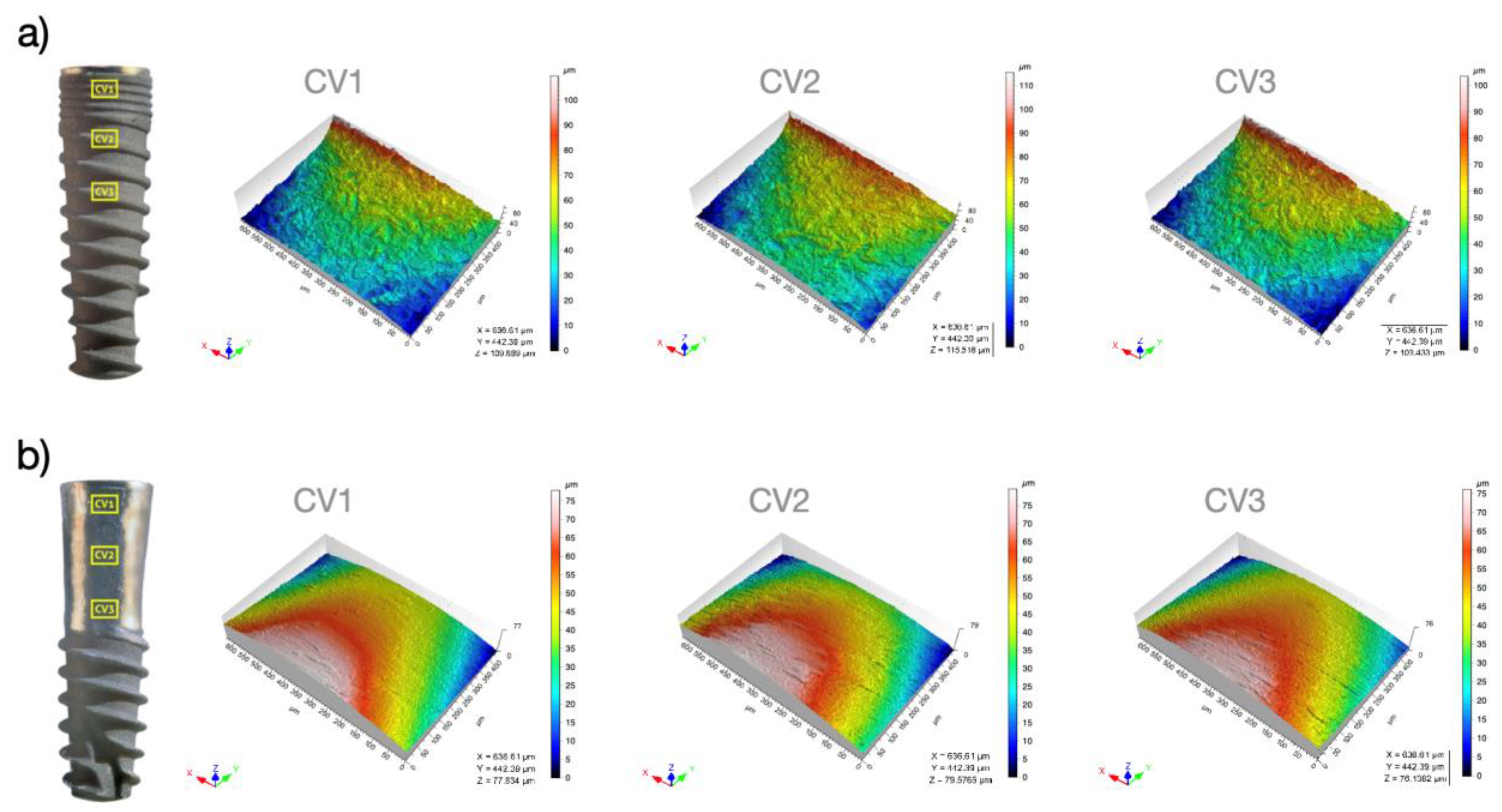
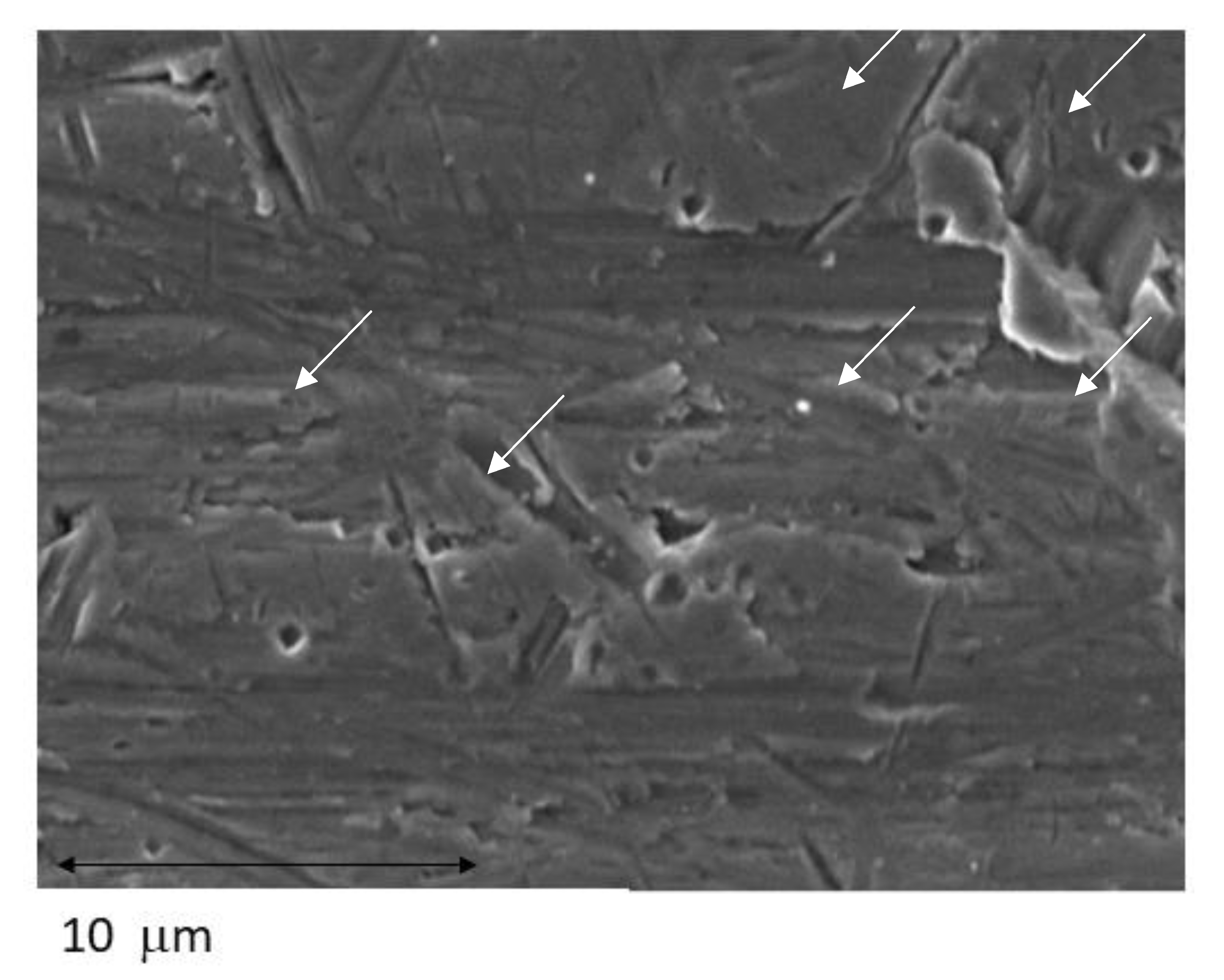
3.1. Surface Topography Analyses
3.2. Fatigue Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012, 23, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 89, 313–318. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Periodontol. 2018, 89, 267–290. [Google Scholar] [CrossRef]
- Persson, G.R.; Roos-Jansåker, A.-M.; Lindahl, C.; Renvert, S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2011, 82, 1267–1278. [Google Scholar] [CrossRef]
- Renvert, S.; Lessem, J.; Dahlén, G.; Lindahl, C.; Svensson, M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J. Clin. Periodontol. 2006, 33, 362–369. [Google Scholar] [CrossRef]
- Persson, G.R.; Samuelsson, E.; Lindahl, C.; Renvert, S. Mechanical non-surgical treatment of peri-implantitis: A single-blinded randomized longitudinal clinical study. II. Microbiological results. J. Clin. Periodontol. 2010, 37, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Nart, J.; Pons, R.; Valles, C.; Esmatges, A.; Sanz-Martín, I.; Monje, A. Non-surgical therapeutic outcomes of peri-implantitis: 12-month results. Clin. Oral Investig. 2020, 24, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. How do implant surface characteristics influence peri-implant disease? J. Clin. Periodontol. 2011, 38, 214–222. [Google Scholar] [CrossRef]
- Figuero, E.; Graziani, F.F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Becker, J. The influence of implantoplasty on the diameter, chemical surface composition, and biocompatibility of titanium implants. Clin. Oral Investig. 2017, 21, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Ghisolfi, M.; Murgolo, N.; Chiapasco, M.; Lops, D.; Vogel, G. Therapy of peri-implantitis with resective surgery. Clin. Oral Implants Res. 2004, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Chan, H.L.; Oh, W.S.; Ong, H.S.; Fu, J.H.; Marius, S.; Marianella, S.; Wang, H.L. Impact of implantoplasty on strength of the implant-abutment complex. Int. J. Oral Maxillofac. Implants 2013, 28, 1530–1535. [Google Scholar] [CrossRef]
- Gehrke, S.; Junior, J.; Dedavid, B.; Shibli, J. Analysis of implant strength after implantoplasty in three implant-abutment connection designs: An in vitro study. Int. J. Oral Maxillofac. Implants 2016, 31, 65–70. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Dal Piva AM de, O.; Shibli, J.A.; Borges, A.L.S.; Tango, R.N. Influence of implantoplasty on stress distribution of exposed implants at different bone insertion levels. Braz. Oral Res. 2017, 31, 96. [Google Scholar] [CrossRef] [Green Version]
- Bertl, K.; Isidor, F.; von Steyern, P.V.; Stavropoulos, A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin. Oral Investig. 2021, 25, 2203–2211. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implants Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure tianium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellon, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosión behavior of Ti6Al4V particles obtained by Implatoplasty. An in vivo study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef] [PubMed]
- Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; Technical Report no. ASTM G5-14e1; ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- ASTM G-102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Slaney, A.M.; Wright, V.A.; Meloncelli, P.J.; Harris, K.D.; West, L.J.; Lowary, T.L.; Buriak, J.M. Biocompatible carbohydrate-functionalized stainless steel surfaces: A new method for passivating biomedical implants. ACS Appl. Mater. Interfaces 2011, 3, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Kilpadi, D.V.; Weimer, J.J.; Lemons, J.E. Effect of passivation and dry heat-sterilization on surface energy and topography of unalloyed titanium implants. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 89–101. [Google Scholar] [CrossRef]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Valderrama, P.; Wilson, T.; Palmer, K.; Thomas, A.; Sridhar, S.; Sadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [Green Version]
- Steinebrunner, L.; Wolfart, S.; Ludwig, K.; Kern, M. Implant-abutment interface design affects fatigue and fracture strength of implants. Clin. Oral Implants Res. 2008, 19, 1276–1284. [Google Scholar] [CrossRef]
- Richter, E.J. In vivo vertical forces on implants. Int. J. Oral Maxillofac. Implant. 1995, 10, 99–108. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Ramel, C.F.; Lüssi, A.; Özcan, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef]
- Burgueño-Barris, G.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. The influence of implantoplasty on surface roughness, biofilm formation, and biocompatibility of titanium implants: A systematic review. Int. J. Oral Maxillofac. Implants 2021, 36, 111–119. [Google Scholar] [CrossRef]
- Beheshti Maal, M.; Aanerød Ellingsen, S.; Reseland, J.E.; Verket, A. Experimental implantoplasty outcomes correlate with fibroblast growth in vitro. BMC Oral Health 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toma, S.; Lasserre, J.; Brecx, M.C.; Nyssen-Behets, C. In vitro evaluation of peri-implantitis treatment modalities on Saos-2osteoblasts. Clin. Oral Implants Res. 2016, 27, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Toma, S.; Behets, C.; Brecx, M.C.; Lasserre, J.F. In vitro comparison of the efficacy of peri-implantitis treatments on the removal and recolonization of streptococcus gordonii biofilm on titanium disks. Materials 2018, 11, 2484. [Google Scholar] [CrossRef] [Green Version]
- Geremias, T.C.; Montero, J.F.D.; Magini, R.S.; Filho, G.S.; Magalhães, E.B.; Bianchini, M.A. Biofilm analysis of retrieved dental implants after different peri-implantitis treatments. Case Rep. Dent. 2017, 2017, 8562050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Chaar, E.; Mohammad, A.; Kefaia, A.; Abdulla, A.; Stephanie, C. Decontamination of the infected implant surface: A scanning electron microscope study. Int. J. Periodontics Restor. Dent. 2020, 40, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ravidà, A.; Siqueira, R.; Saleh, I.; Saleh, M.H.A.; Giannobile, A.; Wang, H.L. Lack of clinical benefit of implantoplasty to improve implant survival rate. J. Dent. Res. 2020, 99, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol. 2021, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Camps-Font, O.; González-Barnadas, A.; Mir-Mari, J.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Fracture resistance after implantoplasty in three implant-abutment connection designs. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e691–e699. [Google Scholar] [CrossRef]
- Leitão-Almeida, B.; Font, O.C.; Correia, A.; Mari, J.M.; Figueiredo, R.; Castellón, E.V. Effect of crown to implant ratio and implantoplasty on the fracture resistance of narrow dental implants with marginal bone loss: An in vitro study. BMC Oral Health 2020, 20, 329. [Google Scholar] [CrossRef]
- Leitão-Almeida, B.; Camps-Font, O.; Correia, A.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of bone loss on the fracture resistance of narrow dental implants after implantoplasty. An in vitro study. Med. Oral Patol. Oral Cir. Bucal 2021, 26, 611–618. [Google Scholar] [CrossRef]
- Jorio, I.C.; Stawarczyk, B.; Attin, T.; Schmidlin, P.R.; Sahrmann, P. Reduced fracture load of dental implants after implantoplasty with different instrumentation sequences. An in vitro study. Clin. Oral Implants Res. 2021, 32, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Brunello, G.; Michelon, F.; Concheri, G.; Graiff, L.; Meneghello, R. Implantoplasty: Carbide burs vs. diamond sonic tips. An in vitro study. Clin. Oral Implants Res. 2021, 32, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.J. Titanium alloys for dental implants: A review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Gil, F.J.; Herrero-Climent, M.; Lázaro, P.; Rios, J.V. Implant-abutment connections: Influence of the design on the microgap and their fatigue and fracture behavior of dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Won, S.Y.; Bae, T.S.; Song, K.Y.; Park, C.W.; Eom, T.G.; Jeong, C.M. Fatigue characteristics of five types of implant-abutment joint designs. Met. Mater. Int. 2008, 14, 133–138. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E.; Levin, L. Effect of dental implant diameter on fatigue performance. Part II: Failure analysis. Clin. Implant Dent. Relat. Res. 2014, 16, 178–184. [Google Scholar] [CrossRef]
- Diéguez-Pereira, M.; Chávarri-Prado, D.; Viteri-Agustín, I.; Montalban-Vadillo, O.; Pérez-Pevida, E.; Brizuela-Velasco, A. Effect of implantoplasty on the elastic limit of dental implants of different diameters. Int. J. Implant Dent. 2021, 7, 88. [Google Scholar] [CrossRef]
- da Rocha Ferreira, J.J.; Machado, L.F.M.; Oliveira, J.M.; Ramos, J.C.T. Effect of crown-to-implant ratio and crown height space on marginal bone stress: A finite element analysis. Int. J. Implant Dent. 2021, 7, 81. [Google Scholar] [CrossRef]
- Lee, C.K.; Karl, M.; Kelly, J.R. Evaluation of test protocol variables for dental implant fatigue research. Dent. Mater. 2009, 25, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Shemtov-Yona, K.; Rittel, D.; Levin, L.; Machtei, E.E. Effect of dental implant diameter on fatigue performance. Part I: Mechanical behavior. Clin. Implant Dent. Relat. Res. 2014, 16, 172–177. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. Fatigue of dental implants: Facts and fallacies. Dent. J. 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemtov-Yona, K.; Rittel, D. Identification of failure mechanisms in retrieved fractured dental implants. Eng. Fail. Anal. 2014, 38, 58–65. [Google Scholar] [CrossRef]
- Shah, S.D. Implant Strength after Implantoplasty. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2019. [Google Scholar]
- Chang, C.-L.; Lu, H.-K.; Ou, K.-L.; Su, P.-Y.; Chen, C.-M. Fractographic analysis of fractured dental implant components. J. Dent. Sci. 2013, 8, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Rzhepishevska, O.; Hakobyana, S.; Ruhala, R.; Gautrotb, J.; Barberoc, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Biomater. Sci. 2013, 1, 589–602. [Google Scholar] [CrossRef] [Green Version]
- Terada, A.; Okuyama, K.; Nishikawa, M.; Tsuneda, S.; Hosomi, M. The effect of surface charge property on Escherichia coli initial adhesion and subsequent biofilm formation. Biotechnol. Bioeng. 2012, 109, 1745–1754. [Google Scholar] [CrossRef]
- Schubert, A.; Wassmann, T.; Holtappels, M.; Kurbad, O.; Krohn, S.; Bürgers, R. Predictability of microbial adhesion to dental materials by roughness parameters. Coatings 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Bollen, C.M.; Papaioanno, W.; Eldere, J.V.; Schepers, E.; Quirynen, M.; Steenberghe, D.V. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin. Oral Implants Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implants 1996, 11, 169–178. [Google Scholar]
- Serino, G.; Turri, A.; Lang, N.P. Maintenance therapy in patients following the surgical treatment of peri-implantitis: A 5-year follow-up study. Clin. Oral Implants Res. 2015, 26, 950–956. [Google Scholar] [CrossRef]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion Behavior of Titanium Dental Implants with Implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed]



| Sa (µm) | Sz (µm) | Ssk | Sdr (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Control | IP | Control | IP | Control | IP | Control | IP |
| 1 | 1.88 | 0.47 | 20.86 | 13.34 | 0.29 | −1.02 | 18.95 | 2.62 |
| 2 | 1.84 | 0.49 | 19.60 | 6.20 | −0.04 | −0.46 | 18.97 | 2.57 |
| 3 | 1.82 | 0.58 | 30.64 | 7.66 | 0.31 | −0.86 | 18.73 | 2.61 |
| 4 | 1.78 | 0.44 | 23.84 | 7.32 | −0.03 | −0.78 | 18.93 | 2.42 |
| 5 | 1.73 | 0.65 | 21.11 | 15.76 | −0.07 | −0.70 | 17.67 | 5.01 |
| 6 | 1.87 | 0.50 | 33.41 | 10.47 | 0.64 | −0.99 | 20.96 | 3.45 |
| 7 | 1.72 | 0.33 | 20.15 | 5.04 | −0.05 | −1.39 | 16.29 | 1.19 |
| 8 | 1.71 | 0.73 | 19.21 | 8.72 | 0.05 | −0.14 | 16.69 | 3.84 |
| 9 | 1.74 | 0.61 | 19.78 | 9.09 | −0.14 | −0.71 | 16.35 | 2.95 |
| 10 | 1.72 | 0.34 | 27.92 | 6.31 | 0.05 | 0.18 | 16.89 | 2.75 |
| Median | 1.76 | 0.49 | 20.98 | 8.19 | 0.01 | −0.74 | 18.20 | 2.67 |
| IQR | 0.11 | 0.16 | 8.14 | 4.16 | 0.34 | 0.53 | 2.26 | 0.87 |
| p-value | <0.001 * | <0.001 * | 0.001 * | <0.001 * | ||||
| Chemical Product | Composition (mM) |
|---|---|
| K2HPO4 | 0.44 |
| KCl | 5.4 |
| CaCl2 | 1.3 |
| Na2HPO4 | 0.25 |
| NaCl | 137 |
| NaHCO3 | 4.2 |
| MgSO4 | 1.0 |
| C6H12O6 | 5.5 |
| % Fmax Total | Peak Load (N) | Number of Cycles | Failure | |
|---|---|---|---|---|
| Location | Description | |||
| Implantoplasty group (n = 10) | ||||
| 95% | 628 | 5,000,000 | Absence of failure | |
| 95% | 628 | 5,000,000 | Absence of failure | |
| 95% | 628 | 102,360 | Implant body | Fracture |
| 90% | 595 | 279,251 | Implant body | Fracture |
| 90% | 595 | 5,000,000 | Absence of failure | |
| 85% | 562 | 318,799 | Implant body | Fracture |
| 85% | 562 | 5,000,000 | Absence of failure | |
| 80% | 529 | 5,000,000 | Absence of failure | |
| 80% | 529 | 5,000,000 | Absence of failure | |
| 80% | 529 | 5,000,000 | Absence of failure | |
| Control group (n = 9) | ||||
| 80% | 735 | 36,364 | Implant body | Fracture |
| 80% | 735 | 66,690 | Implant body | Fracture |
| 70% | 643 | 38,830 | Implant body | Fracture |
| 70% | 643 | 68,519 | Implant body | Fracture |
| 65% | 597 | 112,841 | Implant body | Fracture |
| 65% | 597 | 85,644 | Implant body | Fracture |
| 60% | 551 | 5,000,000 | Absence of failure | |
| 60% | 551 | 5,000,000 | Absence of failure | |
| 60% | 551 | 5,000,000 | Absence of failure | |
| Samples | EOCP (mV) | icorr (μA/cm2) | ECORR (mV) |
|---|---|---|---|
| As-received | −141.7 ± 0.3 | 0.019 ± 0.010 | −380 ± 18 |
| Interface smooth-roughned | −194.3 ± 0.4 | 0.069 ± 0.016 | −450 ± 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camps-Font, O.; Toledano-Serrabona, J.; Juiz-Camps, A.; Gil, J.; Sánchez-Garcés, M.A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants. J. Funct. Biomater. 2023, 14, 61. https://doi.org/10.3390/jfb14020061
Camps-Font O, Toledano-Serrabona J, Juiz-Camps A, Gil J, Sánchez-Garcés MA, Figueiredo R, Gay-Escoda C, Valmaseda-Castellón E. Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants. Journal of Functional Biomaterials. 2023; 14(2):61. https://doi.org/10.3390/jfb14020061
Chicago/Turabian StyleCamps-Font, Octavi, Jorge Toledano-Serrabona, Ana Juiz-Camps, Javier Gil, Maria Angeles Sánchez-Garcés, Rui Figueiredo, Cosme Gay-Escoda, and Eduard Valmaseda-Castellón. 2023. "Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants" Journal of Functional Biomaterials 14, no. 2: 61. https://doi.org/10.3390/jfb14020061
APA StyleCamps-Font, O., Toledano-Serrabona, J., Juiz-Camps, A., Gil, J., Sánchez-Garcés, M. A., Figueiredo, R., Gay-Escoda, C., & Valmaseda-Castellón, E. (2023). Effect of Implantoplasty on Roughness, Fatigue and Corrosion Behavior of Narrow Diameter Dental Implants. Journal of Functional Biomaterials, 14(2), 61. https://doi.org/10.3390/jfb14020061








